Polymer-Cement Composites Glazing by Concentrated Solar Energy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Polymer-Cement Composites Samples
2.2. Coating of MDF Samples
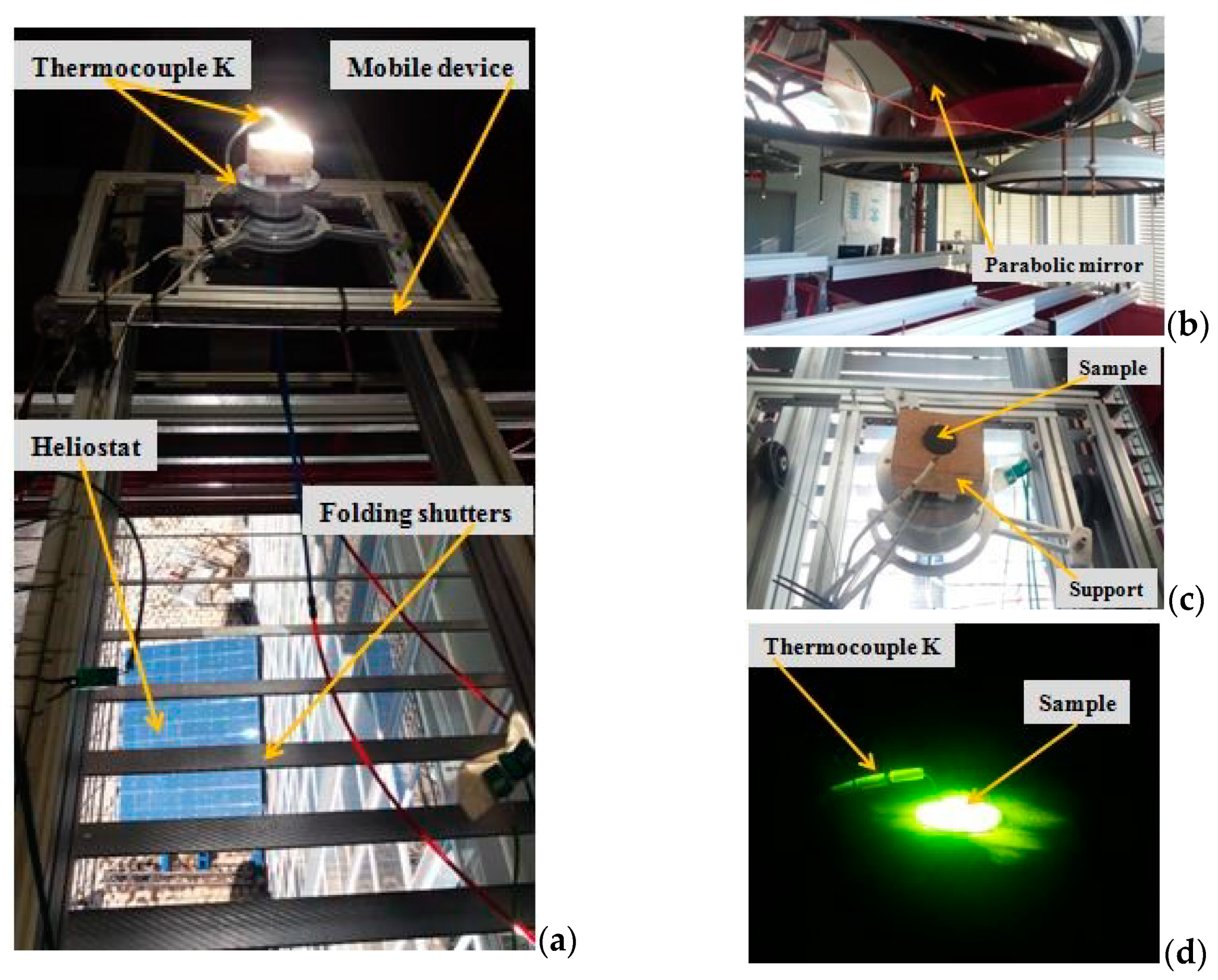
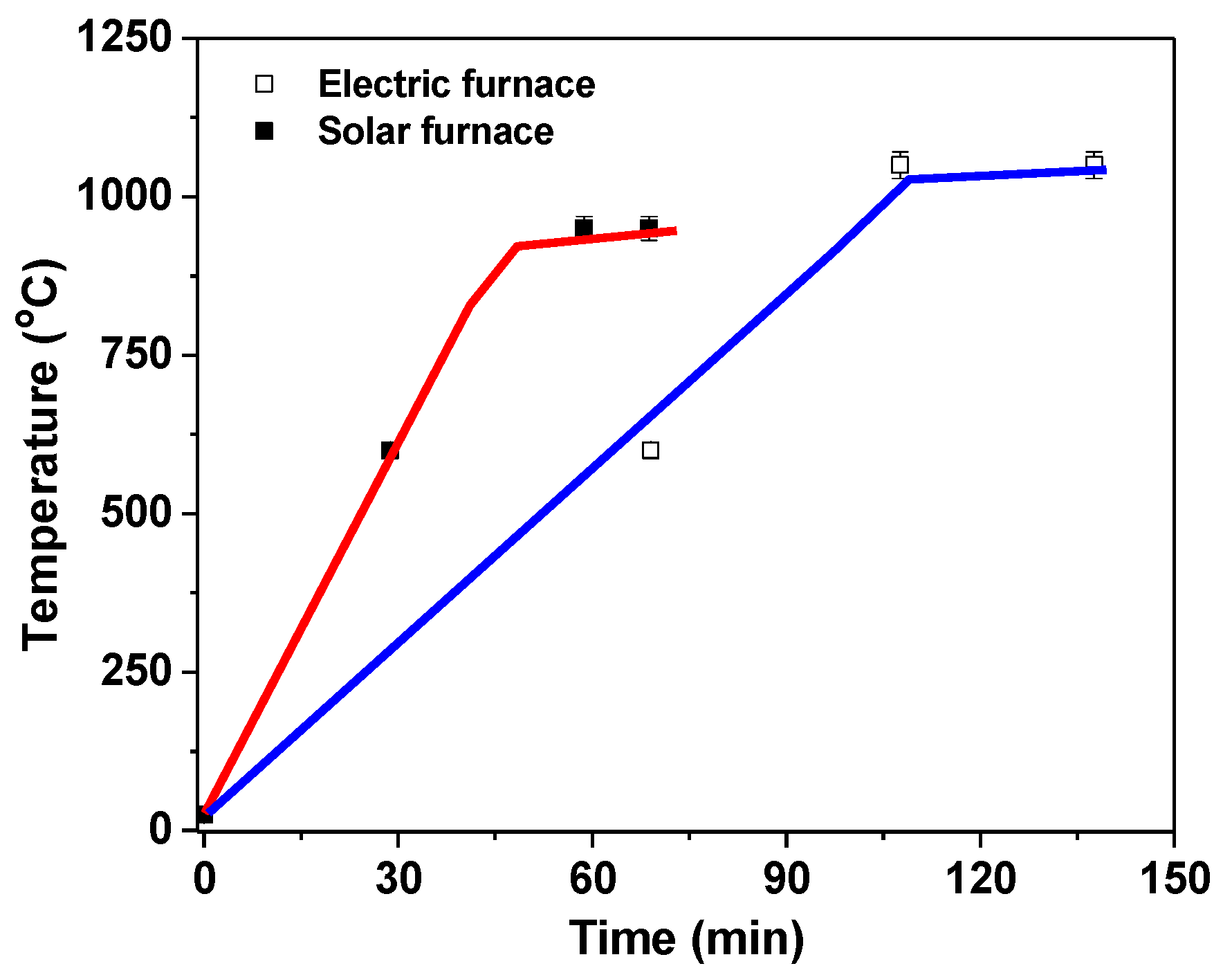
2.3. Glaze Formation by Thermal Treatment
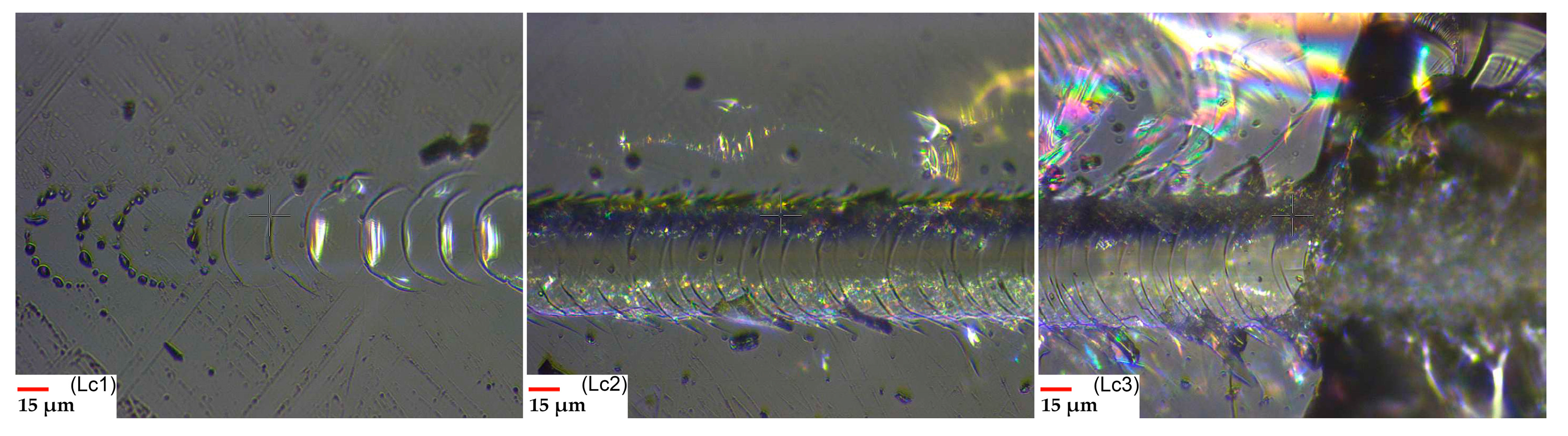
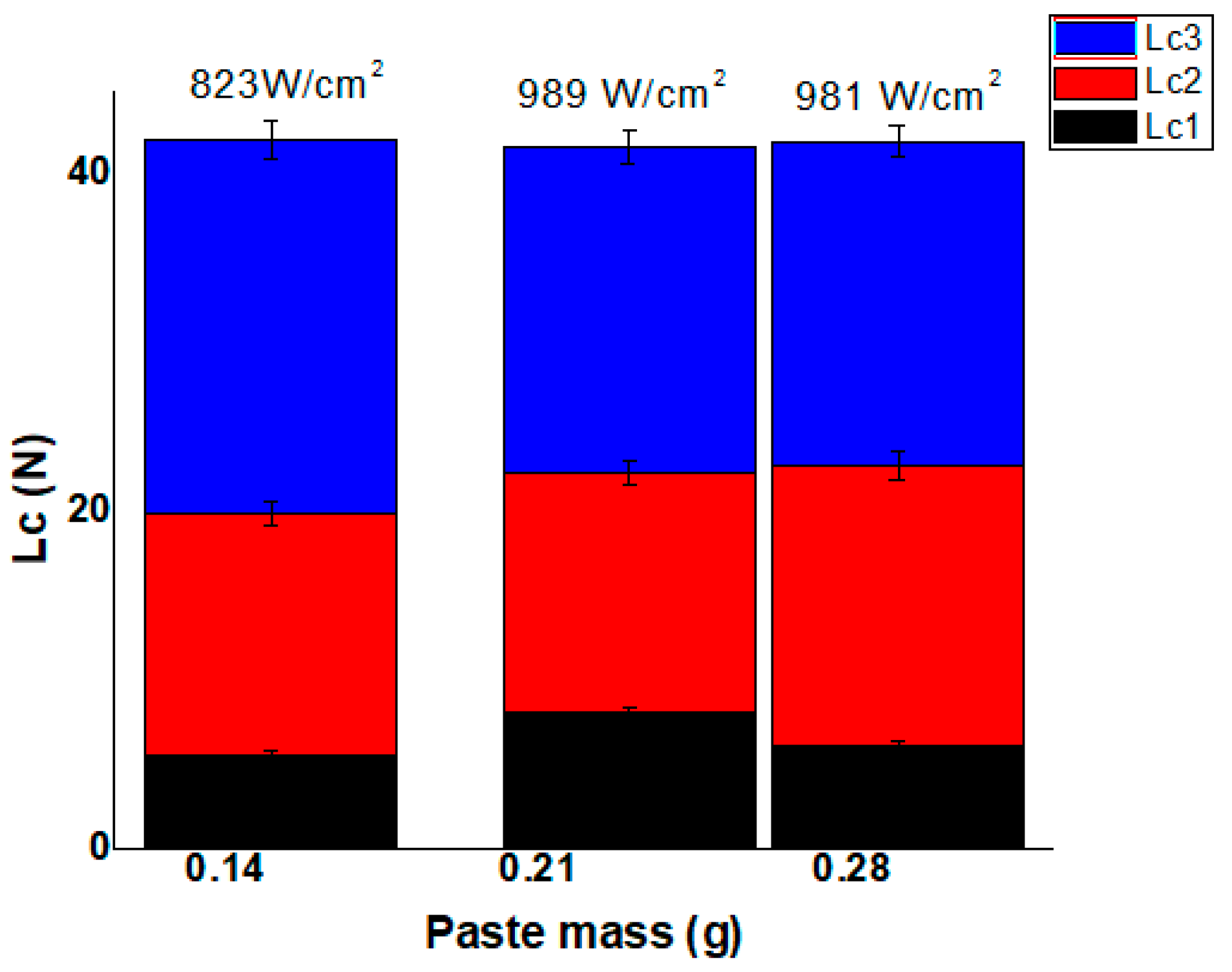
2.4. Scratch Test
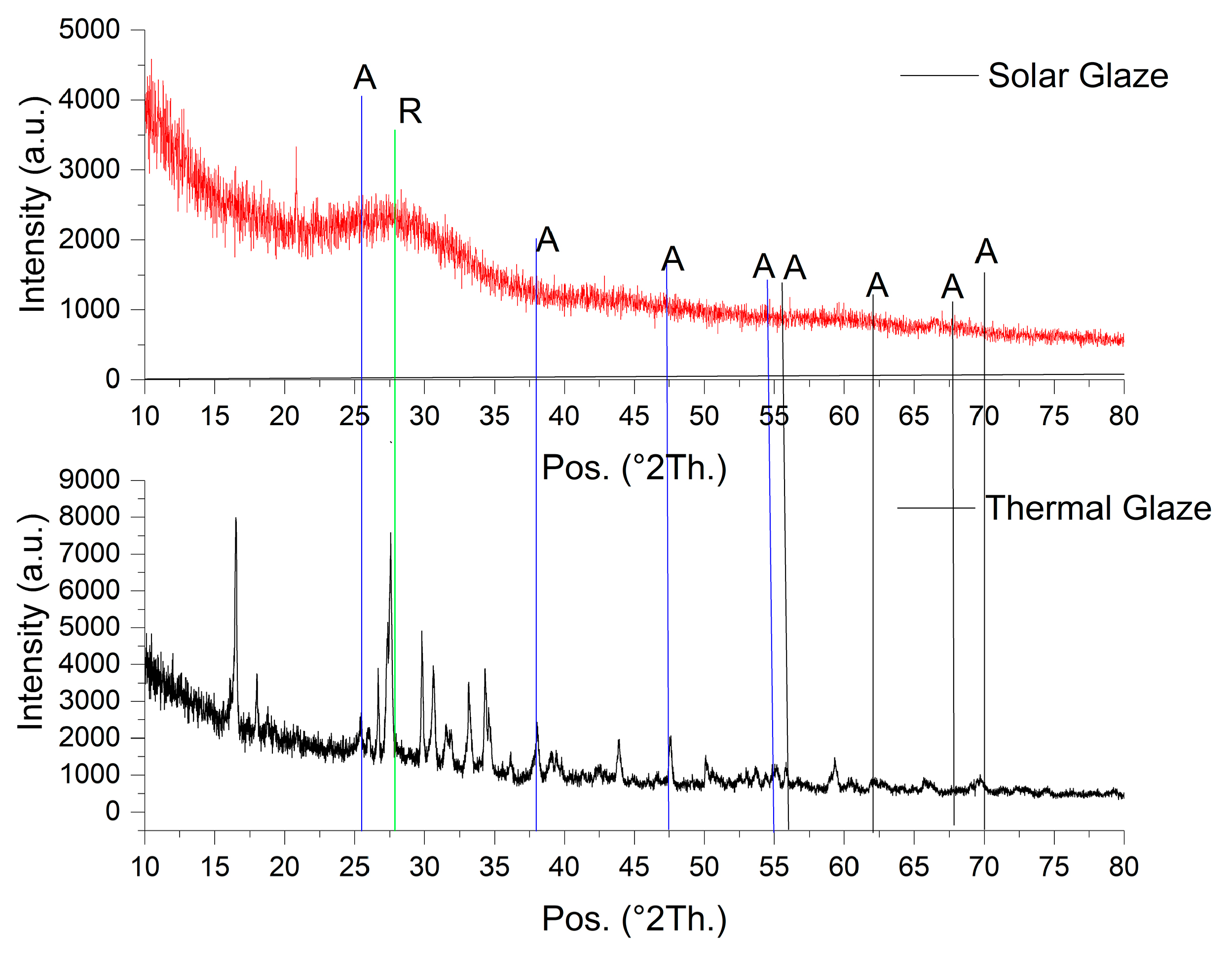
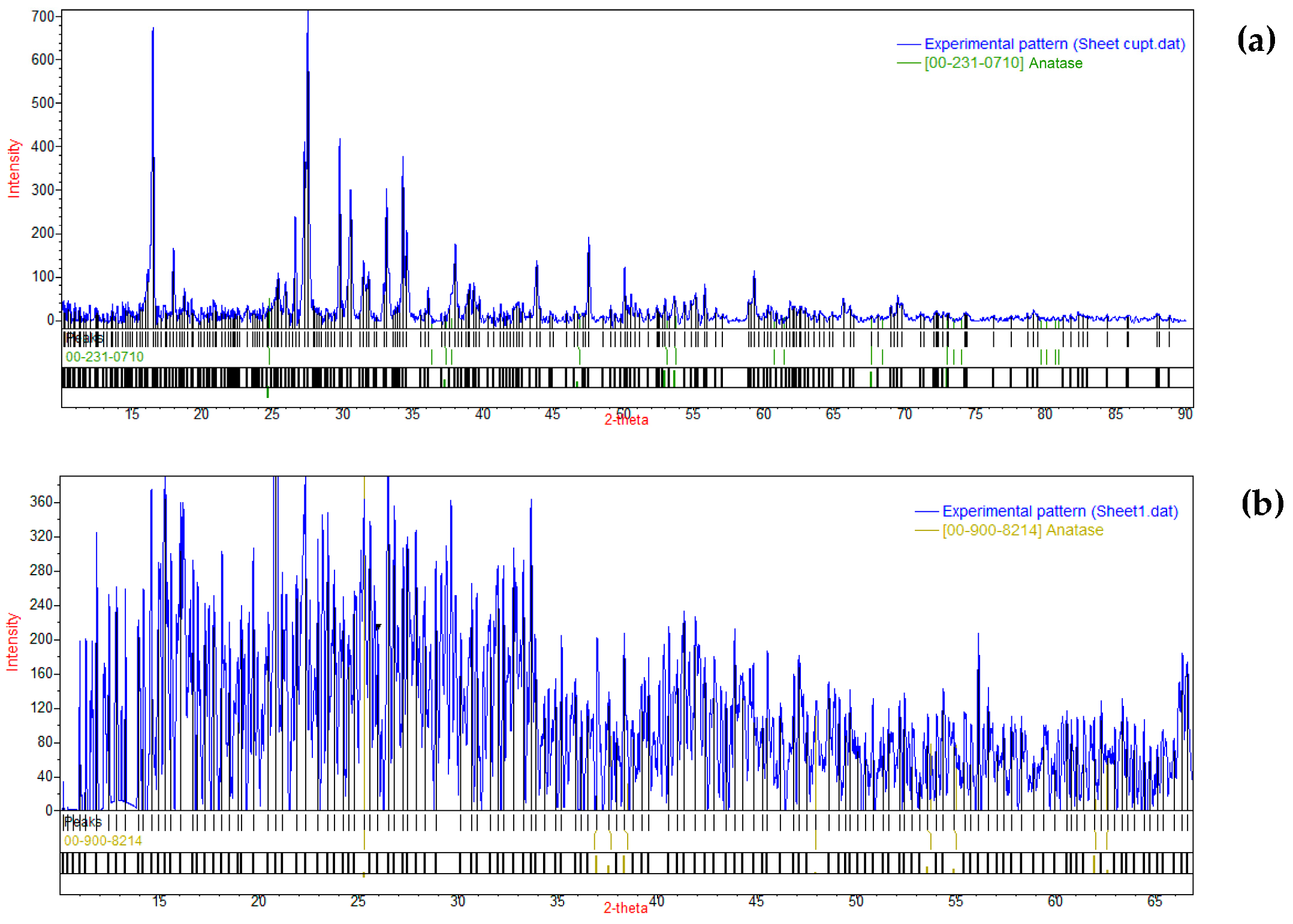
2.5. Scanning Electron Microscopy and X-ray Analysis
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.D.A.; Kim, K.H. Solar energy: Potential and future prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Farjana, S.H.; Huda, N.; Parvez Mahmud, M.A.; Saidur, R. Solar process heat in industrial systems–A global review. Renew. Sustain. Energy Rev. 2018, 82, 2270–2286. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, C.; Mullick, S.C.; Kandpal, T.C. Solar industrial process heating: A review. Renew. Sustain. Energy Rev. 2017, 78, 124–137. [Google Scholar] [CrossRef]
- Lédé, J. Solar thermochemica lconversion of biomass. Sol. Energy 1999, 65, 3–13. [Google Scholar] [CrossRef]
- Epstein, M.; Spiewak, I.; Funken, K.H.; Ortner, J. Review of the technology for solar gasification of carbonaceous materials. Am. Soc. Mech. Eng. Sol. Eng. 1994, 26, 79–97. [Google Scholar]
- Puig-Arnavat, M.; Tora, E.A.; Bruno, J.C.; Coronas, A. State of the art on reactor designs for solar gasification of carbonaceous feedstock. Sol. Energy 2013, 97, 67–84. [Google Scholar] [CrossRef]
- Nzihou, A.; Flamant, G.; Stanmore, B. Synthetic fuels from biomass using concentrated solar energy-A review. Energy 2012, 42, 121–131. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A review of solar thermochemical processes. Renew. Sustain. Energy Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Herranz, G.; Romero, A.; Castro, V.; Rodriguez, G.P. Development of high speed steel sintered using concentrated solar energy. J. Mater. Process. Technol. 2013, 213, 2065–2073. [Google Scholar] [CrossRef]
- Herranz, G.; Romero, A.; Castro, V.; Rodriguez, G.P. Processing of AISI M2 high speed steel reinforced with vanadium carbide by solar sintering. Mater. Des. 2014, 54, 934–946. [Google Scholar] [CrossRef]
- Reyo-Prats, R.; Plaza, A.C.; Faugeroux, O.; Claudet, B.; Soum-Glaude, A.; Hildebrandt, C.; Binyamin, Y.; Aguero, A.; Meißner, T. Accelerated aging of absorber coatings for CSP receivers under real high solar flux. Sol. Energy Mater. Sol. Cells 2019, 193, 92–100. [Google Scholar] [CrossRef]
- Cambronero, L.E.G.; Canadas, I.; Ruiz-Román, J.M.; Cisneros, M.; Corpas Iglesias, F.A. Weld structure of joined aluminium foams with concentrated solar energy. J. Mater. Process. Technol. 2014, 214, 2637–2643. [Google Scholar] [CrossRef]
- Haferkamp, H.; Bunte, J.; Herzog, D.; Ostendorf, A. Laser based welding of cellular aluminium. Sci. Technol. Weld. Join. 2004, 9, 65–71. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Karakizis, P.N.; Kazasidis, M.E.; Karalis, D.G.; Rodrıguez, J. Experimental and numerical investigation of AA6082-T6 thin plates welding using Concentrated Solar Energy (CSE). Sol. Energy Mater. Sol. Cells 2017, 171, 187–196. [Google Scholar] [CrossRef]
- Romero, A.; García, I.; Arenas, M.A.; López, V.; Vázquez, A. Ti6Al4V titanium alloy welded using concentrated solar energy. J. Mater. Process. Technol. 2015, 223, 284–291. [Google Scholar] [CrossRef]
- Flamanta, G.; Ferrierea, A.; Laplazeb, D.; Montya, C. Solar processing of materials: Opportunities and new frontiers. Sol. Energy 1999, 66, 117–132. [Google Scholar] [CrossRef]
- Carle, J.E.; Andreasen, J.W.; Jørgensen, M.; Krebs, F.C. Low band gap polymers based on 1,4-dialkoxybenzene, thiophene, bithiophene donors and the benzothiadiazole acceptor. Sol. Energy Mater. Sol. Cells 2010, 94, 774–780. [Google Scholar] [CrossRef]
- Helgesen, M.; Krebs, F.C. Photovoltaic performance of polymers based on dithienylthienopyrazines bearing thermocleavable benzoate esters. Macro Mol. 2010, 43, 1253–1260. [Google Scholar] [CrossRef]
- Tromholt, T.; Manceau, M.; Helgesen, M.; Carle, J.E.; Krebs, F.C. Degradation of semiconducting polymers by concentrated sunlight. Sol. Energy Mater. Sol. Cells 2011, 95, 1308–1314. [Google Scholar] [CrossRef]
- Boubault, A.; Claudet, B.; Faugeroux, O.; Olalde, G. Aging of solar absorber materials under highly concentrated solar fluxes. Sol. Energy Mater. Sol. Cells 2014, 123, 211–219. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, M.R.; Santana, D.; Olalde, G. Experimental study of honeycomb SiCSi under highly concentrated solar flux. Evolution of its thermo-radiative properties. Sol. Energy Mater. Sol. Cells 2016, 155, 253–263. [Google Scholar] [CrossRef]
- Ceballos-Mendivil, L.G.; Cabanillas-López, R.E.; Tánori-Córdova, J.C.; Murrieta-Yescas, R.; Pérez-Rábago, C.A.; Villafán-Vidales, H.I.; Arancibia-Bulnes, C.A.; Estrada, C.A. Synthesis of silicon carbide using concentrated solar energy. Sol. Energy 2015, 116, 238–246. [Google Scholar] [CrossRef]
- Sierra, C.; Vazquez, A.J. NiAl coatings on carbon steel by self-propagating high-temperature synthesis assisted with concentrated solar energy: Mass influence on adherence and porosity. Sol. Energy Mater. Sol. Cells 2005, 86, 33–42. [Google Scholar] [CrossRef]
- Plaza, D.M.; Cañadas Martinez, I.; Gasch, G.M.; Sufrategui, F.T.; García, J.R. A case study of the feasibility of using solar concentrating technologies for manufacturing ceramics. J. Clean. Prod. 2015, 87, 977–991. [Google Scholar] [CrossRef]
- Roman, R.; Canadas, I.; Rodrıguez, J.; Hernandez, M.T.; Gonzalez, M. Solar sintering of alumina ceramics: Microstructural development. Sol. Energy 2008, 82, 893–902. [Google Scholar] [CrossRef]
- Apostol, I.; Rodríguez, J.; Cañadas, I.; Galindo, J.; Mendez, S.L.; Abreu Martins, P.L.; Cunha, L. Concentrated solar energy used for sintering magnesium titanates for electronic applications. Appl. Surf. Sci. 2018, 438, 594–665. [Google Scholar]
- Rashidi, S.; Tamayol, A.; Valipour, M.S.; Shokri, N. Fluid flow and forced convection heat transfer around a solid cylinder wrapped with a porous ring, a solid cylinder wrapped with a porous ring. Int. J. Heat Mass Transf. 2013, 63, 91–100. [Google Scholar] [CrossRef]
- Rashidi, S.; Bovand, M.; Pop, I.; Valipour, M.S. Numerical simulation of forced convective heat transfer past a square diamond-shaped porous cylinder. Transp. Porous Media 2014, 102, 207–225. [Google Scholar] [CrossRef]
- Rashidi, S.; Esfahani, J.A.; Rashidi, A. A review on the applications of porous materials in solar energy systems. Renew. Sustain. Energy Rev. 2017, 73, 1198–1210. [Google Scholar] [CrossRef]
- Lalau, Y.; Faugeroux, O.; Guillot, E.; Andre, D.; Huger, M.; Proust, A.; Chotard, T.; Claudet, B. Impact: A novel device for in-situ thermo-mechanical investigation of materials under concentrated sunlight. Sol. Energy Mater. Sol. Cells 2017, 172, 59–65. [Google Scholar] [CrossRef]
- Patachia, S.; Moise, G.; Ozkul, M.H.; Ekincioglu, O. Characterization of ecological macro defect free (MDF) cements by infrared spectrometry. Environ. Eng. Manag. J. 2011, 10, 237–240. [Google Scholar] [CrossRef]
- Patachia, S.; Moise, G.; Ozkul, M.H.; Ekincioglu, O.; Croitoru, C. Ecological polymer used for macro-defect-free cements (MDF) production. Environ. Eng. Manag. J. 2009, 8, 679–684. [Google Scholar] [CrossRef]
- Ekincioglu, O.; Ozkul, M.H.; Patachia, S.; Moise, G. Effect of TiO2 Addition on the Properties of Macro Defect Free Cement-Polymer Composites. Restor. Build. Monum. 2013, 19, 125–134. [Google Scholar] [CrossRef]
- Patachia, S.; Moise, G.; Ozkul, M.H.; Ekincioglu, O. Influence of the self cross linkable polymers on the properties of the macro defect free (MDF) cements. Bull. Transilv. Univ. Bras. 2009, 2, 181–186. [Google Scholar]
- Patachia, S.; Moise, G.; Ozkul, M.H.; Ekincioglu, O.; Croitoru, C. Influence of the Obtaining Technology on the Macro Defect Free Cements Characteristics. Pollack Period. 2009, 4, 75–82. [Google Scholar] [CrossRef]
- Patachia, S.; Moise, G.; Ozkul, M.H.; Ekincioglu, O.; Croitoru, C. Morphological Characterization of High Alumina Cement Based Macro Defect Free Cements. Bull. Transilv. Univ. Bras. 2008, 1, 251–254. [Google Scholar]
- Ekincioğlu, Ö.; Özkul, M.H.; Struble, L.J.; Patachia, S. State of the art of macro-defect-free composites. J. Mater. Sci. 2018, 53, 10595–10616. [Google Scholar] [CrossRef]
- Rasteiro, M.G.; Gassman, T.; Santos, R.; Antunes, E. Crystalline phase characterization of glass-ceramic glazes. Ceram. Int. 2007, 33, 345–354. [Google Scholar] [CrossRef]
- Sheikhattar, M.; Attar, H.; Sharafi, S.; Carty, W.M. Influence of surface crystallinity on the surface roughness of different ceramic glazes. Mater. Charact. 2016, 118, 570–574. [Google Scholar] [CrossRef]
- Da Silva, A.L.; Muche, N.F.D.; Dey, M.; Hotza, D.; Castro, H.R.R. Photocatalytic Nb2O5-doped TiO2 nanoparticles for glazed ceramic tiles. Ceram. Int. 2016, 42, 5113–5122. [Google Scholar] [CrossRef]
- Teixeira, M.; Bernardin, A.M. Development of TiO2 white glazes for ceramic tiles. Dyes Pigm. 2009, 80, 292–296. [Google Scholar] [CrossRef]
- Romero, M.; Robla, J.I.; Padilla, I.; García-Hierro, J.; López-Delgado, A. Eco-efficient melting of glass frits by concentrated solar energy. Sol. Energy 2018, 174, 321–327. [Google Scholar] [CrossRef]
- Rey-Garcia, F.; Gutiérrez-Morac, F.; Borrela, C.J.; Estepaa, L.C.; Angurela, L.A. Microstructural characterization and tribological behavior of laser furnace processed ceramic tiles. Ceram. Int. 2018, 44, 6997–7005. [Google Scholar] [CrossRef]
- Izadi, A.; Heidari, B.; Kasrai, S.; Soltanian, A.; Raissi, S. Comparison of surface roughness between CO2 laser and typical glazing on two types of porcelain vita and ivoclar. Biosci. Biotechnol. Res. Commun. 2017, 10, 529–535. [Google Scholar] [CrossRef]
- Baltes, L.; Patachia, S.; Tierean, M.; Ekincioglu, O.; Ozkul, H.M. Photoactive glazed polymer-cement composite. Appl. Surf. Sci. 2018, 438, 84–95. [Google Scholar] [CrossRef]
- Ekincioglu, O.; Ozkul, M.H.; Struble, L.J.; Patachia, S. Optimization of material characteristics of macro-defect free cement. Cem. Concr. Compos. 2012, 34, 556–565. [Google Scholar] [CrossRef]
- Ekincioglu, O.; Ozkul, M.H.; Ohama, Y.; Patachia, S.; Moise, G. Effect of epoxy resin addition on the moisture sensitivity of macro defect free polymer-cement composites. Key Eng. Mater. 2011, 466, 65–72. [Google Scholar] [CrossRef]
- Pradell, T.; Molera, J.; Salvadó, N.; Labrador, A. Synchrotron Radiation Micro-XRD in the Study of Glaze Technology. Appl. Phys. A 2010, 99, 407–417. [Google Scholar] [CrossRef]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. QUALX2.0: A qualitative phase analysis software using the freely available database POW-COD. J. Appl. Crystallogr. 2015, 48, 598–603. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Xu, D.; Wang, D.; Xue, Y.; Su, W. Pressure-induced crystallization of amorphous SiO2 with silicon–hydroxy group and the quick synthesis of coesite under lower temperature. High Press. Res. 2008, 28, 641–650. [Google Scholar] [CrossRef]
- Li, K.; Bao, Y.; Wan, D.; Huo, Y.; Wu, J. Evaluating adhesion of ceramic coatings by scratch testing. Key Eng. Mater. 2012, 512–515, 959–964. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltes, L.S.; Patachia, S.; Ekincioglu, O.; Ozkul, H.; Croitoru, C.; Munteanu, C.; Istrate, B.; Tierean, M.H. Polymer-Cement Composites Glazing by Concentrated Solar Energy. Coatings 2021, 11, 350. https://doi.org/10.3390/coatings11030350
Baltes LS, Patachia S, Ekincioglu O, Ozkul H, Croitoru C, Munteanu C, Istrate B, Tierean MH. Polymer-Cement Composites Glazing by Concentrated Solar Energy. Coatings. 2021; 11(3):350. https://doi.org/10.3390/coatings11030350
Chicago/Turabian StyleBaltes, Liana Sanda, Silvia Patachia, Ozgur Ekincioglu, Hulusi Ozkul, Catalin Croitoru, Corneliu Munteanu, Bogdan Istrate, and Mircea Horia Tierean. 2021. "Polymer-Cement Composites Glazing by Concentrated Solar Energy" Coatings 11, no. 3: 350. https://doi.org/10.3390/coatings11030350
APA StyleBaltes, L. S., Patachia, S., Ekincioglu, O., Ozkul, H., Croitoru, C., Munteanu, C., Istrate, B., & Tierean, M. H. (2021). Polymer-Cement Composites Glazing by Concentrated Solar Energy. Coatings, 11(3), 350. https://doi.org/10.3390/coatings11030350










