Investigations on the Diffusion of Platinum between CMSX-4 Superalloy and Platinum-Enriched Bond Coat
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
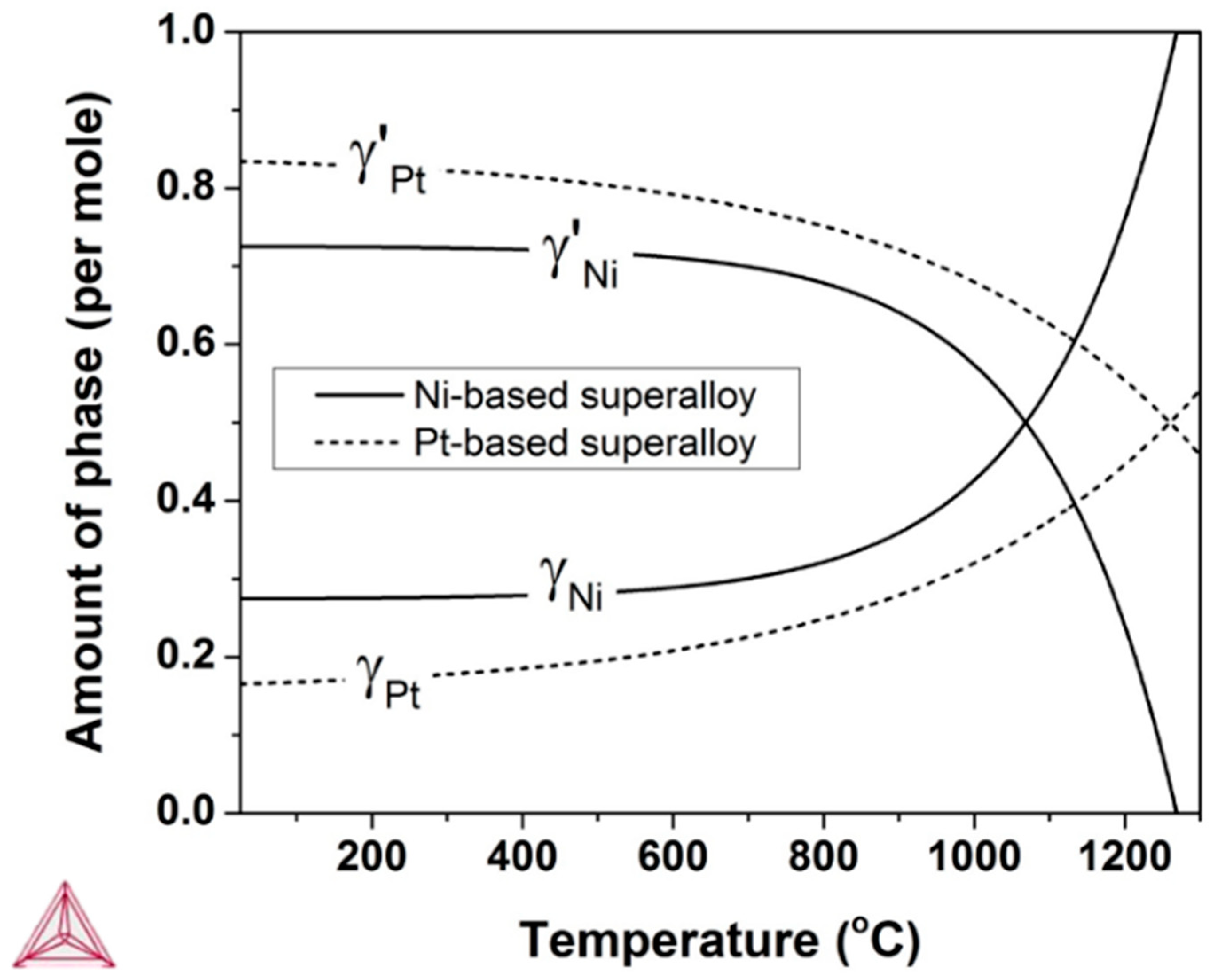
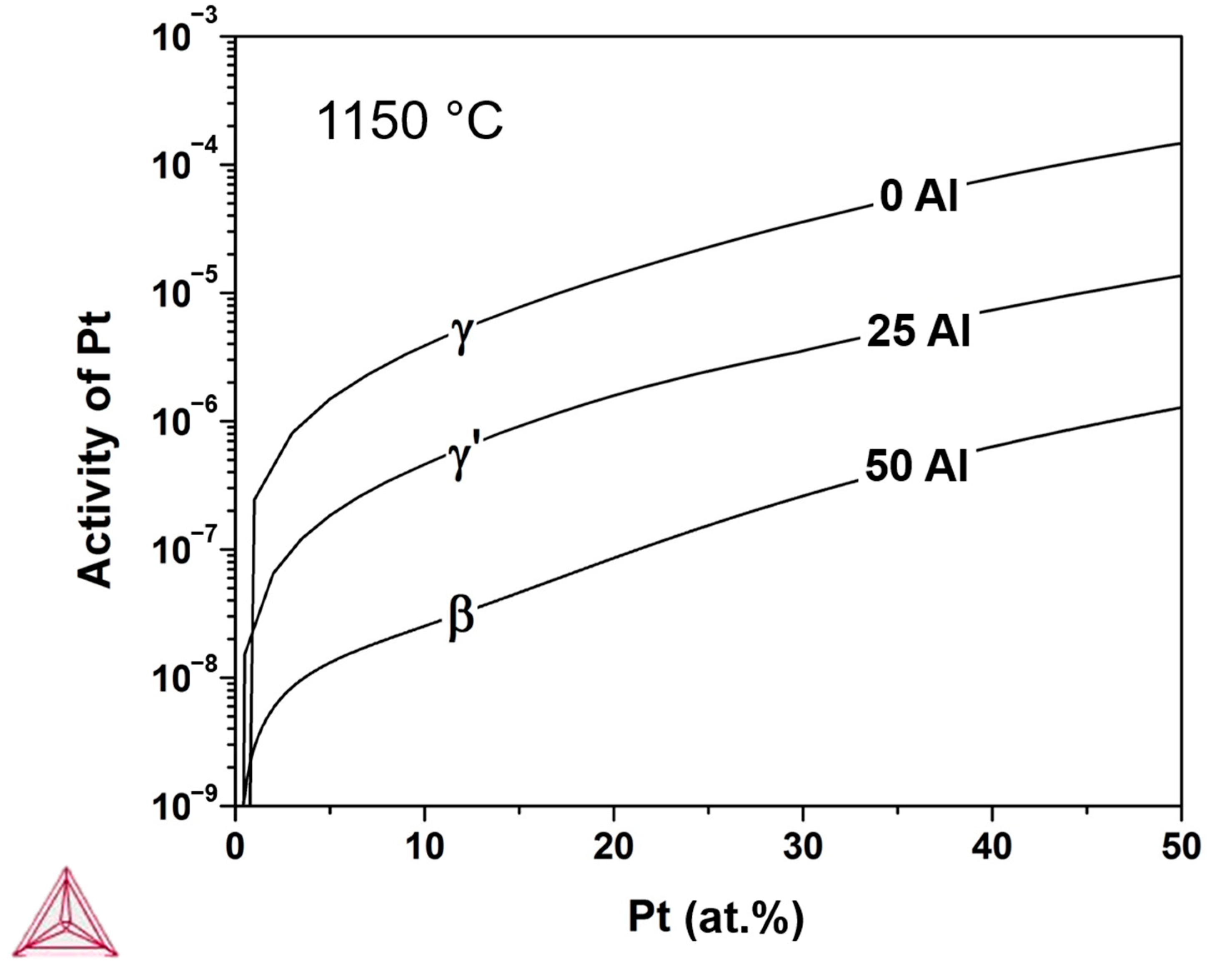
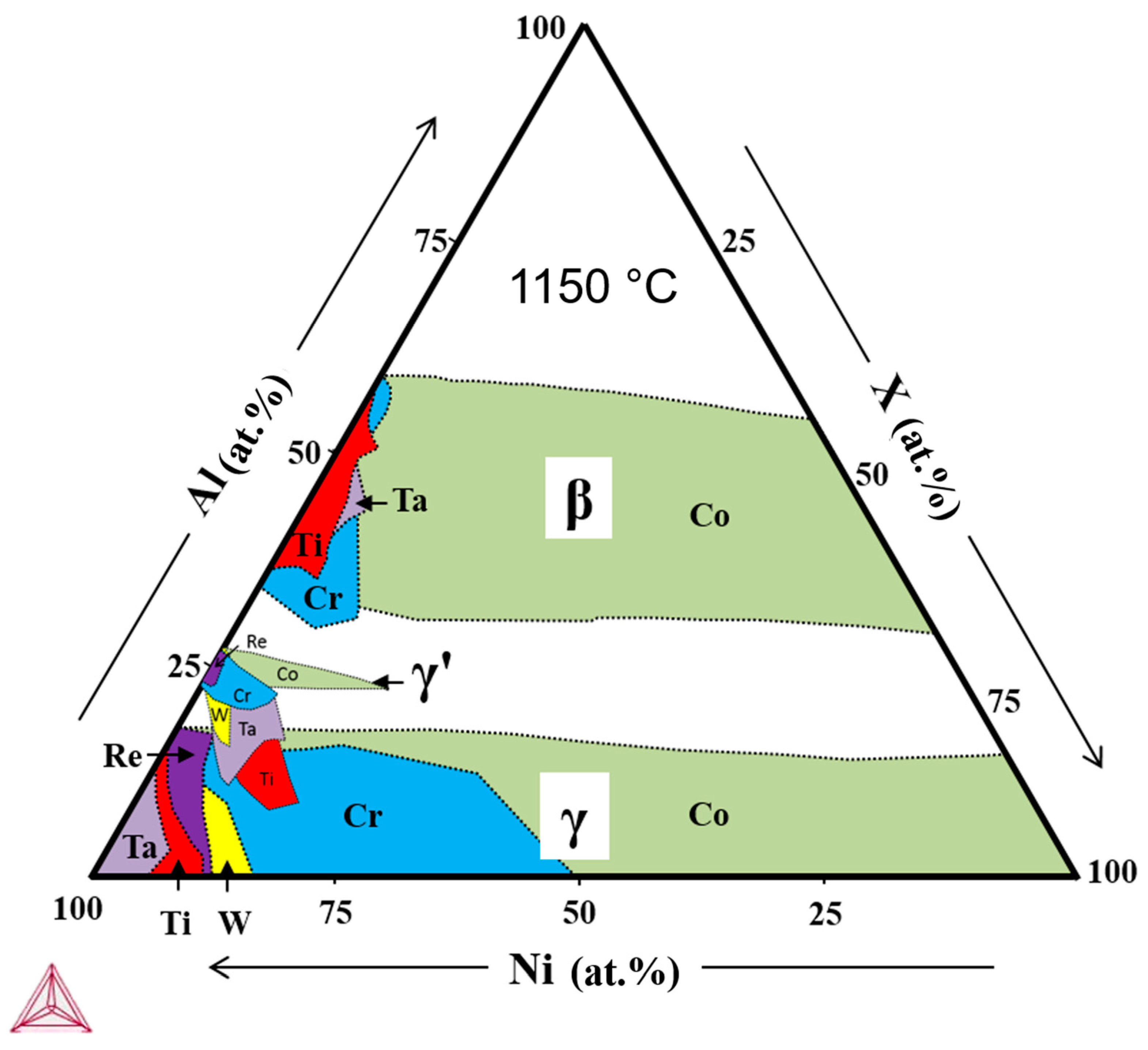
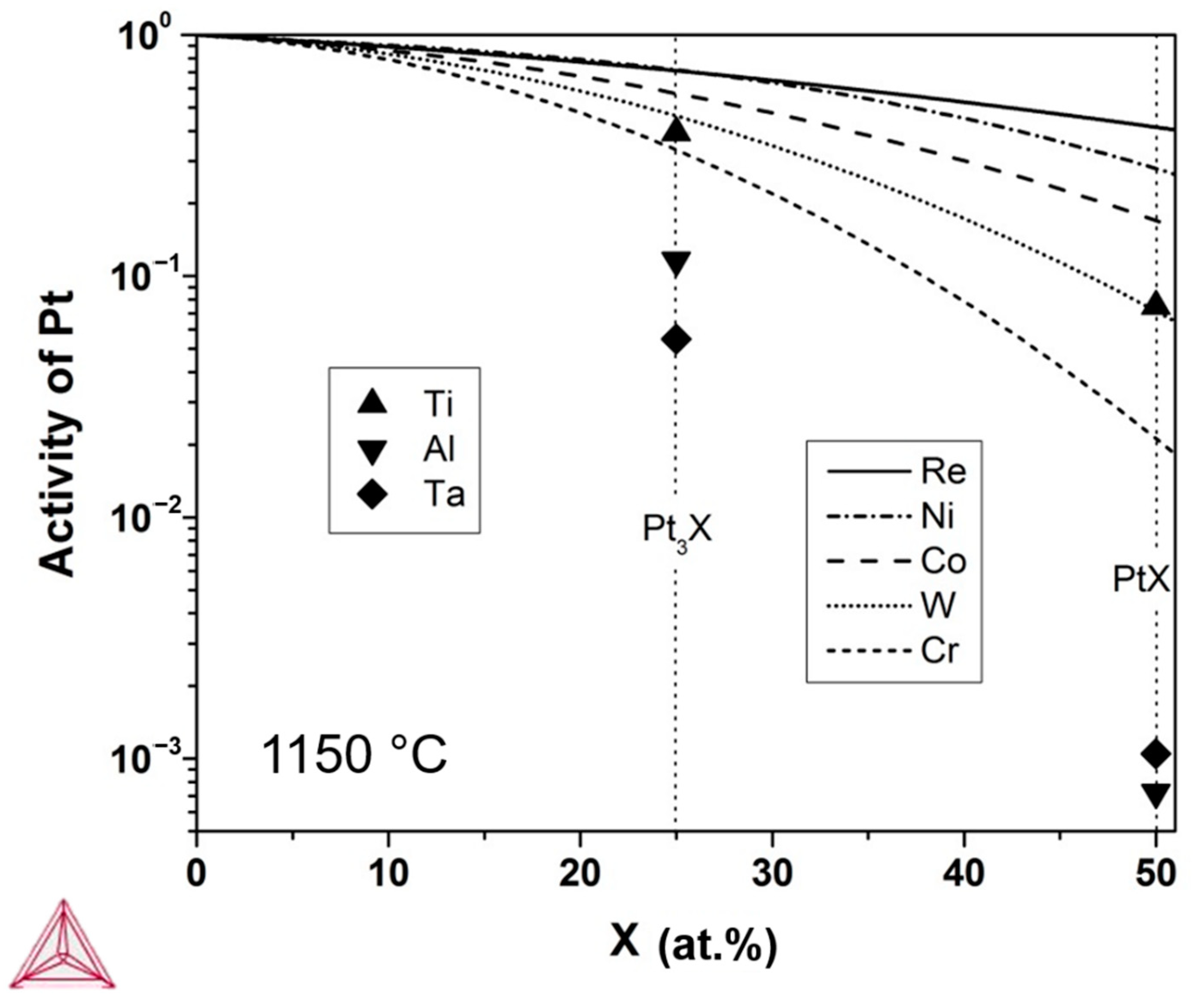
2.3. Thermodynamic Calculations
3. Results
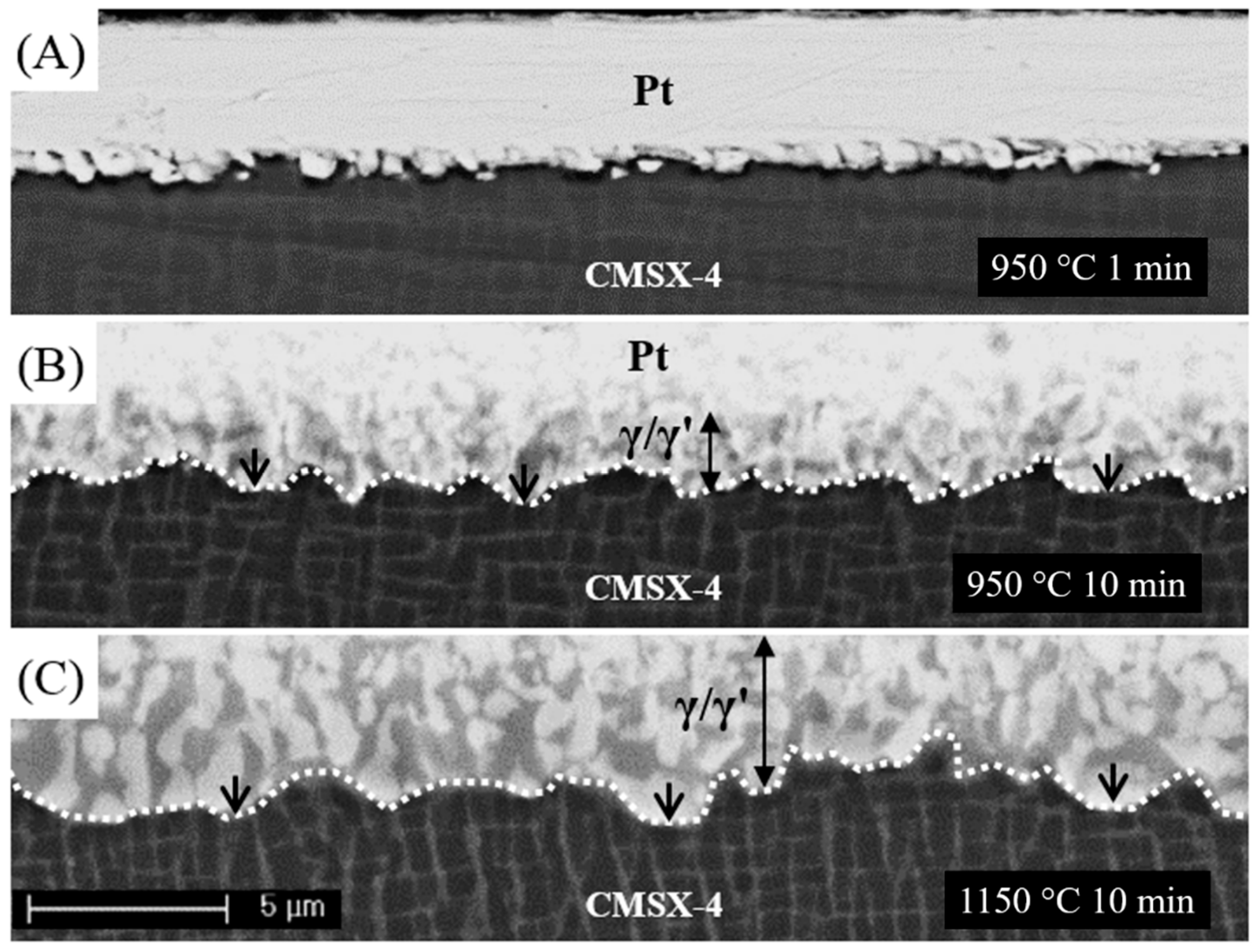
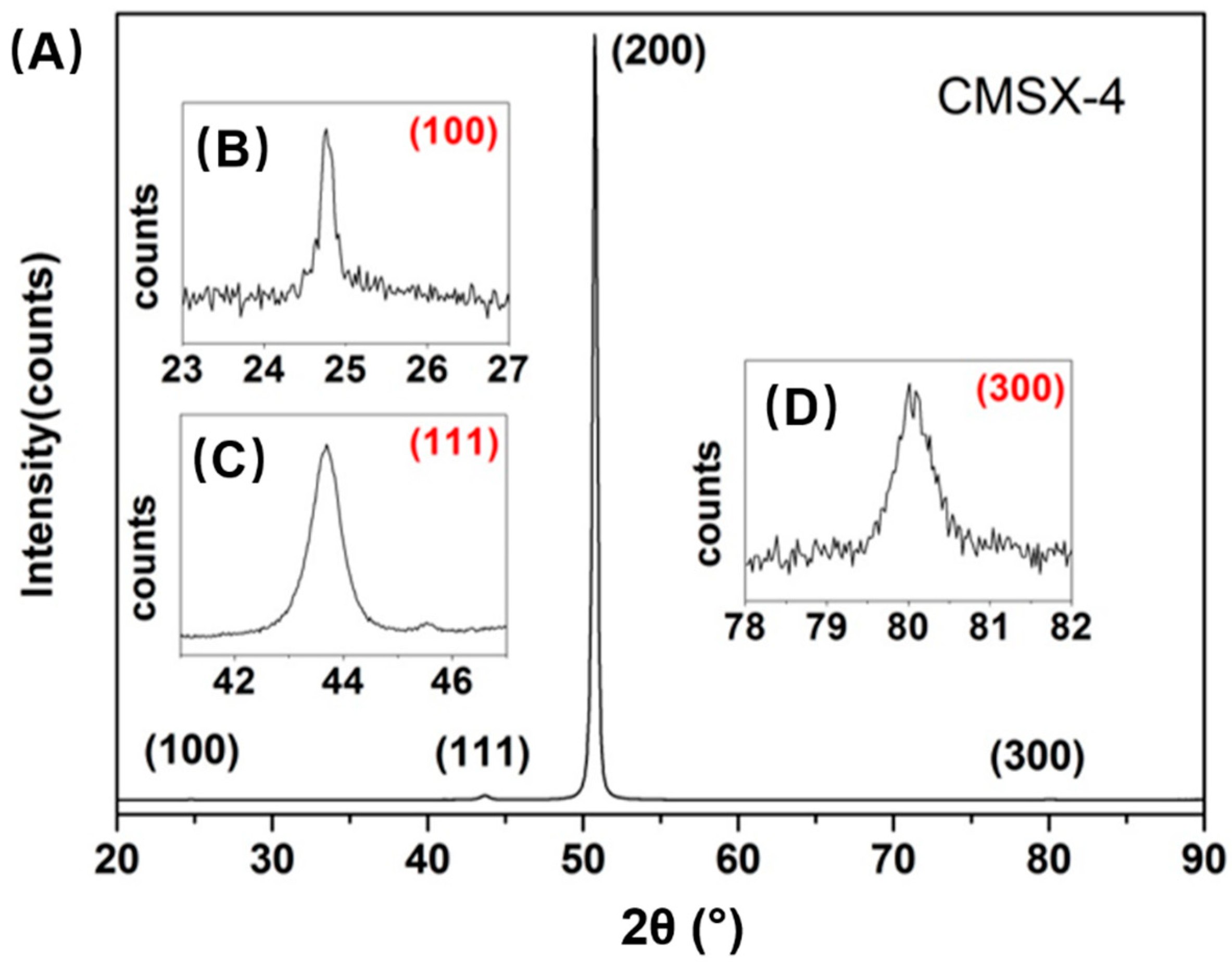
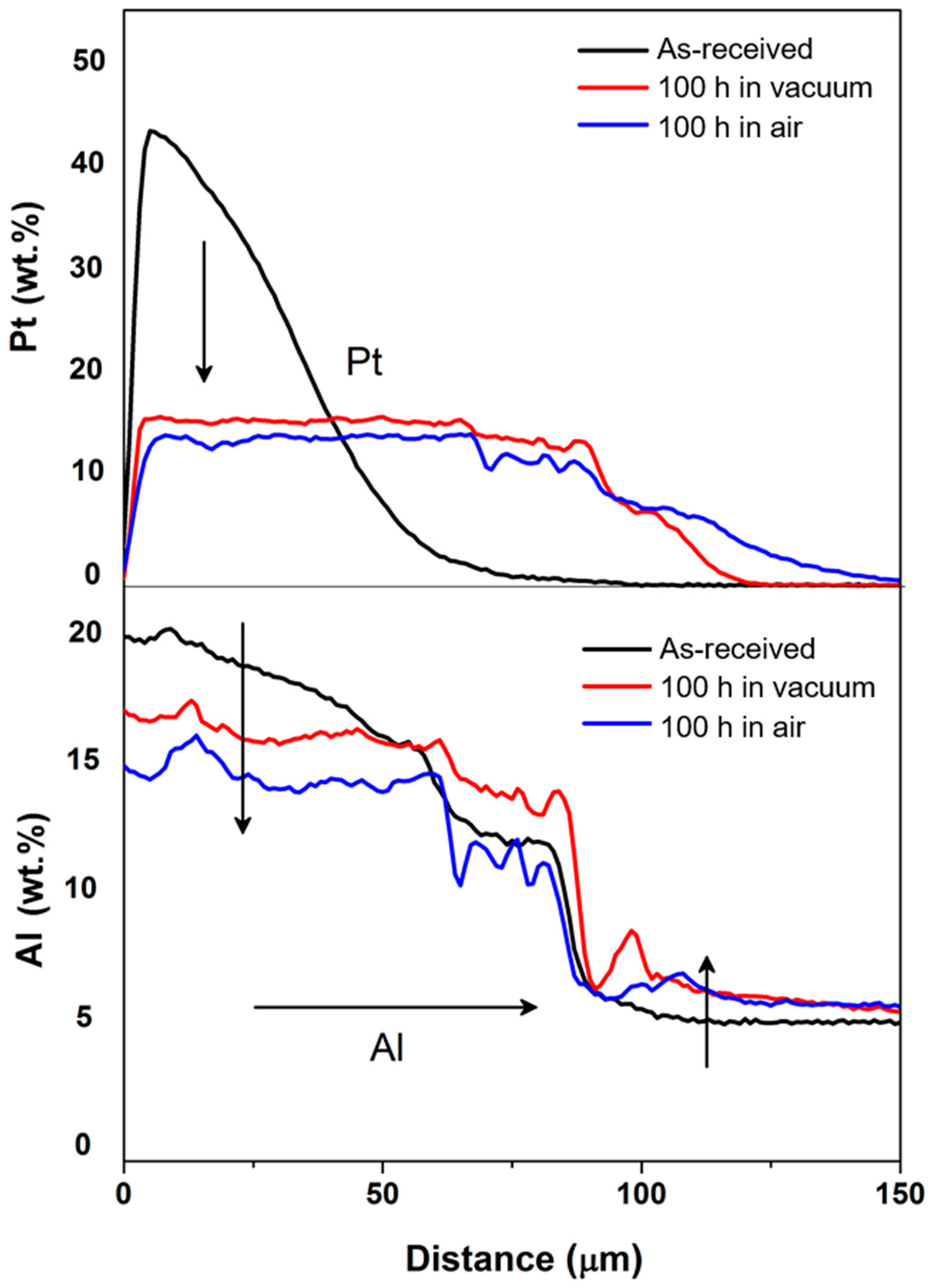
3.1. Diffusion of Pt into Superalloy
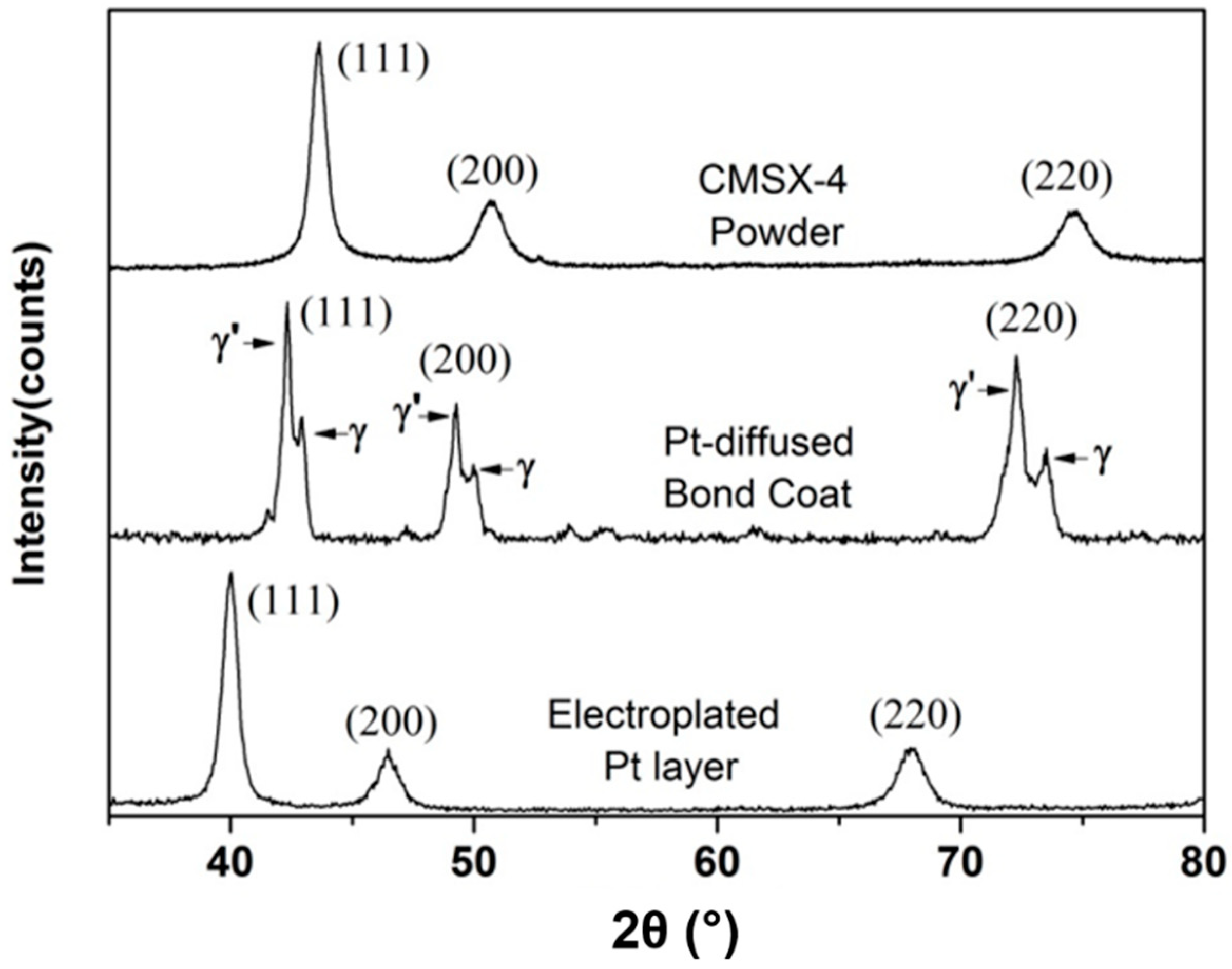
3.2. Diffusion of Pt in γ/γ′ Bond Coat/Superalloy
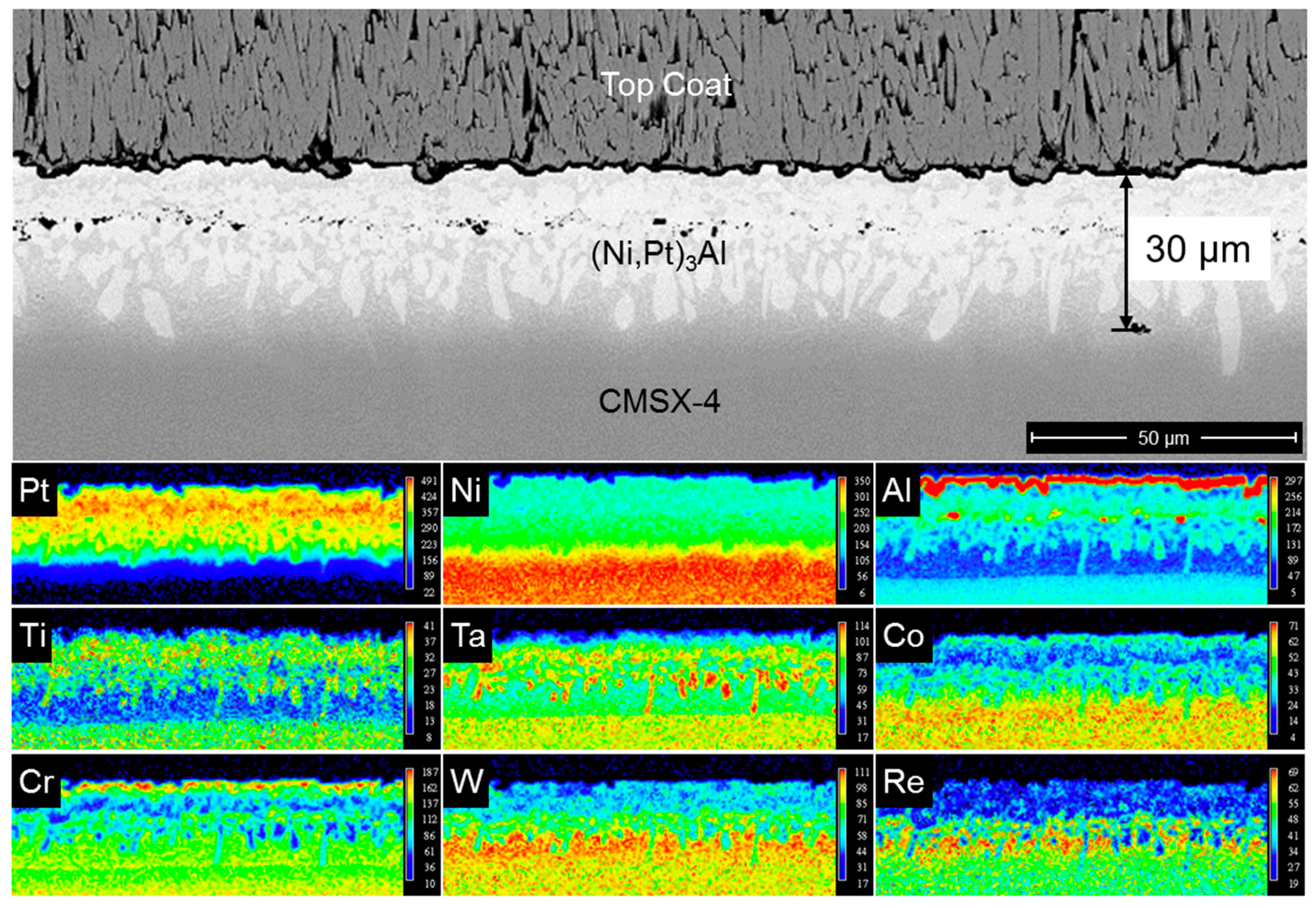
3.3. Diffusion of Pt in β Bond Coat/Superalloy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, A.G.; Mumm, D.R.; Hutchinson, J.W.; Meier, G.H.; Pettit, F.S. Mechanisms controlling the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 505–553. [Google Scholar] [CrossRef]
- Nicholls, J.R. Advances in coating design for high-performance gas turbines. MRS Bull. 2003, 28, 659–670. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials design for the next generation thermal barrier coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Zhang, Y.; Haynes, J.A.; Wright, G.; Pint, B.A.; Cooley, K.M.; Lee, W.Y.; Liaw, P.K. Effects of Pt incorporation on the isothermal oxidation behavior of chemical vapor deposition aluminide coatings. Met. Mater. Trans. A 2001, 32, 1727–1741. [Google Scholar] [CrossRef]
- Cadoret, Y.; Bacos, M.P.; Josso, P.; Maurice, V.; Marcus, P.; Zanna, S. Effect of Pt additions on the sulfur segregation, void formation and oxide scale growth of cast nickel aluminides. Mater. Sci. Forum 2004, 461–464, 247–254. [Google Scholar] [CrossRef]
- Haynes, J.A.; Pint, B.A.; More, K.; Zhang, Y.; Wright, I.G. Influence of sulfur, platinum, and hafnium on the oxidation behavior of cvd nial bond coatings. Oxid. Met. 2002, 58, 513–544. [Google Scholar] [CrossRef]
- Hou, P.; Mccarty, K.F. Surface and interface segregation in β-NiAl with and without Pt addition. Scr. Mater. 2006, 54, 937–941. [Google Scholar] [CrossRef]
- Chen, J.; Little, J. Degradation of the platinum aluminide coating on CMSX4 at 1100 °C. Surf. Coat. Technol. 1997, 92, 69–77. [Google Scholar] [CrossRef]
- Bouchet, R.; Mévrel, R. Influence of platinum and palladium on diffusion in β-NiAl phase. Defect Diffus. Forum 2005, 238–243. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Rickerby, D.; Jones, R.; Xiao, P. Evolution of interfacial toughness of a thermal barrier system with a Pt-diffused γ/γ′ bond coat. Acta Mater. 2011, 59, 6401–6411. [Google Scholar] [CrossRef]
- Hayashi, S.; Sordelet, D.J.; Walker, L.R.; Gleeson, B. Interdiffusion in Pt-containing γ-ni and γ′-ni3al alloys at 1150 °C. Mater. Trans. 2008, 49, 1550–1557. [Google Scholar] [CrossRef]
- Minamino, Y.; Koizumi, Y.; Tsuji, N.; Morioka, M.; Hirao, K.; Shirai, Y. Pt diffusion in B2-type ordered NiAl intermetallic compound and its diffusion mechanisms. Sci. Technol. Adv. Mater. 2000, 1, 237–249. [Google Scholar] [CrossRef]
- Yamane, T.; Hisayuki, K.; Yoshida, H.; Minamino, Y.; Araki, H.; Hirao, K. Diffusion of platinum, vanadium and manganese in Ni3Al phase under high pressure. J. Mater. Sci. 1999, 34, 1835–1838. [Google Scholar] [CrossRef]
- Bouchet, R.; Mevrel, R. Calculating the composition-dependent diffusivity matrix along a diffusion path in ternary systems. Calphad 2003, 27, 295–303. [Google Scholar] [CrossRef]
- Copland, E. Partial Thermodynamic properties of γ′-(Ni,Pt)3Al in the Ni-Al-Pt system. J. Phase Equilibria Diffus. 2007, 28, 38–48. [Google Scholar] [CrossRef]
- Filipek, R.; Datta, P.K.; Danielewski, M.; Bednarz, L.; Best, R.; Rakowska, A. Interdiffusion in the Pt/Beta-NiAl System; Scitec Publications Ltd.: Uetikon-Zuerich, Switzerland, 2001; Volume 194-1, pp. 571–576. [Google Scholar]
- Gleeson, B.; Wang, W.; Hayashi, S.; Sordelet, D. Effects of platinum on the interdiffusion and oxidation behavior of Ni-Al-based alloys. In High Temperature Corrosion and Protection of Materials 6, Prt 1 and 2, Proceedings; Steinmetz, P., Wright, I.G., Meier, G., Galerie, A., Pieraggi, B., Podor, R., Eds.; Trans Tech Publications Ltd.: Zurich-Uetikon, Switzerland, 2004; Volume 461–464, pp. 213–222. [Google Scholar]
- Hayashi, S.; Ford, S.; Young, D.; Sordelet, D.; Besser, M.; Gleeson, B. α-NiPt(Al) and phase equilibria in the Ni–Al–Pt system at 1150 °C. Acta Mater. 2005, 53, 3319–3328. [Google Scholar] [CrossRef]
- Matsumaru, H.; Hayashi, S.; Narita, T. Interdiffusion between Ni based superalloy and diffusion barrier coatings at 1423 K. Mater. Sci. Forum 2006, 522–523, 285–292. [Google Scholar] [CrossRef]
- Karunaratne, M.; Reed, R. Interdiffusion of the platinum-group metals in nickel at elevated temperatures. Acta Mater. 2003, 51, 2905–2919. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Bai, M.; Chandio, A.; Wu, R.; Xiao, P. Effect of platinum addition on oxidation behaviour of γ/γ′ nickel aluminide. Acta Mater. 2015, 86, 319–330. [Google Scholar] [CrossRef]
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Zhao, X.; Cernik, B.; Tang, C.; Thompson, S.; Xiao, P. Stress evolution in a Pt-diffused γ/γ′ bond coat after oxidation. Surf. Coat. Technol. 2014, 247, 48–54. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Qiu, L.; Liu, H.; Bai, M.; Xiao, P. The al-enriched γ′-Ni3Al-base bond coat for thermal barrier coating applications. Corros. Sci. 2020, 167, 108523. [Google Scholar] [CrossRef]
- Bai, M. Fabrication and Characterization of Thermal Barrier Coatings; University of Manchester: Manchester, UK, 2015. [Google Scholar]
- TCS Ni-Based Superalloys Database, version 6.0; Software For thermodynamic calculation; Foundation of Computational Thermodynamics: Stockholm, Sweden, 2011.
- Andersson, J.-O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Bai, M.; Reddy, L.; Hussain, T. Experimental and thermodynamic investigations on the chlorine-induced corrosion of HVOF thermal sprayed NiAl coatings and 304 stainless steels at 700 °C. Corros. Sci. 2018, 135, 147–157. [Google Scholar] [CrossRef]
- Pala, Z.; Bai, M.; Lukac, F.; Hussain, T. Laser clad and HVOF-sprayed stellite 6 coating in chlorine-rich environment with KCl at 700 °C. Oxid. Met. 2017, 88, 749–771. [Google Scholar] [CrossRef]
- Bai, M.; Song, B.; Reddy, L.; Hussain, T. Preparation of MCrAlY–Al2O3 composite coatings with enhanced oxidation resistance through a novel powder manufacturing process. J. Therm. Spray Technol. 2019, 28, 433–443. [Google Scholar] [CrossRef]
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Teatum, E.T.; Gschneidner, K.A., Jr.; Waber, J.T. Compilation of Calculated Data Useful in Predicting Metallurgical Behavior of the Elements in Binary Alloy Systems; Los Alamos Scientific Laboratory of the University of California: Santa Fe, NM, USA, 1960; Volume 2345. [Google Scholar]
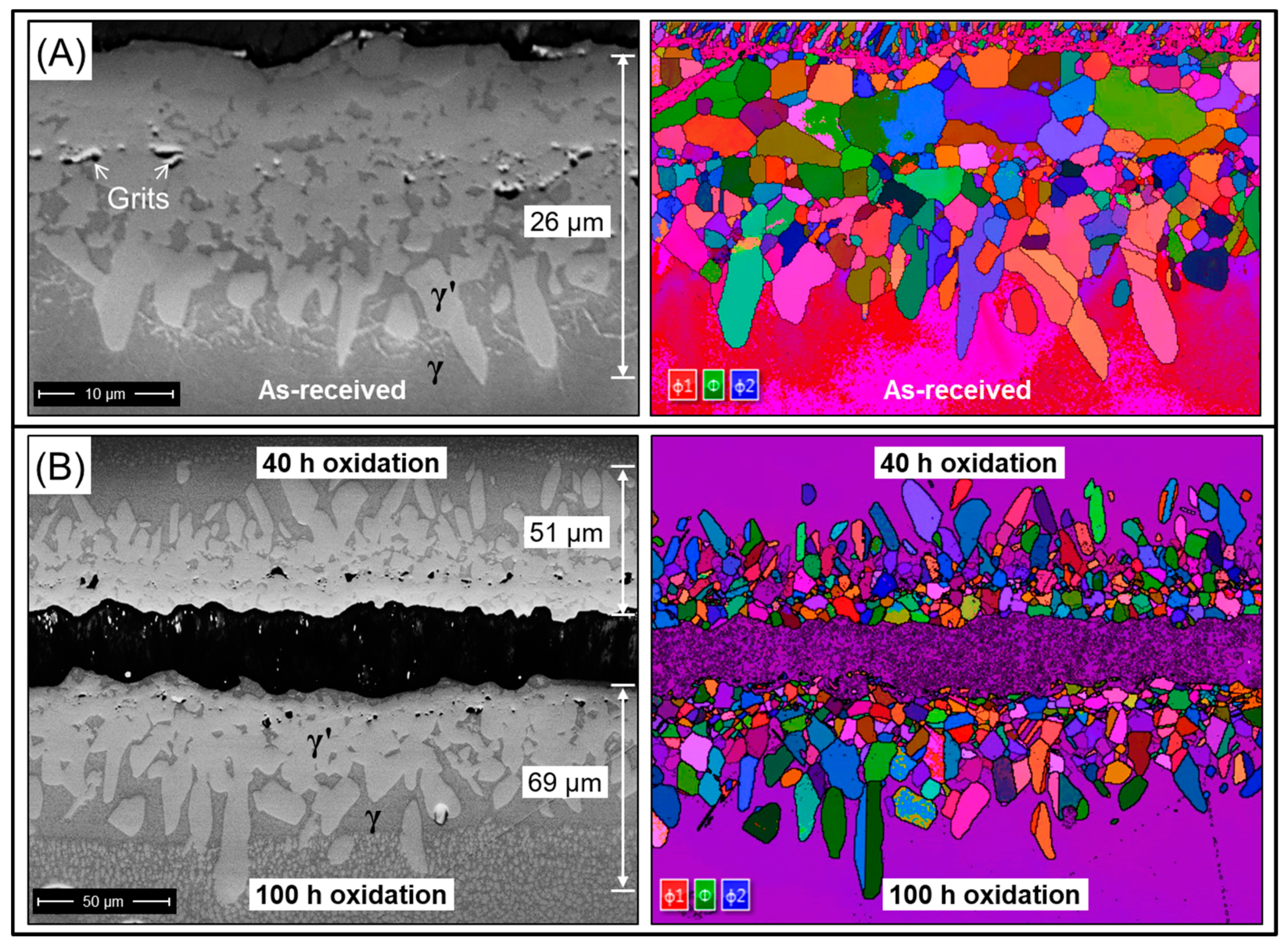
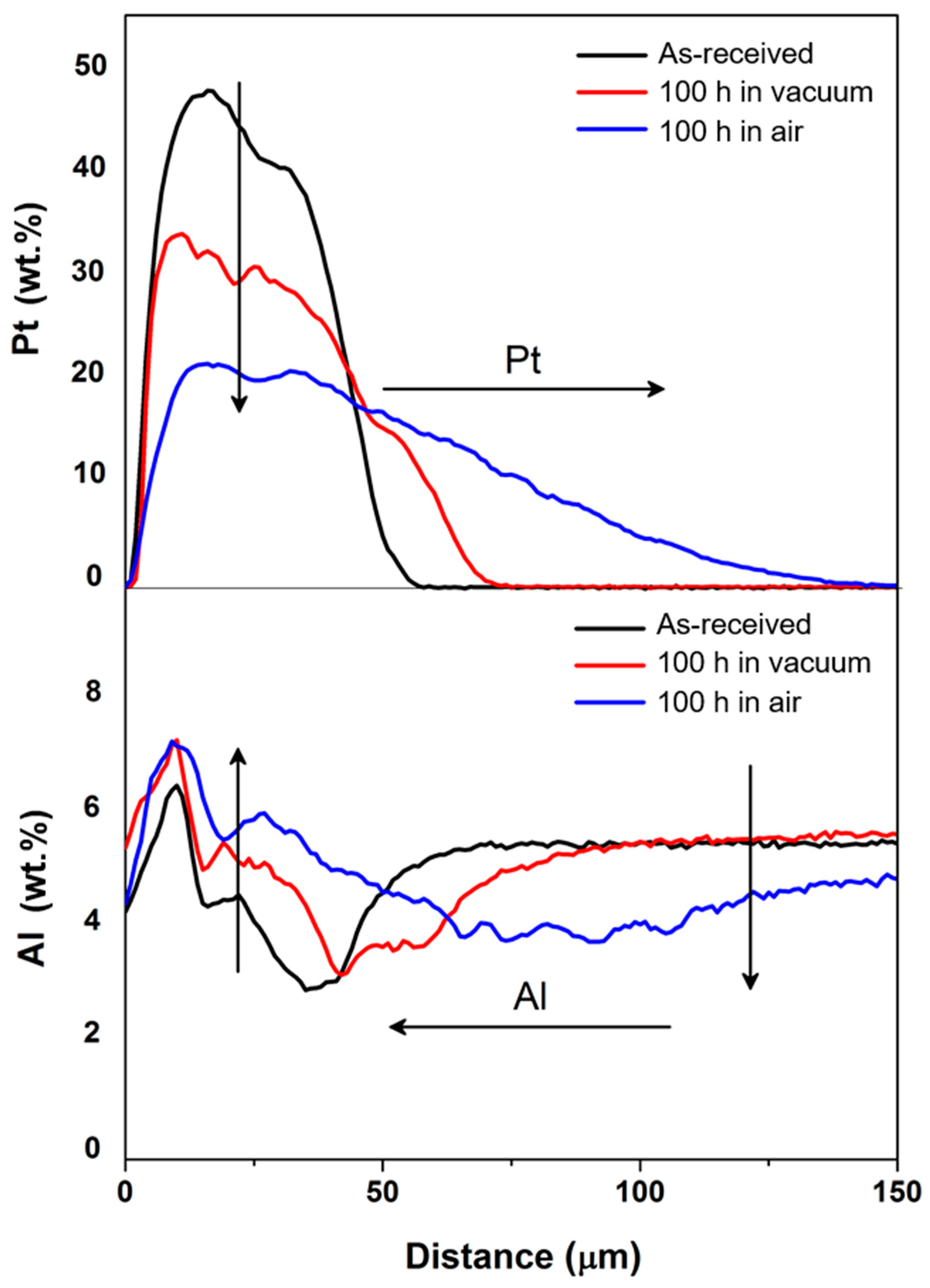
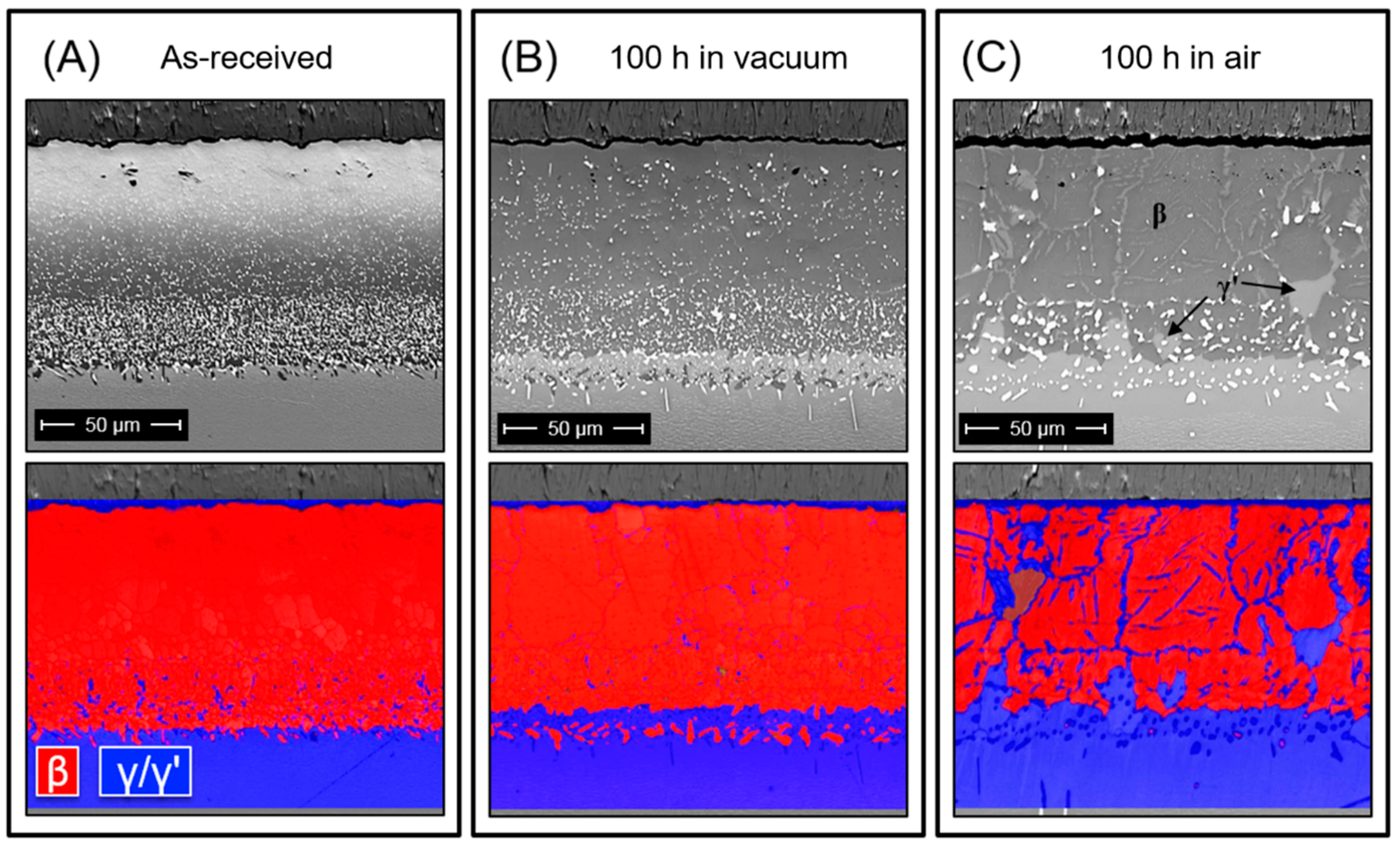
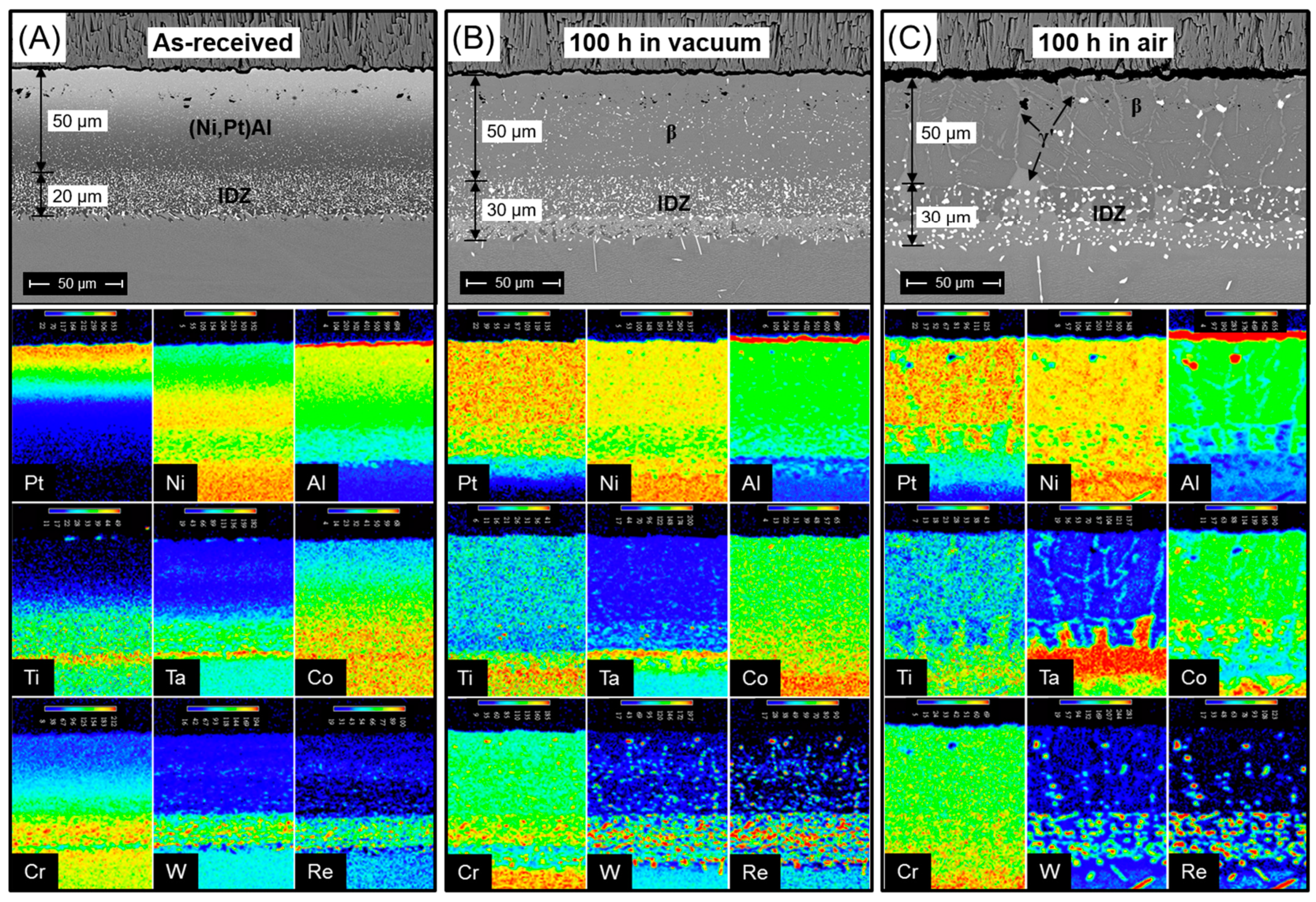
| γ′-Phase | γ-Phase |
|---|---|
| (Ni, Pt)3X (X = Al, Ti or Ta) | Ni (Cr, Co, W, Re) |
| β-Phase | Γ’-Phase | γ-Phase |
|---|---|---|
| (Pt, Ni, Cr, Co) Al | Ni3X (X = Ti, Ta) | Ni (W, Re, Cr, Co) |
| Sample | a (Å) |
|---|---|
| CMSX-4 superalloy | 3.594 |
| CMSX-4 (γ′-cubes) | 3.597 |
| As-electroplated Pt layer | 3.908 |
| Pt-diffused γ′/γ bond coat | a(γ) = 3.643; a(γ′) = 3.698 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, M.; Chen, Y.; Xiao, P. Investigations on the Diffusion of Platinum between CMSX-4 Superalloy and Platinum-Enriched Bond Coat. Coatings 2021, 11, 441. https://doi.org/10.3390/coatings11040441
Bai M, Chen Y, Xiao P. Investigations on the Diffusion of Platinum between CMSX-4 Superalloy and Platinum-Enriched Bond Coat. Coatings. 2021; 11(4):441. https://doi.org/10.3390/coatings11040441
Chicago/Turabian StyleBai, Mingwen, Ying Chen, and Ping Xiao. 2021. "Investigations on the Diffusion of Platinum between CMSX-4 Superalloy and Platinum-Enriched Bond Coat" Coatings 11, no. 4: 441. https://doi.org/10.3390/coatings11040441
APA StyleBai, M., Chen, Y., & Xiao, P. (2021). Investigations on the Diffusion of Platinum between CMSX-4 Superalloy and Platinum-Enriched Bond Coat. Coatings, 11(4), 441. https://doi.org/10.3390/coatings11040441








