Preparation and Application of Polyurethane Coating Material Based on Epoxy Cyclohexane Protective for Paper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Epoxy Cyclohexane Homopolyether
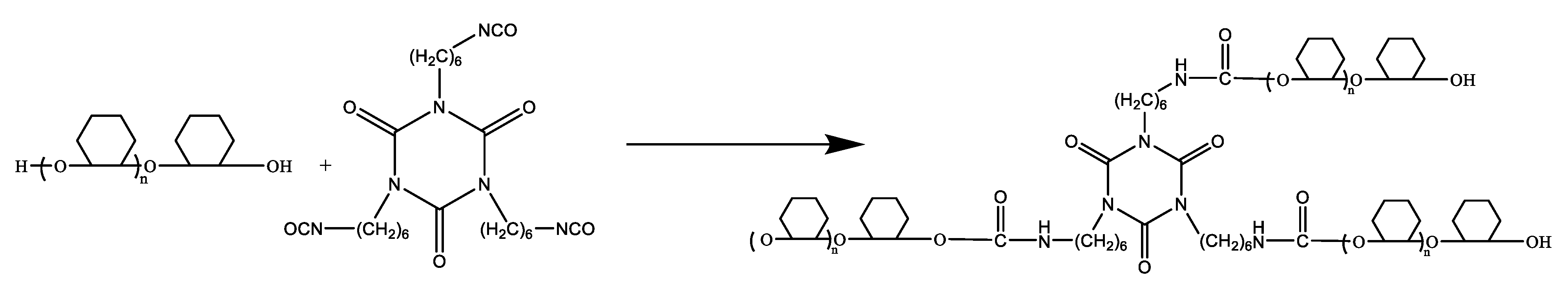
2.3. Preparation of Polyurethane Based on Epoxy Cyclohexane Homopolyetheryl
2.4. Preparation of Paper Samples
2.5. Analysis and Testing Methods
3. Results and Discussion
3.1. The Reaction Mechanism of Epoxy Cyclohexane Homopolyether
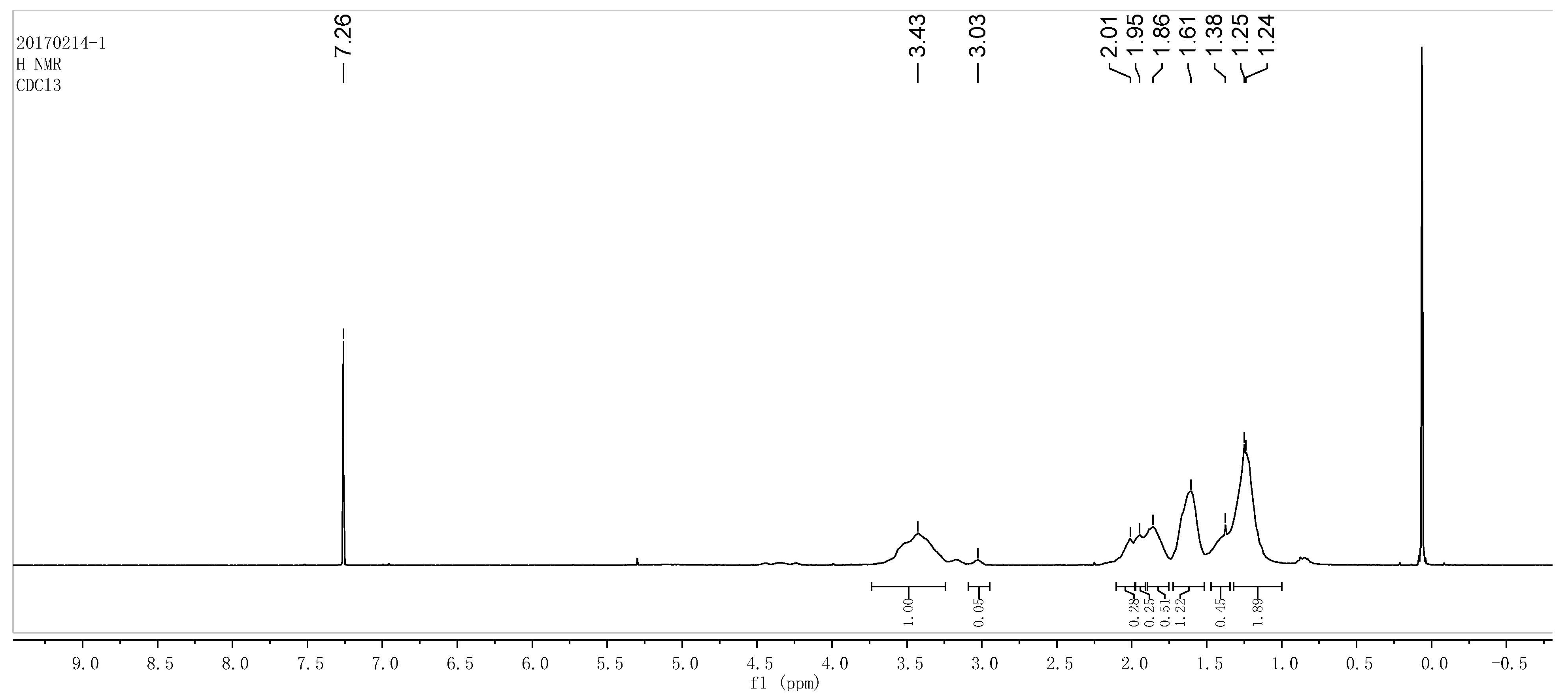
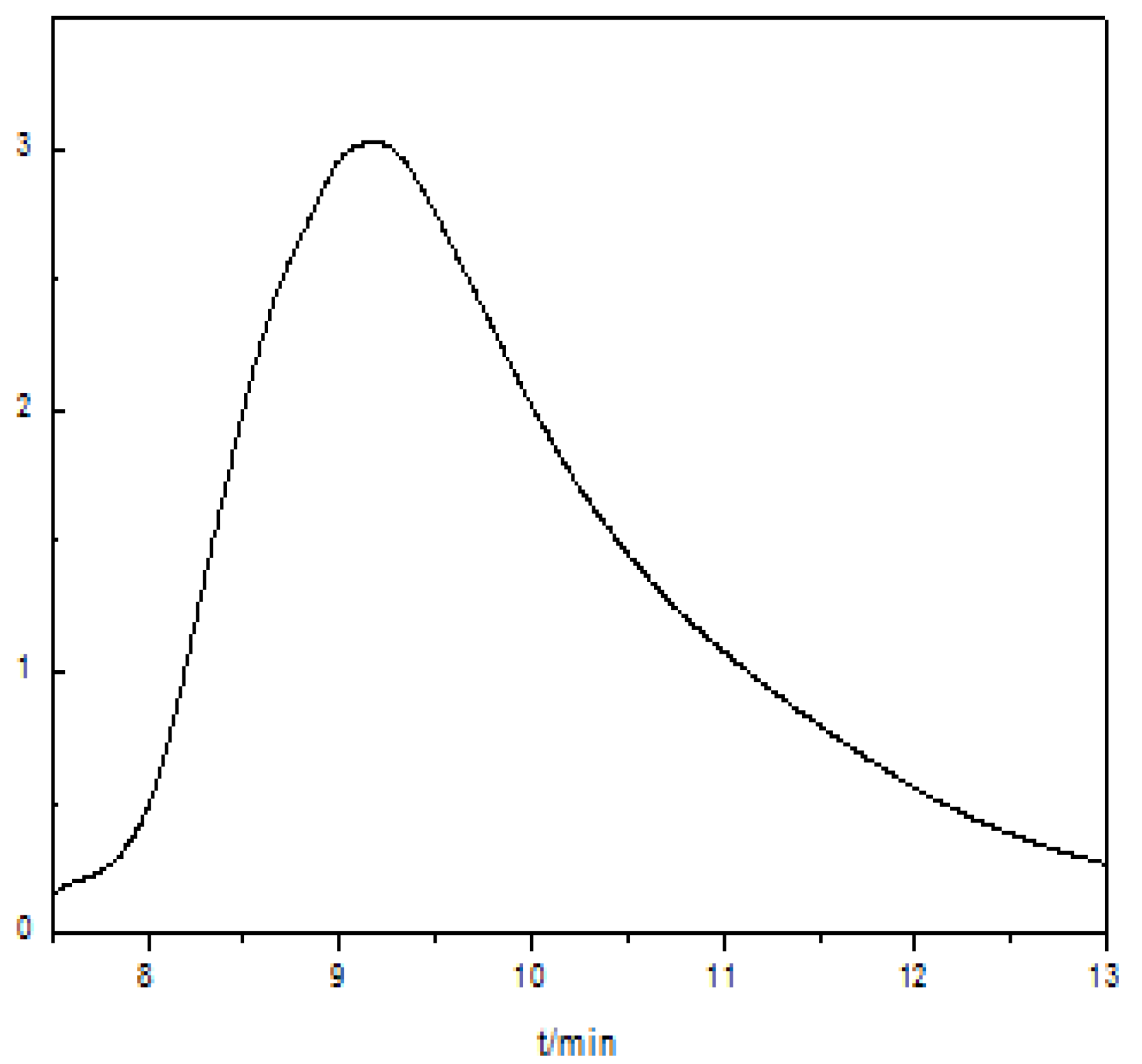
3.2. Properties of Polyurethane Based on Epoxy Cyclohexane Homopolyether
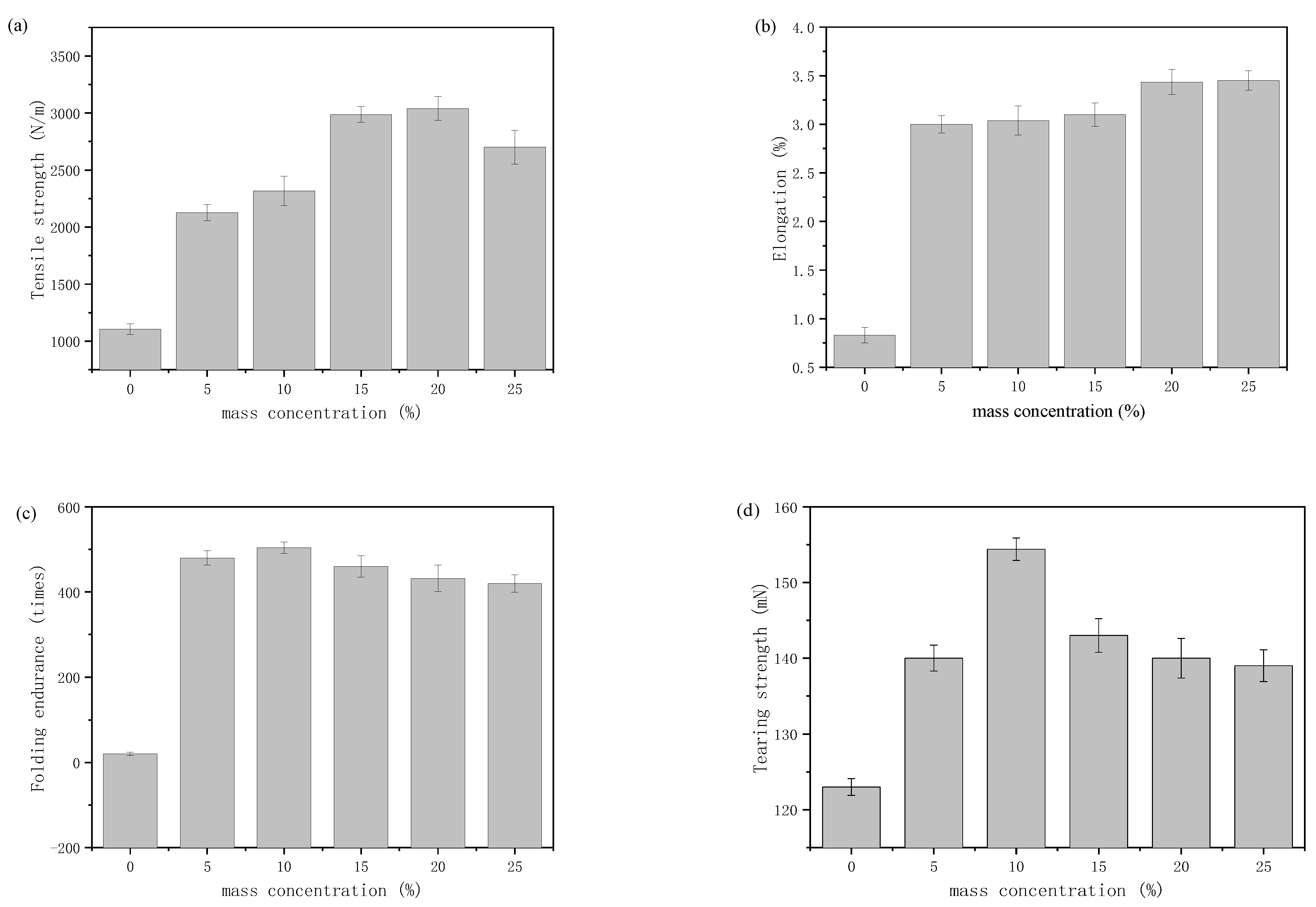
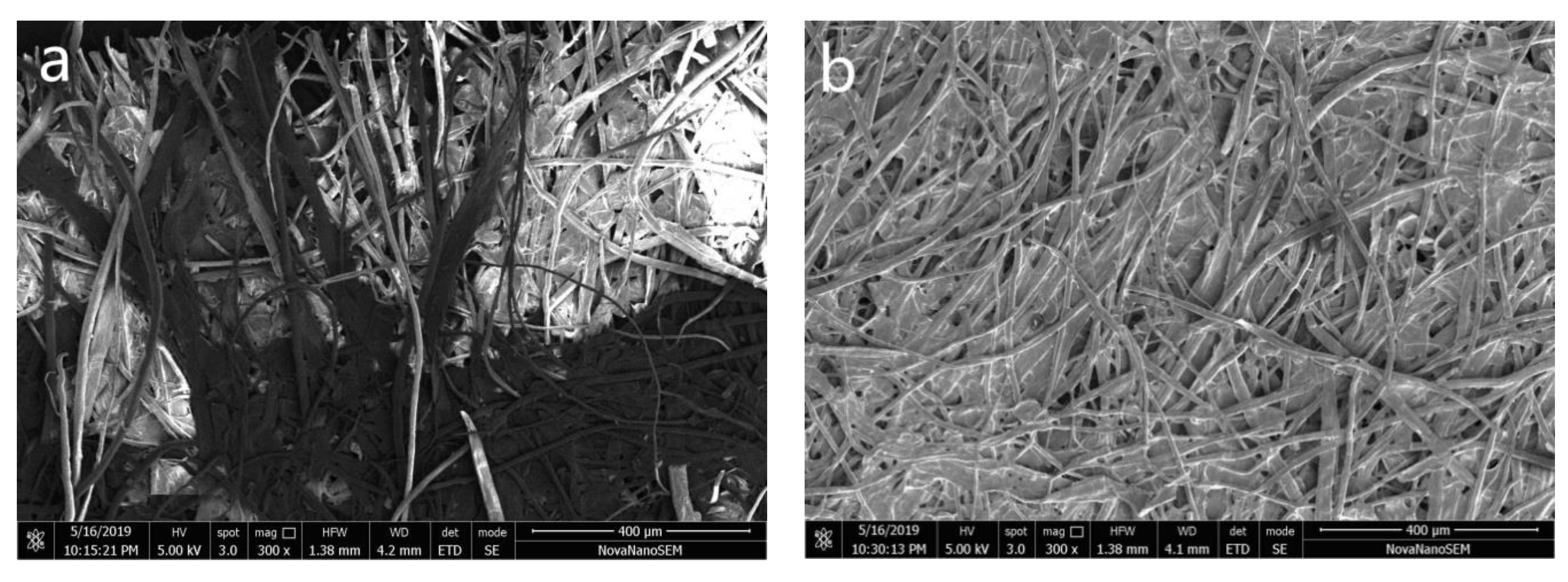
3.3. Application of Polyurethane Based on Epoxy Cyclohexane Homopolyetheryl as Protective Material for Paper Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
Abbreviations
Appendix A

References
- Camargos, C.H.M.; Junior, J.C.F.; Pereira, F.V. Cellulose nanocrystal-based composite for restoration of lacunae on damaged documents and artworks on paper. J. Cult. Herit. 2017, 23, 170–175. [Google Scholar] [CrossRef]
- Vohrer, U.; Trick, I.; Bernhardt, J.; Oehr, C.; Brunner, H. Plasma treatment—An increasing technology for paper restoration? Surf. Coat. Technol. 2001, 142, 1069–1073. [Google Scholar] [CrossRef]
- Qiao, L.; Chen, K.; Zhao, D.; Zhan, Y.; Min, W.; Huang, Q.; Shen, G.; Zhou, J. The application of poly(methyl methacrylate-co-butyl acrylate-co-styrene) in reinforcing fragile papers: Experiments and computer simulations. Cellulose 2017, 24, 5157–5171. [Google Scholar] [CrossRef]
- El-Feky, O.M.; Hassan, E.A.; Fadel, S.M.; Hassan, M.L. Use of ZnO nanoparticles for protecting oil paintings on paper support against dirt, fungal attack, and UV aging. J. Cult. Herit. 2014, 15, 165–172. [Google Scholar] [CrossRef]
- Afsharpour, M.; Imani, S. Preventive protection of paper works by using nanocomposite coating of zinc oxide. J. Cult. Herit. 2017, 25, 142–148. [Google Scholar] [CrossRef]
- Jin, S.; Qi, Y.; Shen, Y.; Li, H. A transparent polyurethane based on nanosilica in reinforcing papers. Nord. Pulp Pap. Res. J. 2020, 36, 82–90. [Google Scholar] [CrossRef]
- Chen, Q.; Wen, W.Y.; Qiu, F.X.; Xu, J.C.; Yu, H.Q.; Chen, M.L.; Yang, D.Y. Preparation and application of modified carboxymethyl cellulose Si/polyacrylate protective coating material for paper relics. Chem. Pap. 2016, 70, 946–959. [Google Scholar] [CrossRef]
- Totolin, M.I.; Neamţu, I. Positive findings for plasma polymer (meth) acrylate thin films in heritage protective applications. J. Cult. Herit. 2011, 12, 392–398. [Google Scholar] [CrossRef]
- Mu, C.S.; Huang, K.L.; Li, K.X.; Luo, S.J.; Liu, Y.H.; Huang, S.S.; He, Y.L.; Li, W.G. Study progress in polyether polyol. Chem. Ind. Dev. 2009, 38, 13–18. (In Chinese) [Google Scholar]
- Qian, B.Z.; Zhu, J.F. Development progress on polyether polyol at home and abroad. Fine Spec. Chem. 2010, 18, 5–12. [Google Scholar]
- Krol, P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007, 52, 915–1015. [Google Scholar] [CrossRef]
- Paik Sung, C.S.; Smith, T.W.; Sung, N.H. Properties of segmented polyether poly(urethaneureas) based of 2,4-toluene diisocyanate. 2. infrared and mechanical studies. Macromolecules 1980, 13, 117–121. [Google Scholar] [CrossRef]
- Adhikari, R.; Gunatillake, P.A.; McCarthy, S.J.; Meijs, G. Mixed macrodiol-based siloxane polyurethanes: Effect of the comacrodiol structure on properties and morphology. J. Appl. Polym. 2015, 78, 1071–1082. [Google Scholar] [CrossRef]
- Wang, C.B.; Cooper, S.L. Morphology and properties of segmented polyether polyurethaneureas. Macromolecules 1983, 16, 775–786. [Google Scholar] [CrossRef]
- Miller, J.A.; Lin, S.B.; Hwang, K.K.; Wu, K.S.; Gibson, P.E.; Cooper, S.L. Properties of polyether-polyurethane block copolymers: Effects of hard segment length distribution. Macromolecules 1985, 18, 32–44. [Google Scholar] [CrossRef]
- Corcuera, M.A.; Rueda, L.; d’Arlas, B.F.; Arbelaiz, A.; Marieta, C.; Mondragon, I.; Eceiza, A. Microstructure and properties of polyurethanes derived from castor oil. Polym. Degrad. Stab. 2010, 95, 2175–2184. [Google Scholar] [CrossRef]
- Yang, B. Progress of the polyether polyols synthesis. Guangdong Chem. Ind. 2009, 36, 78–80. (In Chinese) [Google Scholar]
- Jin, S.S.; Qi, Y.P.; Shen, Y.F.; Li, H. Study on the application of oligomers in paper reinforcement protection. J. Chem. 2018, 1, 229–233. [Google Scholar]
- Jin, S.S.; Qi, Y.P.; Shen, Y.F.; Li, H. The application of organosilicon modified polyurethane in reinforcing traditional paper. Nord. Pulp Pap. Res. J. 2019, 34, 485–494. [Google Scholar] [CrossRef]
- Yang, N. Study on Synthesis and Properties of Transparent Polyurethane Materials Based on Epoxy Cyclohexane. Master’s Thesis, Zhengzhou University, Zhengzhou, China, April 2016. (In Chinese). [Google Scholar]
- GB/T12914-2008. Paper and Board-Determination of Tensile Properties; Standards Press of China: Beijing, China, 2008. [Google Scholar]
- GB/T 457-2008. Paper and Board-Determination of Folding Endurance; Standards Press of China: Beijing, China, 2008. [Google Scholar]
- GB/T 455-2002. Paper and Board-Determination of Tearing Resistance; Standards Press of China: Beijing, China, 2002. [Google Scholar]
- GB/T 8941-2013. Paper and Board-Measurement of Specular Gloss; Standards Press of China: Beijing, China, 2013. [Google Scholar]
- GB/T 7974-2013. Paper, Board and Pulps-Measurement of Diffuse Blue Reflectance Factor-D65 Brightness; Standards Press of China: Beijing, China, 2013. [Google Scholar]
- Li, Y.; Li, Z.; Shen, G.; Zhan, Y. Paper conservation with an aqueous NaOH/urea cellulose solution. Cellulose 2019, 26, 4589–4599. [Google Scholar] [CrossRef]
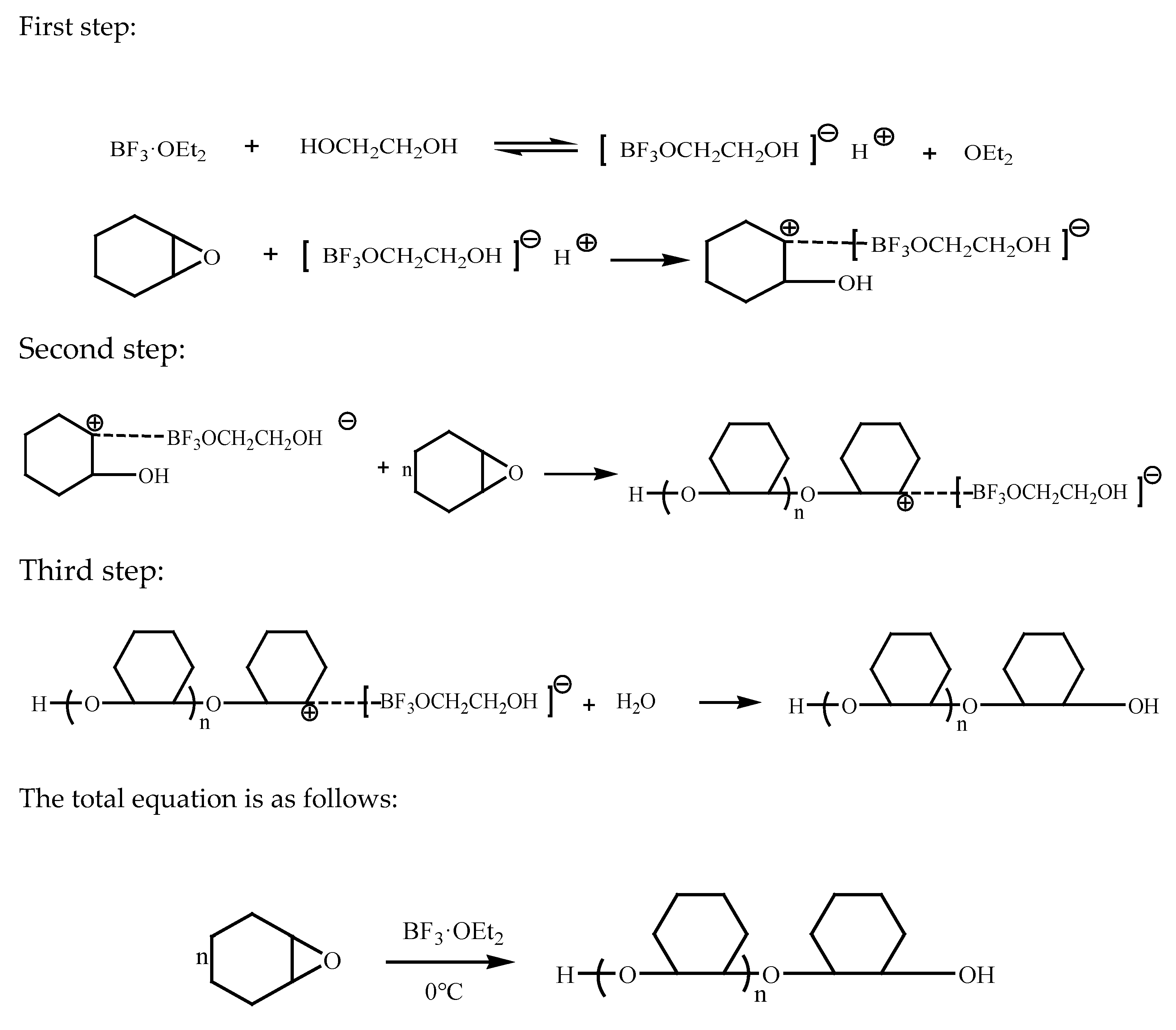


| Sample | 5% | 10% | 15% | 20% | 25% |
|---|---|---|---|---|---|
| Storagestability (>1 month) | stable | stable | stable | stable | lamination |
| Viscosity/mPa·s | 0.9 | 1.16 | 1.24 | 1.3 | 2.0 |
| Mass Concentration (%) | 0 | 5 | 10 | 15 | 20 | 25 |
|---|---|---|---|---|---|---|
| R457—brightness (%) | 75.9 ± 0.1 | 74.4 ± 0.1 | 73.0 ± 0.1 | 72.9 ± 0.1 | 72.6 ± 0.1 | 69.9 ± 0.1 |
| G75—gloss | 5.0 ± 0.1 | 5.0 ± 0.1 | 4.9 ± 0.1 | 4.8 ± 0.1 | 4.6 ± 0.1 | 4.5 ± 0.1 |
| Sample | Thickness/mm | Average/mm | |||
|---|---|---|---|---|---|
| A | 0.087 | 0.088 | 0.088 | 0.089 | 0.0880 |
| B | 0.089 | 0.089 | 0.090 | 0.091 | 0.0898 |
| Thickness difference | 0.002 | 0.001 | 0.002 | 0.002 | 0.0018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Jin, S.-S.; Qi, Y.-P.; Shen, Y.-F.; Li, H. Preparation and Application of Polyurethane Coating Material Based on Epoxy Cyclohexane Protective for Paper. Coatings 2021, 11, 431. https://doi.org/10.3390/coatings11040431
Liu J, Jin S-S, Qi Y-P, Shen Y-F, Li H. Preparation and Application of Polyurethane Coating Material Based on Epoxy Cyclohexane Protective for Paper. Coatings. 2021; 11(4):431. https://doi.org/10.3390/coatings11040431
Chicago/Turabian StyleLiu, Juan, Shan-Shan Jin, Ying-Ping Qi, Yong-Feng Shen, and Hua Li. 2021. "Preparation and Application of Polyurethane Coating Material Based on Epoxy Cyclohexane Protective for Paper" Coatings 11, no. 4: 431. https://doi.org/10.3390/coatings11040431
APA StyleLiu, J., Jin, S.-S., Qi, Y.-P., Shen, Y.-F., & Li, H. (2021). Preparation and Application of Polyurethane Coating Material Based on Epoxy Cyclohexane Protective for Paper. Coatings, 11(4), 431. https://doi.org/10.3390/coatings11040431




