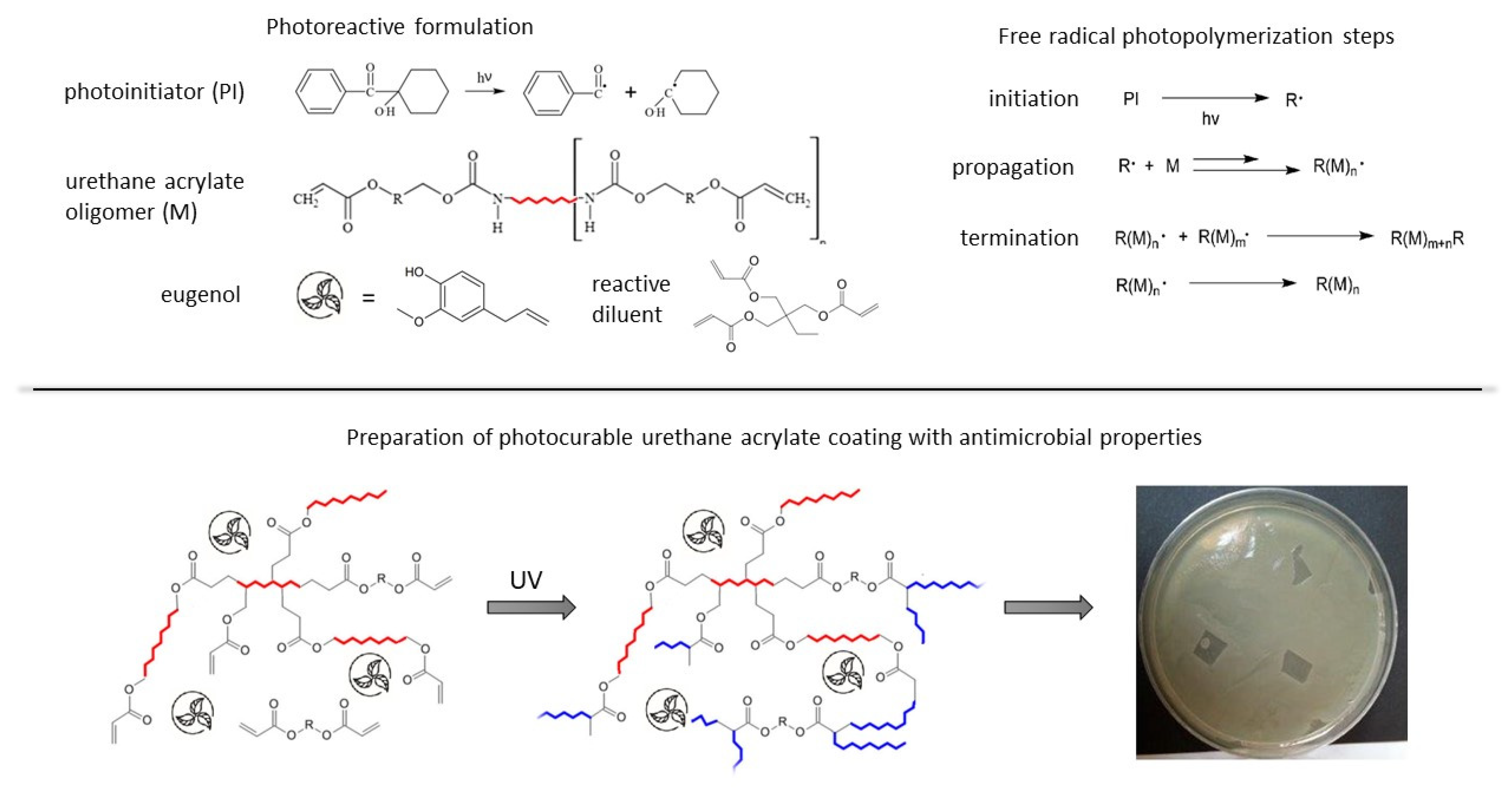
1. Introduction
Photocurable coatings containing compounds of natural origin (for example, limonene and eugenol) are very attractive intermediates for various applications in green materials chemistry. At present, medical applications for this type of materials are of the greatest interest. Mainly, it is connected with the low toxicity of the final product [
1,
2]. High-efficiency photocurable coatings are obtained by the photopolymerization reaction. The popularity of UV-driven chemical reactions has grown very significantly in recent years, mainly due to the fact that such reactions allow to obtain a high conversion of a raw material in a very curing short time [
3]. This method also does not require the use of solvents, and it consumes little energy, because the reaction is carried out at a low temperature [
4,
5,
6]. The varnishes curable by ultraviolet (UV) are composed of oligomers, monomers, or reactive diluents, photoinitiators, and additives. The physical and tensile properties of the obtained polymeric films depended on the coating formulation and the type of radiation used [
7,
8]. The urethane acrylate oligomer was used as the film former, and it is usually composed of highly viscous resins [
9,
10,
11]. The monomer is added to these polymers to blend other ingredients and control the formulation viscosity (the reactive diluent). Still, it affects both the cure speed and the polymerization extent, as well as the properties of the cured film [
12,
13]. In addition, the varnish includes the radical photoinitiator that initiates the polymerization reaction [
14,
15,
16,
17].
Urethane acrylates possess the best balance between hardness, toughness, and chemical and abrasion resistance on the one hand, and flexibility and elasticity on the other. Possibilities of changeability are generally very vast so that the tough and abrasion-resistant films can be produced as well as the incredibly soft and elastic ones. Many of these properties are based on the ability of urethane groups to form the intermolecular hydrogen bonds, which, however, simultaneously increase the viscosity of the formulation [
18]. If the properties of the films were the sole consideration, urethane acrylates would undoubtedly be the most frequently used oligomers in the radiation-curable formulations. However, the high viscosity significantly reduces the range of opportunities for their widespread use. In view of this fact, in this publication, we used the reactive diluent in the form of a trifunctional acrylate monomer [
19,
20]. The most reactive systems are composed of aliphatic urethane with the high functionality and low viscosity. This class of oligomers produces the films with the highest quality. Properties such as adhesion, hardness, and abrasion-resistant combined with high elasticity and flexibility are excellent, the same as resistance to weathering and yellowing. As a result of these outstanding properties, approximately 80% of the urethane acrylates are based on aliphatics [
18]. Poly(urethane acrylates) copolymers are prepared directly from the monomers by the photopolymerization. The obtained copolymers may be used as the active environment for the later radical copolymerization initiated by the UV radiation [
21]. As a result of the photochemically initiated reaction of the dissociation of the double bonds of acrylate groups, the curing of the polymer coating is observed.
Today, essential oils enjoy great interest and are widely used in medicine, in the perfume industry, in cosmetics, in the food industry, in organic syntheses, and in the production of polymeric materials. For example, an orange oil obtained from orange peels, containing 98% of limonene, was used in the production of photocurable varnishes. This application of limonene was described in our previous work [
22] in which we obtained photocurable varnishes with pleasant smells that showed the bactericidal and biostatic properties. Another compound of natural origin that is currently of great interest to researchers is eugenol. It is the main component of the clove essential oil, which is isolated from flower buds of
Syzygium aromaticum (family:
Myrtaceae). Many medical applications described for this essential oil is connected with the presence of eugenol in its composition [
23,
24,
25,
26,
27,
28]. Eugenol (4-allyl-2-methoxyphenol) can be used for example as the antiseptic agent in dental and medical practice [
23,
27,
29]. Eugenol is the aromatic compound that is more complex taking into account its structure than limonene. In addition to the allylic group, the eugenol molecule also has the hydroxyl and methoxy groups attached to the aromatic ring. The limonene molecule has the different, cyclic but not aromatic structure. In the side chain there is one double bond in the limonene molecule, while the other occurs in the cyclic ring. Thus, application of eugenol in the production of photocurable varnishes can cause formation of a product with different properties than the varnishes obtained with limonene molecules.
In this work the preparation of the urethane acrylate resin modified with the different amounts of eugenol was reported. For the preparation of the photoreactive coating compositions the radical photoinitiator was also used. The coatings were created with the use of the UV radiation. The obtained materials were tested for the kinetics of the photopolymerization process and the properties of the cured coatings. The studies also focused on testing the antimicrobial activity of the obtained photocurable UA coatings, particularly against Gram-negative and Gram-positive bacteria (
Escherichia coli and
Staphylococcus epidermidis) as well as the representative of the yeastlike fungus (
Candida albicans). This work aimed to examine the influence of the eugenol molecules concentration on the chemical, physical, thermal, and mechanical properties and antimicrobial properties of the obtained coatings. In addition, the aim was to find such conditions at which the optimal properties for industrial applications could the prepared coatings be achieved. Coatings of this type are suitable for high-grade exterior applications due to the fact of their resistance to weathering and yellowing. Above all, they could be used to protect a substrate against microorganisms. They can be applied to all potential substrates such as plastics, metal, wood, paper, textiles, and leather [
18]. Considering the properties of the used biologically active compound (eugenol), these coatings can find applications in the furniture industry, for production of everyday objects, and they can also find special applications, e.g., in medicine and in the cosmetics industry.
2. Materials and Methods
2.1. Materials
The following raw materials were used for the studies: aliphatic urethane acrylate (Genomer 4425, Rahn, Zurich, Switzerlandt), 1-hydroxycyclohexyl phenyl ketone (Irgacure 184, BASF, Ludwigshafen, Germany), trimethylolpropane triacrylate as a reactive diluent (TMPTA, BASF), and eugenol (>98%, Sigma, Buchs, Switzerland).
2.2. Preparation of Coatings
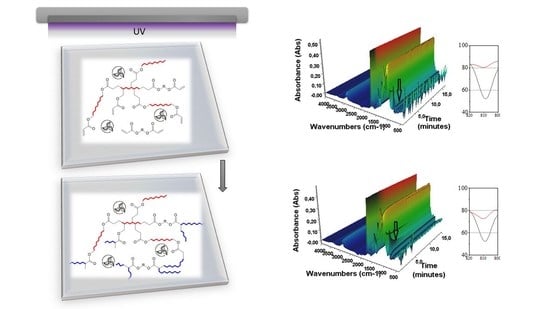
The preparation of the photocurable urethane acrylate coatings with the different amounts of the unsaturated biologically active compound (eugenol) allowed for the obtention of the hybrid polymer with help of the photochemically initiated reaction. For this purpose, coating compositions were prepared by homogenization (using a mechanical homogenizer) the resin and eugenol (in the concentration of 1–10 wt%, thus reducing the proportion of UA resin) at the ambient temperature and under dark conditions. In the next stage, the photoinitiator (3 wt% in relation to the photoreactive mixture with eugenol) was mixed with the reactive acrylic diluent (TMPTA 10 wt%), until the homogenous solution was obtained. Thus, the names of the photoreactive systems were PRE0 for the non-eugenol system, PRE1 for the 1 wt% eugenol system, etc. Subsequently, the curing solution was applied to the glass substrates by means of a gap applicator (120 µm). The polymeric film was cured under a light source for 120 s and with an intensity of 10 mW/cm
2. The distance from the UV source (λ = 350–400 nm, 36 W) to the photoreactive cure layer was 5 cm. The way of the preparation of the photocurable urethane acrylate coatings containing eugenol applied in our work was presented in
Figure 1.
2.3. Spectroscopy
The Fourier-transformed infrared (FTIR) spectroscopy in the attenuated total reflectance mode (ATR) was measured on an Nicolet iS5 instrument (Thermo Electron Corporation, Waltham, MA, USA). Sixteen scans were averaged for each sample in the range of 4000–400 cm−1 at the room temperature. The infrared spectrums were used to characterize the obtained UA with the different amounts of eugenol. In addition, the series real-time IR (RT-IR) were used to determine the conversion of the unsaturated chemical bonds. More importantly, this spectroscopic technique permits in situ monitoring of the chemical processes via mimicking the disappearance of the characteristic bands of the reactive compounds subjected to UV exposure. The photoreactive mixture was placed in the mold made from glass slides and spacers of 15 mm in diameter and 0.5 in thickness. The samples were placed in the compartment of the Fourier transform infrared spectrometer and were simultaneously exposed to the UV radiation source (mercury UV lamp, Indigo, Łódź, Poland, 36 W, 280–400 nm, 10 mW/cm2) and the IR analysis light beam. The absorbance change of the acrylate and eugenol double bond (C=C) peak area was correlated to the extent of the polymerization. The following relations can express the degree of conversion (DC): DC (%) = (A0−At)·100/A0, where A0 is the initial peak area before the irradiation and At is the peak area at time t. The photopolymerization rate (Rp) was calculated by the following relations: Rp = dDC/dt, where t is the time of the irradiation.
2.4. Thermal Properties
The decomposition profile of the cured samples was thermogravimetrically analyzed using TG 209 F1 LIBRA NETZSCH (Selb, Germany) analyzer. Film samples ranging from 4 to 6 mg were placed in the corundum sample pan and heated from 25 to 800 °C, under an air atmosphere at a heating rate of 10 °C/min, and the weight loss and temperature differences were recorded as the function of temperature.
2.5. Viscosity
The viscosity of the obtained photoreactive systems was determined with a Bohlin Visco 88 (Malvern Panalytical, Malvern, UK) viscometer. The measurement was carried out at the temperature of 20 °C using the C14 geometry at the speed of 20 rpm. The presented viscosity values represent the average values made from two measurements, the error of which did not exceed 3%.
2.6. Properties of the Cured Coatings
The following tests were performed in order to evaluate the mechanical properties of the cured coatings: tack-free time, pendulum hardness test, adhesion, gloss, and yellowness index. Tack-free time was measured as the surface cure time according to ISO 9117. It is when the coating was deemed to be properly adhered to and achieved the final technical parameters. The presented values represent the average values made from two measurements. The hardness of coatings was tested using the König pendulum hardness tester according to ASTM D 4366 with respect to pendulum oscillation times on the coatings on the glass substrate. Hardness test was carried out after 24 h and after 2 months. The presented values represent the average values made from three measurements, the error of which did not exceed 5%. Adhesion of the coatings to the glass substrate was measured using the pull-off adhesion tester according to the national and international standards, including ASTM D4541/D7234 and ISO 4624/16276-1. The presented values represent the average values made from seven measurements, the error of which did not exceed 10%. Gloss was measured by spectrometer GLS (SADT Development Technology Co. Ltd., Beijing, China) according to ASTM D523. The presented values represent the average values made from three measurements, the error of which did not exceed 5%. Yellowness index is the number calculated from spectrophotometric data that describes the change in color of the tested samples. This parameter was measured according to ASTM E313 and using precision colorimeter NH-145 (3NH Technology Co., Ltd., Shenzhen, China). The presented values represent the average values made from three measurements, the error of which did not exceed 5%.
2.7. Antimicrobial Properties
The antimicrobial properties of coatings with eugenol were also examined. Test organisms used in this study were as follows: Escherichia coli strain K12 (ATCC 25922), Staphylococcus epidermidis (ATCC 49461), Candida albicans (culture isolated from immunocompromised patients). The E. coli bacteria were cultured on PCA (Plate Count Agar, BTL Poland), S. epidermidis on BHI agar (Brain Heart Infusion Agar, BTL, Łódź, Poland), Candida albicans on SDA (Sabouraud Dextrose Agar, BIOMAXIMA, Lublin, Poland). The antibacterial and antifungal properties tests of coatings based on urethane acrylates were carried out according to the European Pharmacopoeia 5.0 standards. The disc diffusion method was used.
Before the experiments, bacteria were precultured in appropriate liquid media: E. coli in Enriched Broth (BIOMAXIMA Poland) at 37 °C and S. epidermidis in Brain Heart Infusion Broth (BTL, Poland) at 37 °C. The bacterial cultured was diluted to the final cell density 0.50 in McFarland standard (bioMérieux, Marcy l’Etoile, France), corresponding to bacterial cell number approximately 1.5 × 108 CFU mL−1. Two-day-old yeast culture on MEA slants at 25 °C for C. albicans was used for preparing the yeast suspension. Sterile sodium chlorine buffer (0.85% NaCl) was added to the slant, and the culture was appropriately diluted to obtain an inoculation level of appx 106–107 CFU mL−1 (corresponded to 0.55 in McFarland standard, bioMérieux, Marcy l’Etoile, France). Three hundred microliters of microbial suspension were spread on a media suitable for tested microorganism. Three samples of each varnish samples supplemented with from 1 to 10 wt% of eugenol were put on agar plates. A varnish without eugenol was used as a control experiment. The plates were incubated at a temperature of 37 °C from 24 (bacteria) to 48 h (Candida sp.). The inhibition zone around the paper discs or lacquer samples was measured with a ruler using aCOLyte camera 7500 (Symbios, Frederick, MD, USA).
The minimum inhibitory concentration (MIC) was determined. Three sterile paper discs (Whatman No. 1, Little Chalfont, Buckinghamshire, UK diameter 5 mm) were impregnated with 5 µL of the suitable solution. As a solvents water, 1.0% ethyl acetate, methanol and 10% DMSO were used. The prepared solutions’ concentration were as follows: 100%, 75%, 50%, 30%, 25%, 20%, 15%, 10%, 5%, and 1% of eugenol. The swabbed discs were placed on the proper media. The disc swabbed with ethyl acetate solution was used as a control. The plates were incubated at the appropriate temperature (37 °C for E. coli and S. epidermidis, 25 °C for C. albicans) for 24–48 h. The inhibition zone around the paper discs was measured with a ruler using a COLyte 7500 camera (Symbios, USA). All tests were repeated three times.
The diameter of the inhibition zones classified the sensitivity to the tested agents: not sensitive (–) for diameters less than 5 mm; sensitive (+) for diameters 5–10 mm; very sensitive (++) for diameters larger than 10 mm and less than 15 and super sensitive for diameters larger than 15 mm (+++).
3. Results and Discussion
In this study, urethane acrylate resins were modified with eugenol in order to give them antimicrobial properties. In this studied eugenol was also used as the biologically active diluent to reduce the high viscosity. The second reactive diluent was the trifunctional acrylate monomer. The tested systems had the viscosity of 0.65 Pa·s for PRE0 to 0.61 Pa·s for PRE10.
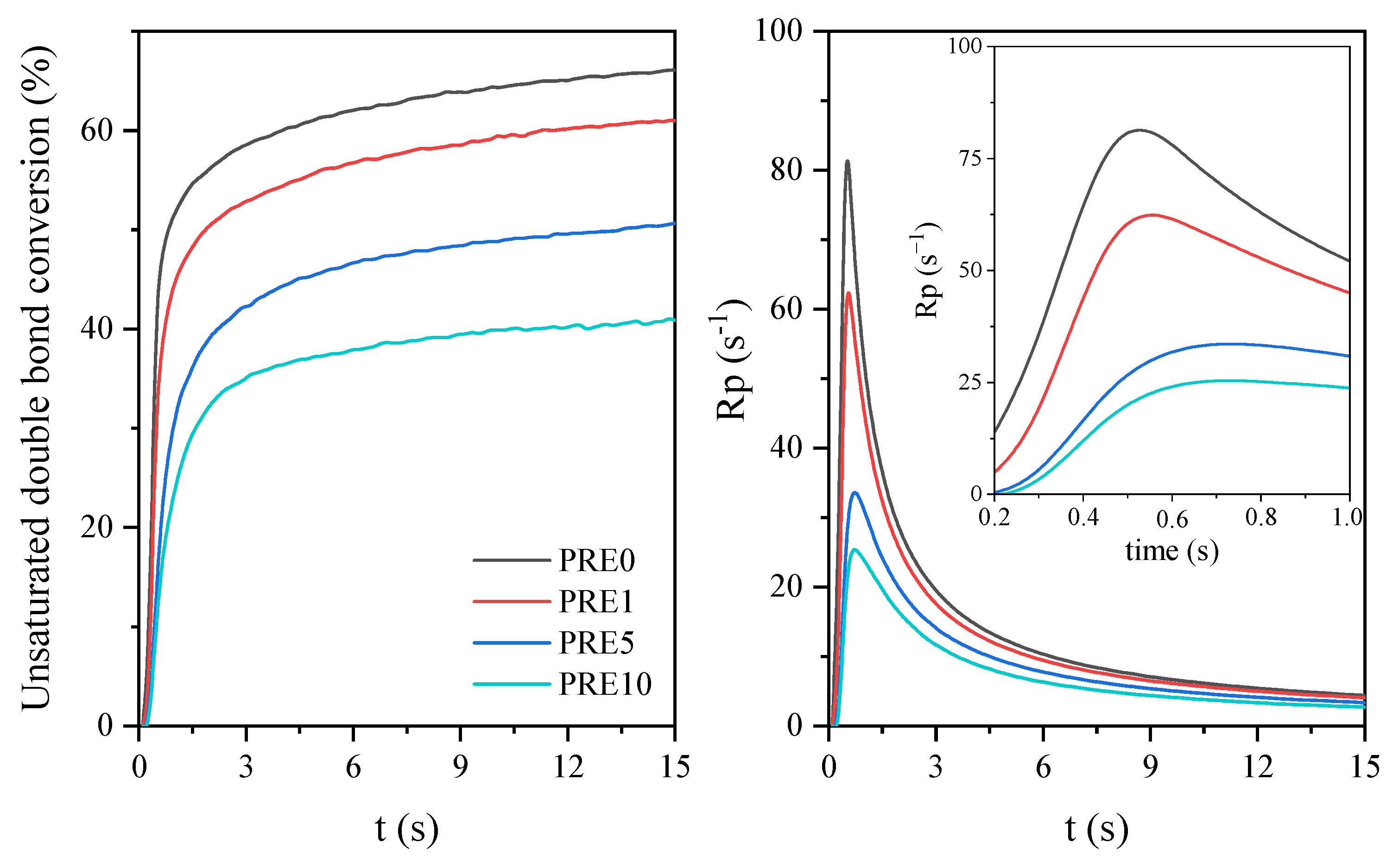
Figure 2 shows the FTIR spectrum of eugenol and the photoreactive systems based on urethane acrylate resin before and after the modification. These systems were presented in the liquid form (i.e., before irradiation as well as after 15 min of the UV irradiation.
The synthesis of monomers derived from eugenol-containing polymerizable functional groups has been widely studied in recent years. The (meth)acrylation of eugenol, isoeugenol, and dihydroeugenol was performed and the solution homopolymerization of these biobased monomers was studied. Moreover, aiming to prepare functional polymers, the introduction of epoxy and cyclic carbonate groups was executed via modification of the allylic double bond present in eugenol-derived methacrylate [
30]. However, it is known from the literature that phenols are strong radical scavengers. Hence, eugenol and its derivatives did not polymerize readily by the photopolymerization, as with many other bio-based monomers. Therefore, it was seen in studies present in the scientific literature that suitable functional groups are added to eugenol and its derivatives to prevent the inhibition. However, when this situation was evaluated in terms of time, energy, and waste generation, it was evident that it brings additional costs and disadvantages [
31]. Polymerization studies after the modification of eugenol are frequently encountered in the literature but, in this form, they usually lose their antimicrobial properties. It is known that polymerization of pure eugenol on its own is very difficult both from the allyl group and its phenolic structure. Hence, in the FTIR spectrum of unmodified (PRE0) and modified (PRE10) resins, similar bands are observed before and after the photopolymerization process. However, a lesser extent of the disappearance of absorption peak corresponding to C=C stretching vibrations developing at 809 cm
−1 in the case of the modified system would suggest a lower degree of conversion of unsaturated acrylate bonds. Such conclusions can also be drawn based on real-time monitoring of the photopolymerization course using the spectroscopic method (
Figure 3). For this purpose, the C=C unsaturated bond disappearance at 809 cm
−1 was measured. On the basis of the obtained data, the course of conversion and the speed of the photopolymerization during exposure to the UV radiation were determined. Based on the received data, the course of conversion and the photopolymerization rate during exposure to the UV radiation were determined. On the basis of the course of conversion and the speed of the selected systems, it was confirmed that the presence of eugenol results in a lower conversion of unsaturated bonds and slows down the photopolymerization process. Hence, it is presumed that eugenol is trapped in the structure of the polymer network and it can be trapped in the urethane acrylate shell via e.g., hydrogen bonds derived from the hydroxyl group.
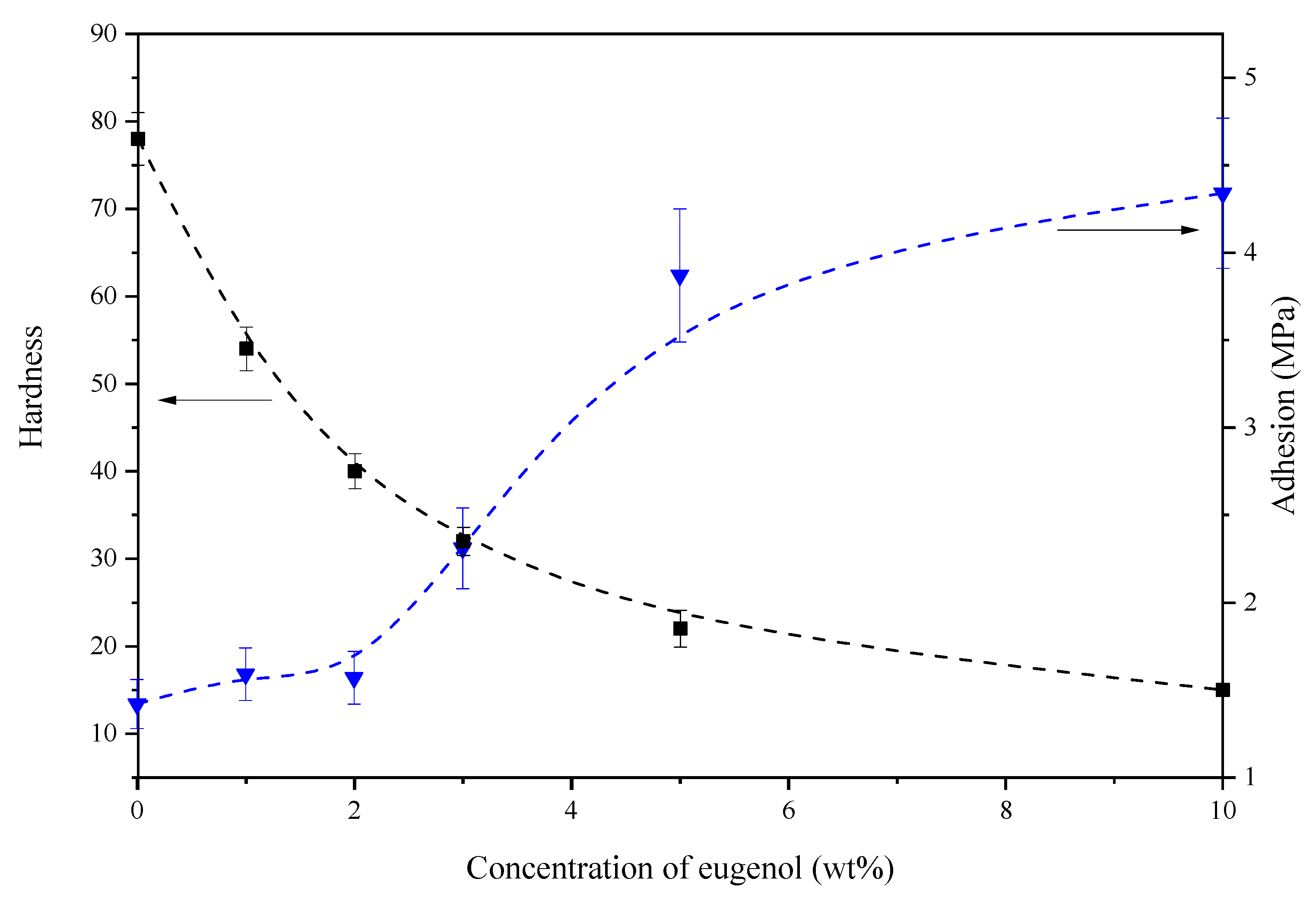
During the studies, the increase in the amount of eugenol in the polymeric film decreased the hardness and increased the adhesion strength to the substrate. However, the most preferred protective coatings were those which formed a hard coating with high adhesion. It is assumed that coatings with hardness below 30 were flexible, which limits their use as the protective coatings. On the other hand, adhesion below 2 MPa may be insufficient to obtain durable coatings on a proper substrate. Therefore, the most beneficial results characterize the coating with 3 wt% of eugenol related to the adhesion and hardness, which is shown as the intersection of the two curves in
Figure 4.
After analyzing the results, the following question arises: why did adhesion increase when eugenol was added? We can answer that eugenol can play the role of the so-called plasticizer. Without reacting chemically with other ingredients in the composition, eugenol “softens” the coating layer, increasing adhesion in a natural way. The second question is: why did surface hardness decrease when eugenol was added? On this question, we can answer that the addition of eugenol increases the adhesion and, thus, reduces the hardness of the cross-linked (hardened) coating.
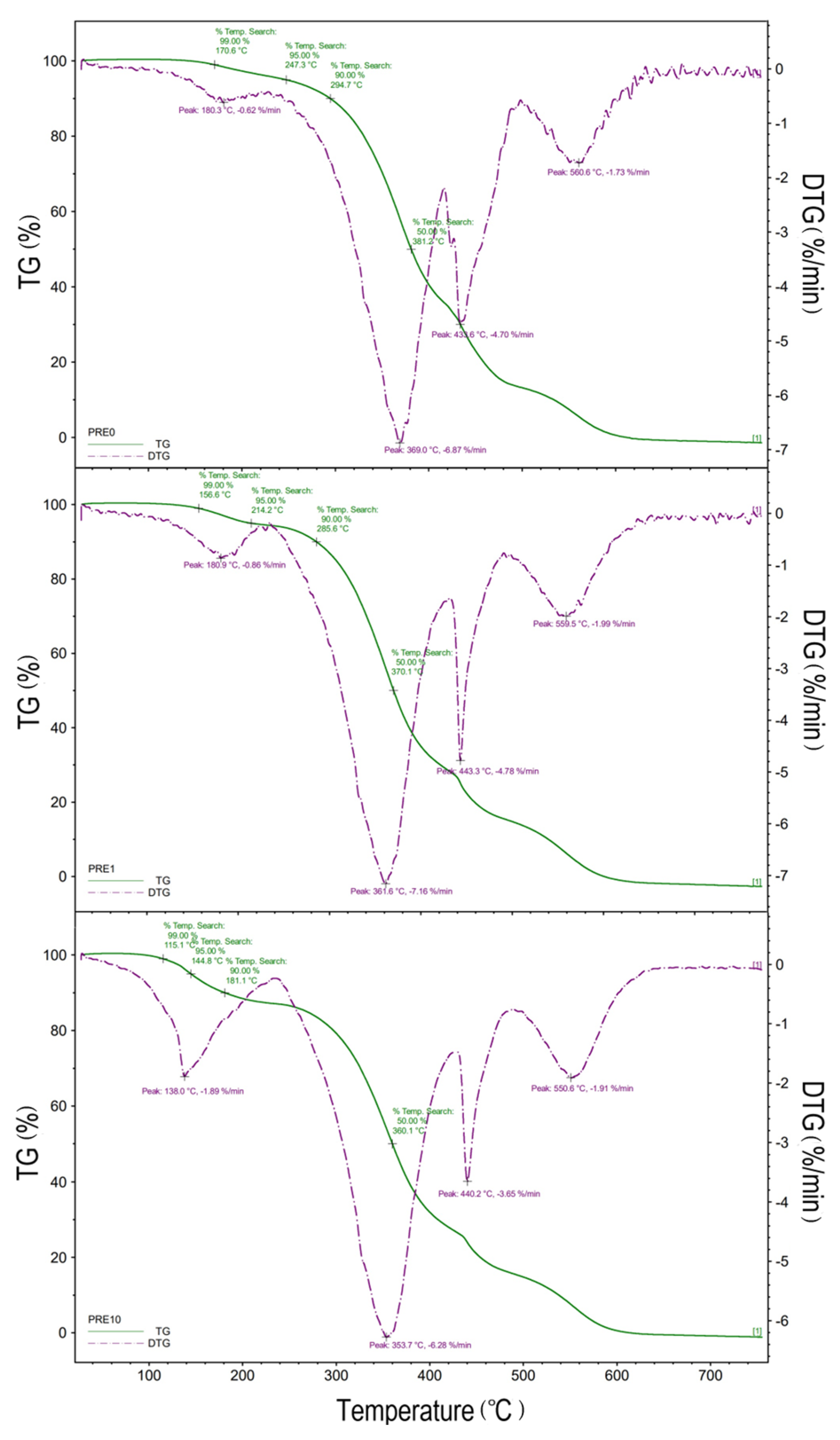
The comparative analysis of the TG curves of the varnish samples with different eugenol content showed that the higher the eugenol content, the lower the stability of the samples.
The most meaningful data from the TGA curves (i.e., the initial decomposition temperature (IDT), the temperatures at which 5%, 10%, and 50% of the specimen decomposed thermally, and the temperature with the maximum weight loss) were collected in
Table 1. The TGA curves all show three (for UV-cured urethane acrylates without eugenol) and four (for UV-cured urethane acrylates with eugenol) distinct regions of weight loss irrespective of formulation, indicating a four-stage degradation process (
Figure 5). The first weight loss observed in the range of 100–150 °C is probably connected with the evaporation of TMPTA not bounded with eugenol. The decomposition temperature corresponding to 5% weight loss is from 144.8 to 214.2 °C. In
Table 1, PRE 1 showed the highest thermal stability.
The addition of eugenol reduces the thermal stability of the urethane acrylates varnishes. The rate of the decomposition of varnishes for the concentration of eugenol in the range of 2–6 wt% is similar, but for the concentrations of eugenol 7–10 wt%, an increase in the rate of the weight loss (decomposition) is observed. The initial temperature of the decomposition for the lacquer PRE3 is amounted to approximately 130 °C, and the change in the stability is probably connected with the evaporation of the diluent and eugenol. The highest stability at the higher temperatures was observed for the varnish without eugenol and the lowest for the varnish with eugenol in amount of 10 wt%.
At a concentration of 10 wt% of eugenol in the coating structure having a thickness of 1 mm, the inhibition zone was observed, which as approximately 0.9 cm in diameter in the case of tests carried out on
Candida albicans. In tests with bacterial cultures, it was found that coating with eugenol was not conducive to colonization because under the surface of coating, the growth of colonies was not observed. No growth of
Staphylococcus epidermidis under the surface of the coating was observed even at a concentration of 1 wt% of eugenol, whereas in the case of
Escherichia coli at the concentration of 3 wt% (
Figure 6). It was believed that the best use of coating with eugenol as the antimicrobial coating will be their local application, especially at the interface between the coating surface with a substrate, they cause a local disappearance of fungi yeast and bacteria colonies, both Gram-negative and -positive. A moderate antimicrobial activity against
E. coli,
P. aeruginosa,
S. aureus, and
B. cereus was detected for the eugenol-rich PLA-based materials [
32,
33]. Our findings showed that eugenol enclosed in varnish structures has antimicrobial properties.
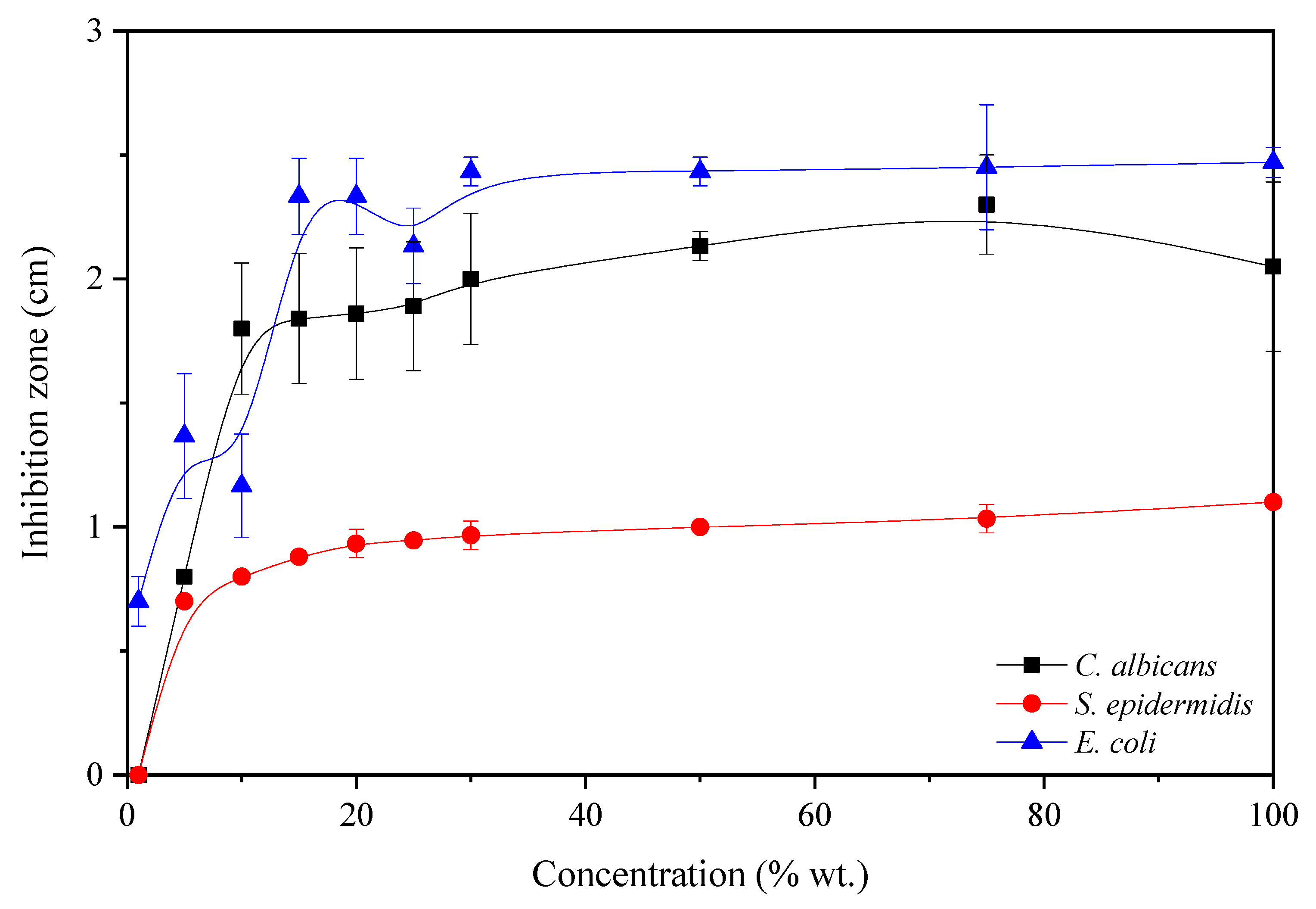
The size of microbial growth inhibition zones depended on the solvent used in the dissolution of eugenol. The minimum inhibitory concentration of eugenol tested as solutions with ethyl acetate is in the form of the inhibition zone of the growth of microorganisms. The results are shown in
Figure 7.
E. coli bacteria are sensitive to the 1 wt% solution of eugenol, while
C. albicans and
S. epidermidis have the inhibition zone for 5 wt% solutions. The 15 wt% solutions of eugenol did not significantly change the inhibition zones for all tested microorganisms. According to Nejad et al. (2017) [
34] eugenol has shown an inhibitory effect on the growth of most gram-negative (
Escherichia coli;
Salmonella typhi;
Pseudomonas aeruginosa) and gram-positive (
Staphylococcus epidermidis,
Streptococcus pneumoniae, and
Streptococcus pyogenes) bacteria at the concentration of 1000 μg/mL. Among known natural products extracted from clove eugenol has the lowest antimicrobial effect. However, a synergistic antibacterial activity between eugenol and other substances (natural or additives) was observed [
28,
35]. The mechanism of eugenol’s antimicrobial action is still under discussion. It seems, however, that it induces protein, lipid leakage of cell membranes, increases the transport of potassium and ATP out of the cells, or disturbs the bacterial cell structure [
33,
35]. The antibacterial action of eugenol is most likely related to the presence of the phenol group in the eugenol molecule, which was discussed in detail in the work of Breloy et al. [
36]. It is reported in this paper that phenol groups are responsible for the disruption of the bacterial membrane and that the differences in the bactericidal effect can be explained by the difference in cell–wall structures between bacteria. Gram-negative bacteria (
E. coli) are protected by a thin layer of peptidoglycans and liposaccharides. Gram-positive bacteria (
S. epidermidis) have cell wall consist of thicker and more protective peptidoglycans. Additionally, the surface of Gram-negative bacteria is more hydrophobic than Gram-positive ones, which favor their contact with hydrophobic surfaces. According to the research of. Breloy et al. [
36] eugenol-derived coating exhibits a high hydrophobicity. Our research results are in line with these conclusions. In our work, the best results in microbiological tests were also obtained for Gram-negative bacteria (
E. coli).