1. Introduction
Ever since the discovery of a TiO
2-based memristor [
1], metal oxide thin films, such as TiO
2, HfO
2, ZnO, Ta
2O
5, have been extensively studied as promising building blocks for electronic devices [
2,
3,
4,
5]. The switch between low and high resistive states results in a bipolar hysteresis current/voltage IV loop, pinched at 0 V and 0 A [
6]. The resistive switch mechanism in TiO
2 based devices (as well as other metal oxides) relies on forming a nanofilament that contains oxygen vacancies responsible for a low resistive state. To develop the electrical devices, a detailed insight in the resistive switch is still of demand, and several theoretical studies have been conducted [
7,
8,
9]. Driven by this interest, various thin films with a typical thickness of around tens of nm were fabricated by methods where synthesis is achieved by chemical reactions with specific precursors or exploiting direct physical deposition of material in a vacuum. In particular, TiO
2 thin films were successfully fabricated by atomic layer deposition (ALD) [
10,
11], reactive magnetron [
12], ion beam [
13], radio frequency (R.F.) sputtering [
14] and laser ablation [
15]. In addition to these methods, TiO
2 thin films were fabricated by the sol–gel approach [
16] and metal organic chemical vapor deposition [
17].
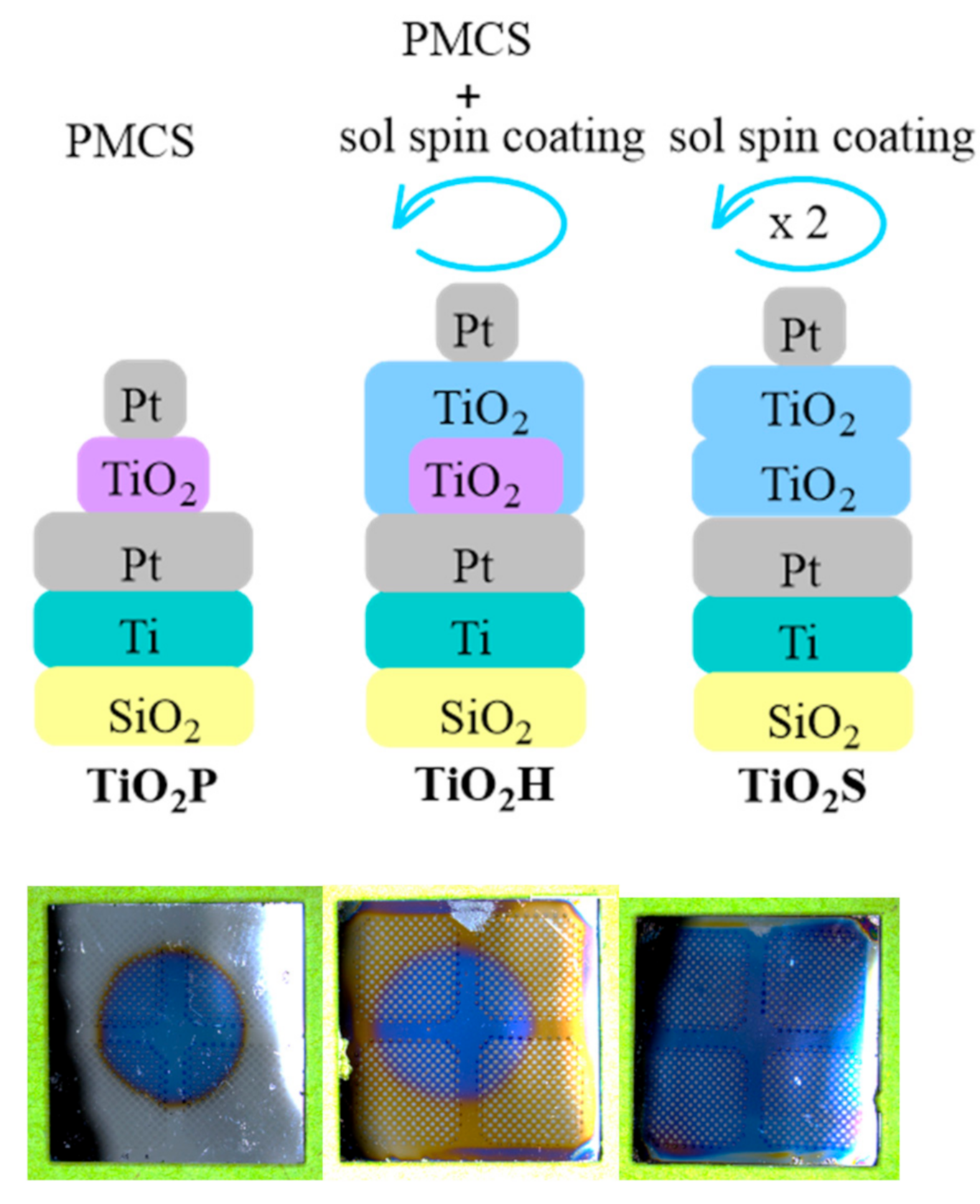
Each method has its own limitations ranging from the process costs, temperature conditions, stoichiometry control, uniformity, and deposition area. For example, the sol–gel technique is a low-temperature, low-cost process that may produce smooth and uniform films. Nevertheless, even though the stoichiometry control of sol–gel derived materials is possible by doping or processing the films in a selected atmosphere, precise control over oxygen atomic percentage is hard to achieve. On the other hand, employing the pulsed microplasma cluster source (PMCS) technique it is possible to produce nanocrystalline TiO
2 thin films at room temperature [
18,
19], with a precise control on oxide stoichiometry, consistent biocompatibility with neuronal tissues and satisfactory memristive response [
20]. The typical high porosity of PMCS films is ideal for sensing applications [
21], but could lead to short circuits in electronic devices when a top electrode (TE) coating is required [
11]. We have already extensively studied the oxide films developed by the PMCS and the sol–gel approaches independently, optimizing the respective process parameters to develop reliable memristive switching devices.
In this work, we propose merging the PMCS and the sol–gel techniques to profit from the methods’ advantages and overcome the specific limitations. We present a proof of concept and feasibility study showing that this hybrid method is a versatile tool for developing the target TiO2 thin films with a memristive response. A side-by-side comparison of the TiO2 thin films produced by the PMCS, the sol–gel, and the PMCS/sol–gel hybrid method (HM) shows that the new approach leads to materials with memristive switching, a large reduction of electrical shorts probability, and improved device electrical performances.
2. Materials and Methods
We developed all TiO
2 films on a SiO
2/Ti/Pt bottom electrode (BE) that was fabricated as reported previously [
16]. Before all the depositions, the SiO
2/Ti/Pt electrodes (1.5 × 1.5 cm
2) were cleaned with acetone, rinsed with isopropanol, DI water, and dried in a stream of nitrogen. In addition to cleaning, oxygen downstream plasma etching of electrodes was performed to produce the sol–gel-derived TiO
2S samples.
The details of the titania sol preparation and a spin coating recipe were published previously [
16]. The two-layered sol–gel derived TiO
2 thin films, TiO
2S, were used as one of the two models to investigate the samples prepared by the HM.
Analogously, we used the PMCS method to prepare TiO
2P model samples by a previously reported procedure [
20]. Briefly, the deposition process is based on the ablation of a titanium rod by a pulsed plasma, created by the ionization of a pulsed high pressure gas (30 bar) triggered by an electric discharge at 1.2 kV. The mixture of clusters and inert gas is extracted in vacuum through a nozzle to form a seeded supersonic molecular beam. The TiO
2 films with different thicknesses were deposited at room temperature using O
2/He gas mixture percentages of 0.1/99.9%. Typical pulsed gas electro-valve opening time and frequency were 700 µs and 4 Hz respectively, while the delay between the electric discharge and valve opening was set at 700 µs.
The TiO
2H sample fabrication started with the deposition of a TiO
2 layer by the PMCS method onto the clean electrodes. After that, spin coating of the titania sol on TiO
2 layer was performed in a clean room (class 100–1000 equipped for MEMS technology). The fresh sol was transferred into a glass syringe and filtered through Millipore Millex-FG Hydrophobic PTFE (Teflon) 0.2 mm before the deposition on the TiO
2 layer. The filtered sol was spin-coated with the following protocol: 1300 rpm for 2 s (0→1300: 2 s), followed by 2000 rpm for 2 s (1300→2000: 2 s), and finally with 3000 rpm for 49 s (2000→3000: 1 s). The resulting TiO
2H samples were heat treated for 1 h at 150 °C and annealed in air atmosphere for 1 h at 400 °C, following optimized conditions published elsewhere [
5,
16].
Platinum (270–300 µm in diameter, 40–50 nm thick) circular shaped electrodes were hence deposited by the electron beam evaporation onto TiO2S, TiO2P, and TiO2H using a shadow mask with dish-shaped patterns. The Pt films were evaporated from a Pt rod (99.99% purity, supplied by Umicore, Pforzheim, Germany) in a vacuum chamber using an e-beam evaporator EBX-16C (Ulvac, Methuen, MA, USA). The pressure during deposition was adjusted to 2.4 × 10−7 torr with the evaporation rate of 0.1 nm min−1.
The FE-SEM characterization was performed by SEM (JSM-7401 FIB-SEM, JEOL, Tokio, Japan) with accelerating voltage of 3 ÷ 5 KV, 10° sample tilt, working distance of 5 ÷ 6 mm and a secondary electrons signal. The surface roughness was evaluated from SEM images. Optical images were obtained by a monocular inspection microscope SKU:H800 (AmScope) coupled with a MU800 digital camera (AmScope). The thickness measurements were carried out by the profilometry technique using a TENCOR P6 mechanical profilometer (Milpitas, CA, USA).
X-ray photoelectron spectroscopy (XPS) was carried out in an ultra-high-vacuum (UHV) system (custom made), equipped with a non-monochromatized X-ray source (Mg Kα photon at 1253.6 eV) and a VSW HA100 hemispherical analyzer, with a total energy resolution of 0.86 eV [
22]. The binding energy (BE) scale of XPS spectra was calibrated by using the Au 4f peak at 84.0 eV as a reference. The core level analysis has been performed by Voigt line-shape deconvolution after background subtraction of a Shirley function. Stoichiometry was evaluated using sensitivity factors corrected by analyzer electron transmission.
Current-controlled electrical testing was accomplished with NI PXIe-1073 chassis connected to a PC through a PCI-express card and controlled by a software (v. 2014) user interface developed within the Labview environment. The chassis was equipped with a NI-PXIe-4139 source measure unit capable of current or voltage controlled supply and measurement. The I-V curves were recorded in an ambient atmosphere at RT. The BE was the SiO2/Ti/Pt substrate, while the deposited Pt discs were the TE.
3. Results
The novel hybrid deposition method consists of two sequential procedures: PMCS followed by a spin coating deposition of the titania sol. We previously extensively studied and reported each of these two optimized procedures for the development of TiO
2 thin films with a memristive behavior [
16,
18,
19,
20,
23]. The first oxide layer deposited on a SiO
2/Ti/Pt electrode by the PMCS method is about 30 nm thick with a 2.8 ± 0.1 nm roughness (estimated from SEM images, not shown,
Table 1). The PMCS-derived TiO
2 films display large roughness, as expected. In our previous studies, for the sol–gel derived oxide films, we observed that the two-layered smooth and dense films displayed a reduced number of short circuits due to the elimination of surface defects upon introducing the second layer [
16,
23]. Thus, the titania sol was spin coated onto the first PMCS-derived TiO
2 layer to improve homogeneity, roughness, and porosity (
Figure 1).
A single sol–gel derived TiO
2 layer, with a typical thickness of about 30 nm was deposited on the PMCS TiO
2 film. After curing, the TiO
2H film has a total thickness of 50.5 nm with a roughness of 1.1 ± 0.1 nm (
Table 1).
To investigate the TiO
2H electrical properties, two TiO
2 based devices were fabricated exclusively by the PMCS (TiO
2P) and by the sol–gel method (TiO
2S) to serve as models (
Figure 1). The TiO
2P set of samples was prepared by the PMCS method under the same conditions used for TiO
2H, yielding nanocrystalline TiO
2 anatase thin films [
18,
19] of about 50 nm with a roughness of 2.9 ± 0.1 nm (
Table 1,
Figure 1). The sol–gel derived double-layered TiO
2S thin films have a thickness of 52 nm and roughness of 0.7 ± 0.1 nm and consist predominantly of an anatase phase (
Table 1,
Figure 1) [
23]. The thickness of the TiO
2 layers is a crucial parameter for the memristive switching response; thus, TiO
2S, TiO
2P, and TiO
2H were developed to maintain comparable thickness through the series (
Table 1).
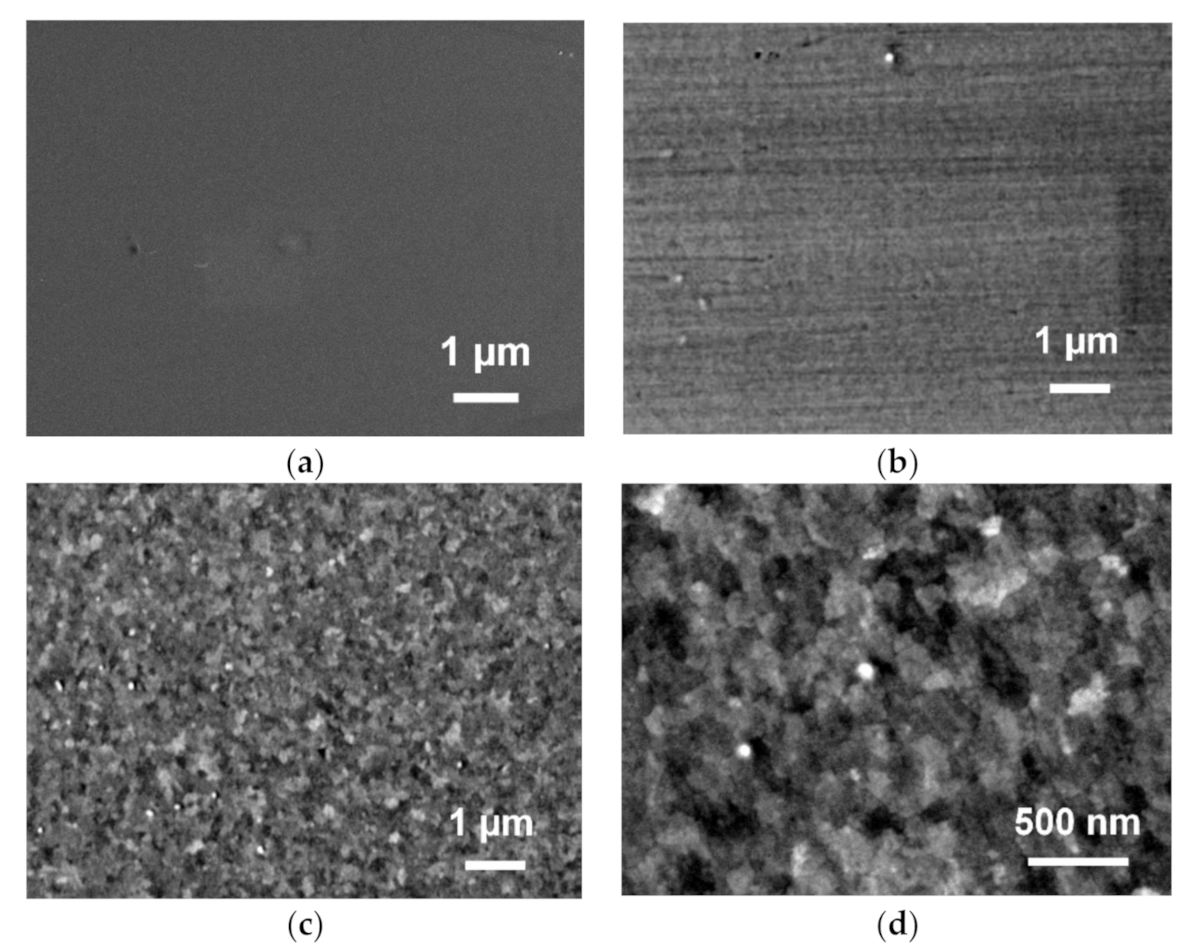
The surfaces of the fabricated films were studied by FE-SEM (
Figure 2). At ×10 K magnification, no microdefects, such as holes, cracks or peeling of the TiO
2 film from the surface of Pt/Ti/SiO
2 were observed in all cases (
Figure 2a–c). We recall that images at higher magnifications of a semiconductor surface of TiO
2P, already reported in literature, reveal nanocrystallites’ presence on the surface [
18,
19]. Meanwhile, a previously published FE-SEM image of TiO
2S [
16] displayed a smooth homogeneous surface with nanocrystallites. A higher magnification image (×30 K) of a TiO
2H semiconductor layer confirms that the sample’s pristine surface is covered with crystallites of TiO
2 (
Figure 2d). Homogeneous brightness on adjacent areas, about a few hundred nanometers wide, are likely produced by different surface angles facing the detector in a chamber or by different crystallite orientations.
We previously studied the chemical properties of TiO
2S and TiO
2P [
18,
19,
20,
23]. Thus, we analyzed only the TiO
2H surface by XPS.
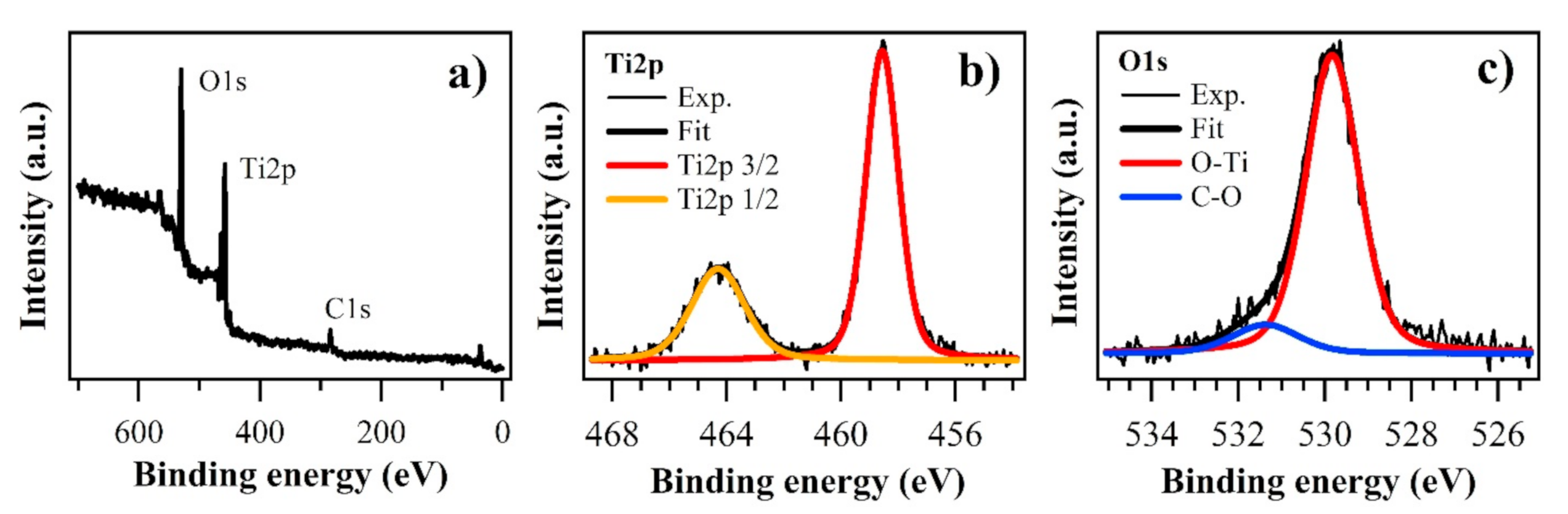
Figure 3a shows a wide range spectrum, evidencing the presence of O1s, Ti2p, and C1s core levels. Ti2p feature is composed of a 3/2–1/2 doublet, located at 458.56 and 464.30 eV (
Figure 3b), the typical BEs of TiO
2 [
18,
19,
20,
23]. No other components are present, suggesting the absence of other types of titanium oxides. O1s core level (
Figure 3c) is characterized by the main peak at 529.83 eV, related to oxygen in TiO
2, and a second feature at 531.37 eV, due to CO groups in agreement with the presence of the C1s core level. The energy difference between Ti2p 3/2 peak and O1s component related to Ti–O bond is 5.72 eV, while the O/Ti stoichiometry ratio is 2.03. All data are in good agreement with the expected values for TiO
2 [
24].
Concerning analysis of electrical properties, as the metal oxide’s initial resistance is typically higher than 10
10 Ω, a voltage-controlled electroforming procedure is applied to observe the resistance switching behavior. The electroforming is performed by applying to a TE (grounding the BE) a sweep voltage from 0 to −10 V (with a current compliance of 0.5 mA, to avoid a hard dielectric breakdown), where the conductive filament is produced, then from −10 to 0 V and from 0 to +1.5 V (with a current compliance of 100 mA) and then back to 0 V [
23,
25], to break the new filament. The performance of the devices varies greatly depending on the voltage/current parameters used for forming the conducting filament [
7,
26]. Therefore, we decided to apply an identical electroforming procedure for all TiO
2 based devices to ensure the memristive response’s reproducibility. The I-V curves (shown in
Figure 4,
Figure 5 and
Figure 6) were obtained by sweeping the activated devices in a low resistance state. At first, upon the negative voltage sweep, the activated devices reach V
OFF, at which the material switches from a low to a high resistance state, then a sweep to positive voltage values is applied until V
ON is reached, at which a high to a low resistance state switch occurs. Finally, by turning back to 0 V, we complete the hysteresis loop with a (V,I) = (0,0) pinch. The compliance current is set to 0.5 mA, the sweep rate is set to 0.02 Vs
−1. In all electrical studies, the first consecutive hysteresis cycles are shown, and all R
ON (resistance in the high conductivity state) and R
OFF (resistance in the low conductivity state) values are measured at 0.1 V. No significant physical deformation of the TE structure was observed for any the devices.
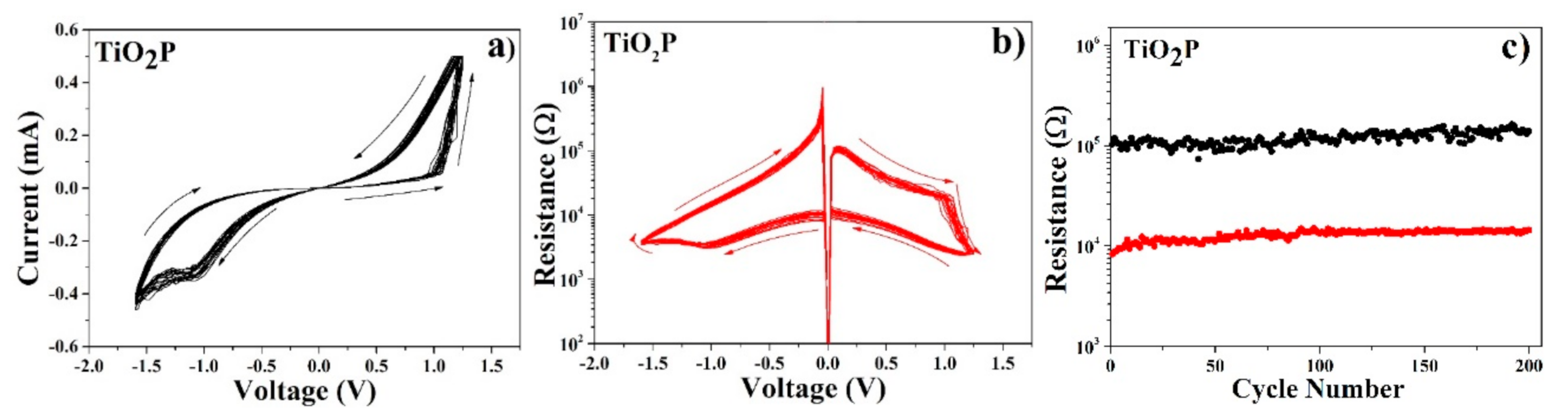
Figure 4 shows 20 typical I-V (a) and R-V (b) curves for the TiO
2P film. V
ON value is about 1.00 ± 0.10 V, while V
OFF is about −1.00 ± 0.10 V. Resistance in the low and high conductivity states are about 10
5 and 10
4 Ω, respectively, with a R
OFF/R
ON ratio of about 10. The typical percentage of circular TE showing shorts with the BE is about 90 ± 2%. Nevertheless, device life is typically higher than 200 cycles (
Figure 4c).
Figure 5 shows hysteresis loops acquired for the TiO
2S devices (I-V (a), R-V (b) curves). V
ON and V
OFF are at about 0.75 ± 0.10 V and −0.62 ± 0.10 V, correspondingly, R
OFF and R
ON values are about 5 × 10
4 and 5 × 10
3 Ω, with a R
OFF/R
ON ratio of about 10. The percentage of the devices showing shorts is about 5 ± 2%, with most of the devices exceeding 50 cycles (
Figure 5c).
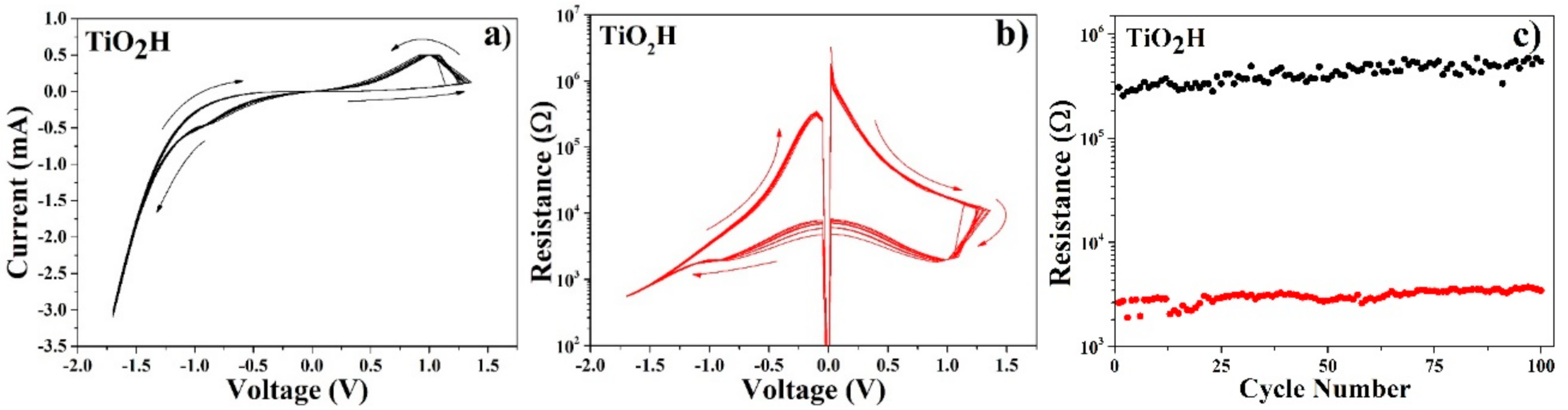
In
Figure 6, I-V (a) and R-V (b) curves for the TiO
2H devices are shown. V
ON is at 1.27 ± 0.10 V, V
OFF is at −0.90 ± 0.10 V. Resistance values for R
OFF and R
ON are about 4 × 10
5 and 3 × 10
3 Ω, correspondingly, with a R
OFF/R
ON ratio higher than 100. The percentage of the devices with TE-BE shorts is about 40 ± 2%, while a typical device life exceeds 100 cycles (
Figure 6c). The key resistive switching parameters are summarized in
Table 2.
4. Discussion
Chemical and structural properties of the TiO
2 films in TiO
2P and TiO
2S devices have already been extensively studied [
5,
16,
18,
19,
23], both showing anatase crystal structure with aggregates of tens and hundreds of nanometers, respectively, while TiO
2P is characterized by high porosity. TiO
2 in TiO
2H shows a surface structure and a roughness similar to the metal oxide film in TiO
2S, suggesting that at least from a morphological point of view, the underlying TiO
2P film does not influence the sol–gel topmost layer morphology significantly. Conversely, it is difficult to identify the effect of processing thermal treatments on the underlying PMCS TiO
2 film. The controlled deposition of nanometric aggregates with a highly defined ordered structure achievable by PMCS at room temperature is one of this technique’s main advantages, as demonstrated for different materials and growth parameters [
27,
28,
29]. Post-deposition thermal annealing at 450 °C in vacuum was reported for PMCS TiO
2 [
19], with the source working parameters optimized for gas sensing, i.e., with very high porosity [
18]. Even though these conditions differ from the one used in this work, the nanocrystallites previously described in literature did not show any change in structure and dimension upon the thermal treatment, but only a slight loss of oxygen due to the annealing in vacuum. This should not occur in TiO
2H where annealing is performed in air. Thus, we can assume that the properties observed for TiO
2P are preserved in the TiO
2 layer developed by the PMCS in TiO
2H.
TiO
2H surface has stoichiometric titanium dioxide chemical properties, with no oxygen defects and absence of other oxides fingerprints. XPS data agree with those of TiO
2S [
24], being XPS, a technique with high surface sensitivity and sampled depth of about 4–8 nm for this material, i.e., the topmost TiO
2 layer realized by sol–gel. This confirms we achieved high reproducibility and control of the sol–gel approach.
The electrical properties of TiO
2S are very similar to already published results, even though in a different crossbar architecture [
23], suggesting satisfactory repeatability of the sol–gel approach for the development of the devices. Concerning TiO
2P, all main parameters (V
ON, V
OFF, R
ON and R
OFF) have slightly higher values than in previously published works [
25]. This is attributed to the different TiO
2 synthesis parameters since in the previously published data, a pure Helium transport gas instead of the 0.1% oxygen gas mixture applied in this work. Moreover, in the previous studies, all resistive switching loops were measured using a Pt wire. In this work, Pt dishes were used as a TE. The electrical properties of all studied devices are summarized in
Table 2. The memristive performance of the TiO
2S device differs from the TiO
2P one. The TiO
2S shows lower switching voltages and ON-OFF resistances, lower durability, and a considerably lower probability of short circuits of the devices than TiO
2P. However, R
OFF/R
ON ratio is the same. Since the film thickness, chemical, and structural properties are very similar in the two model devices, the observed differences in electrical performance are probably due to the intrinsic nanostructure as observed in TiO
2 [
1] and other materials [
30]. Likewise, the high percentage of devices with electrical shorts in the TiO
2P set has the same origin, i.e., the higher porosity of the TiO
2 layer compared to the sol–gel derived metal oxide. The number and size of grain borders in the PMCS films favors an efficient Pt percolation from the TE to the BE inside the film, upon the deposition of the topmost electrode. A more detailed understanding of the different electrical characteristics of the three types of devices, even though quite interesting, would require a more detailed analysis and modeling of the memristive mechanism that goes beyond the scope of this study.
The acquired hysteresis loops (
Figure 4a,
Figure 5a and
Figure 6a) are bipolar and (V,I) = (0,0) pinched; thus, we can classify all the devices as a generic memristor [
31], with only TiO
2P showing a symmetrical shape. It is important to note that the device produced by the hybrid method, TiO
2H, displays memristive switching properties after electroforming by analogy with the two models. Therefore, the hybrid deposition method combining the PMCS and the sol–gel techniques for forming the metal oxide layer is a new versatile tool for developing memristive devices.
The detailed insight into the TiO
2H electrical performances shows that V
ON and V
OFF resemble TiO
2P rather than those of TiO
2S. R
ON in TiO
2H devices is lower than in both model devices. Conversely, TiO
2H has higher resistance in the insulating state, an effect that could be due to the formation of a complex interface between the two metal oxide layers, characterized by different morphology and structure. The percentage of the TiO
2H working devices is significantly higher than for PMCS-derived TiO
2P; thus, the topmost sol–gel derived TiO
2 layer’s deposition was beneficial for preventing the presence of short circuits related to the porosity of the titanium dioxide layer in TiO
2P (
Table 2). The loop’s highly asymmetric shape can probably be converted to a more symmetrical one by an electroforming procedure that should be optimized for the hybrid structure [
7,
26]. However, we did not consider modifying the electroforming step to make the comparison of the devices based on three different processes more understandable and fairer. The TiO
2H device life cycle is higher than for TiO
2S, and R
OFF/R
ON ratio is one order of magnitude higher than the two model devices. Considering all TiO
2H device electrical features is difficult to identify the switching position in the layer. e.g., close to the BE or TE. However, there is a clear role of the interface between the PMCS and sol–gel derived layers. We conclude that the interface leads to higher/lower resistances in non conducting/conducting states, respectively, with significant improvement of R
OFF/R
ON, one of the main parameters defining memristive switching quality.
From the properties discussed above and the comparison of the performances of the three different memristive devices studied, we believe in having proved to be able to combine and exploit the best properties achievable independently by the PMCS and sol–gel methods compensating their specific weaknesses. Hence, the proposed HM approach is well suited to fabricate memristive devices with improved performances.
5. Conclusions
This paper successfully proposes and demonstrates the hybrid deposition method for developing the TiO2 films combining PMCS and sol–gel, two established techniques for metal oxides synthesis in vacuum and wet chemistry, respectively. While these two methods independently produce TiO2 films showing satisfactory memristive properties, their specific morphological and structural characteristics lead to drawbacks that can affect the overall device performances. We showed that these weaknesses can be strengthened or overcome by developing a novel hybrid method merging the sol–gel and the PMCS techniques. We analyzed the approach’s performances in detail, comparing the PMCS (TiO2P) and the sol–gel (TiO2S) derived devices to the hybrid TiO2H, where the PMCS film was deposited on the BE, and the sol–gel TiO2 was deposited on the top of it. The new material shows typical memristive switching, a reduced percentage of electrical shorts to TiO2P, and higher life cycles than TiO2S, benefitting from both techniques and showing a reliable approach for memristive electronic devices.
Additionally, we found that TiO2H gives a ROFF/RON, the most crucial parameter for a memristor, one order of magnitude higher than that for both the PMCS and sol–gel techniques used independently. Since the PMCS growth method precisely controls the composition of TiO2, tuning the oxygen atomic percentage from under to over stoichiometry while giving a fine control of the film thickness, its use paves the way to create interfaces between titanium oxides with a range of vacancies distribution. This represents a novel strategy for modulating the position of the switching zone in the conducting filament. Moreover, based on the achieved results, we envisage the perspective of developing stacked structures of different oxides thanks to the versatility of both the PMCS and the sol–gel methods. The functionalization of the bottom film with organic molecules that can dope the interface with charge donors, acceptors, could further tune the material’s memristive properties.