Detection of Virulence Genes and Biofilm Forming Capacity of Diarrheagenic E. coli Isolated from Different Water Sources
Abstract
1. Introduction
2. Material and Method
2.1. Water Samples Collection
2.2. Isolation and Biochemical Identification of E. coli
2.3. Extraction of DNA
2.4. Polymerase Chain Reaction (PCR)
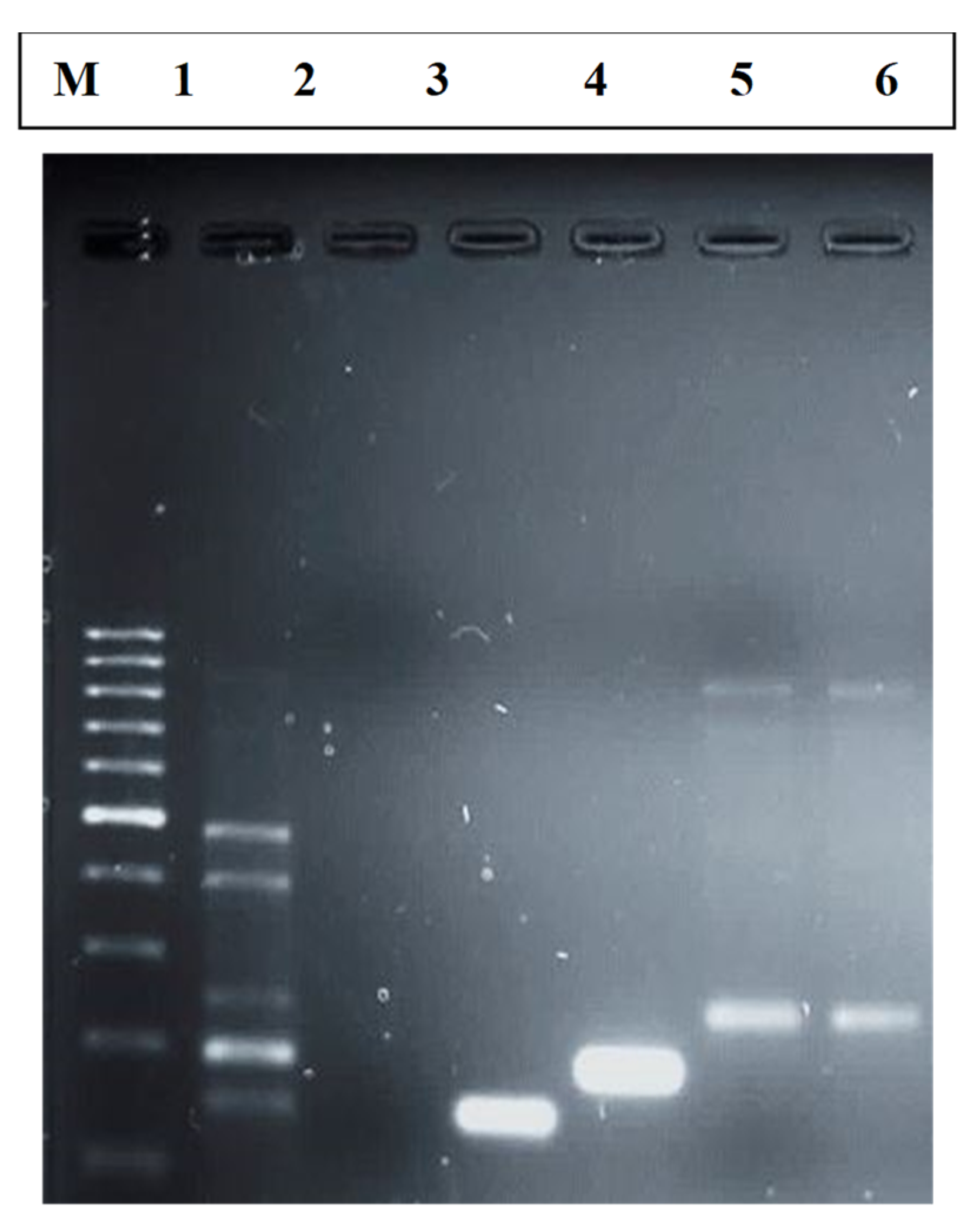
2.5. Visualization of PCR Products
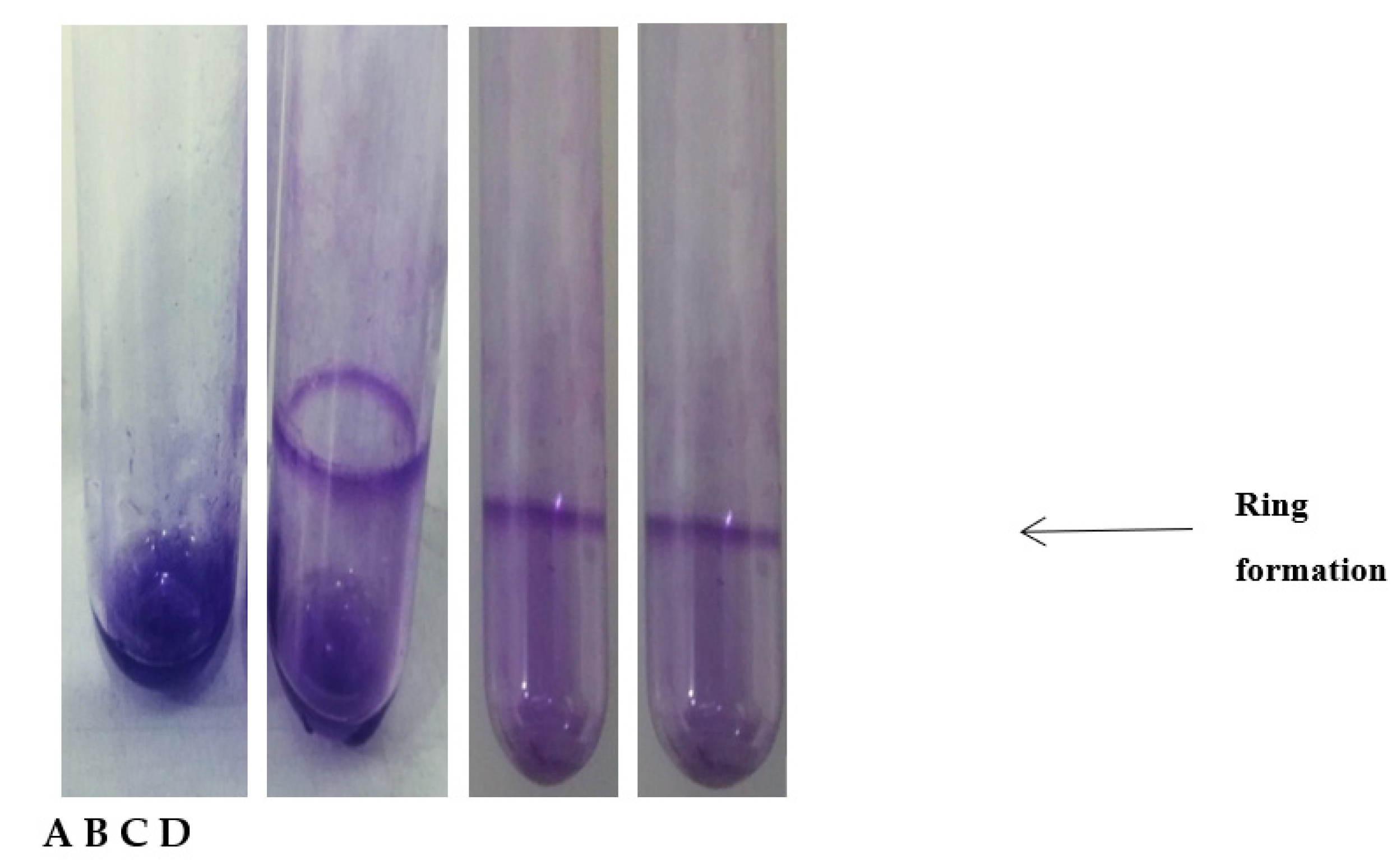
2.6. Biofilm Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Distribution of Isolated Organisms
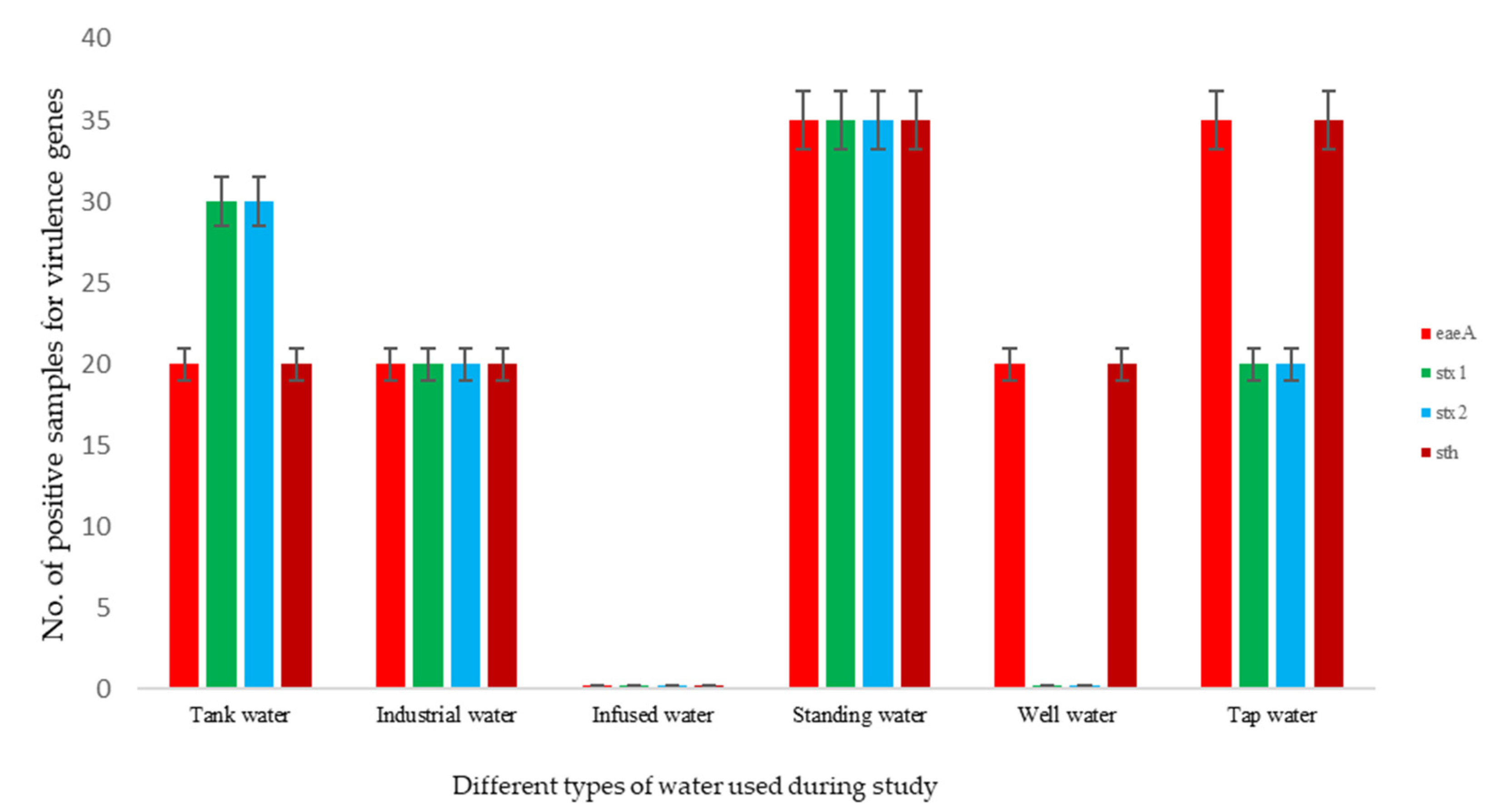
3.2. Prevalence of Virulence Genes from E. coli Isolates
3.3. Biofilm Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.; Jini, D.; Aravind, M.; Parvathiraja, C.; Ali, R.; Kiyani, M.Z.; Alothman, A. A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis. Arab. J. Chem. 2020, 13, 8717–8722. [Google Scholar] [CrossRef]
- Aravind, M.; Ahmad, A.; Ahmad, I.; Amalanathan, M.; Naseem, K.; Mary, S.M.M.; Zuber, M. Critical green routing synthesis of silver NPs using jasmine flower extract for biological activities and photocatalytical degradation of methylene blue. J. Environ. Chem. Eng. 2021, 9, 104877. [Google Scholar] [CrossRef]
- Pervaiz, M.; Ahmad, I.; Yousaf, M.; Kirn, S.; Munawar, A.; Saeed, Z.; Rashid, A. Synthesis, spectral and antimicrobial studies of amino acid derivative Schiff base metal (Co, Mn, Cu, and Cd) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khan, A.J.; Arshad, M.; Javed, M.S.; Ahmad, A.; Shah, S.S.A.; Qiao, G. Charge storage in binder-free 2D-hexagonal CoMoO4 nanosheets as a redox active material for pseudocapacitors. Ceram. Int. 2021, 47, 8659–8667. [Google Scholar] [CrossRef]
- Zhan, M.; Hussain, S.; AlGarni, T.S.; Shah, S.; Liu, J.; Zhang, X.; Liu, G. Facet controlled polyhedral ZIF-8 MOF nanostructures for excellent NO2 gas-sensing applications. Mater. Res. Bull. 2021, 136, 111133. [Google Scholar] [CrossRef]
- Kashif, M.; Ngaini, Z.; Harry, A.V.; Vekariya, R.L.; Ahmad, A.; Zuo, Z.; Alarifi, A. An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO2) towards solar cell application. Appl. Phys. A 2020, 126, 1–13. [Google Scholar] [CrossRef]
- Saleem, M.; Irfan, M.; Tabassum, S.; Albaqami, M.D.; Javed, M.S.; Hussain, S.; Zuber, M. Experimental and theoretical study of highly porous lignocellulose assisted metal oxide photoelectrodes for dye-sensitized solar cells. Arab. J. Chem. 2021, 14, 102937. [Google Scholar] [CrossRef]
- Kashif, M.; Jaafar, E.; Bhadja, P.; Low, F.W.; Sahari, S.K.; Hussain, S.; Al-Tamrah, S.A. Effect of potassium permanganate on morphological, structural and electro-optical properties of graphene oxide thin films. Arab. J. Chem. 2021, 14, 102953. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Xu, P.H.; Liu, G.W.; Ahmad, A.; Chen, X.H.; Zhu, Y.L.; Qiao, G.J. Synthesis, characterization and wettability of Cu-Sn alloy on the Si-implanted 6H-SiC. Coatings 2020, 10, 906. [Google Scholar] [CrossRef]
- Fallah, Z.; Zare, E.N.; Ghomi, M.; Ahmadijokani, F.; Amini, M.; Tajbakhsh, M.; Varma, R. Toxicity and remediation of pharmaceuticals and pesticides using metal oxides and carbon nanomaterials. Chemosphere 2021, 275, 130055. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Al Kahtani, A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Ahmad, I.; Jamal, M.A.; Iftikhar, M.; Ahmad, A.; Hussain, S.; Asghar, H.; Khan, S. Lanthanum-zinc binary oxide nanocomposite with promising heterogeneous catalysis performance for the active conversion of 4-nitrophenol into 4-aminophenol. Coatings 2021, 11, 537. [Google Scholar] [CrossRef]
- Javed, M.S.; Khan, A.J.; Ahmad, A.; Siyal, S.H.; Akram, S.; Zhao, G.; Alfakeer, M. Design and fabrication of bimetallic oxide nanonest-like structure/carbon cloth composite electrode for supercapacitors. Ceram. Int. 2021, 47, 30747–30755. [Google Scholar] [CrossRef]
- Javed, M.S.; Najim, T.; Hussain, I.; Batool, S.; Idrees, M.; Mehmood, A.; Shah, S.S.A. 2D V2O5 ultrathin nanoflakes as a binder-free electrode material for high-performance pseudocapacitor. Ceram. Int. 2021, 47, 25152–25157. [Google Scholar] [CrossRef]
- Beena, V.; Rayar, S.L.; Ajitha, S.; Ahmad, A.; Albaqami, M.D.; Alsabar, F.A.A.; Sillanpää, M. Synthesis and characterization of Sr-doped ZnSe nanoparticles for catalytic and biological activities. Water 2021, 13, 2189. [Google Scholar] [CrossRef]
- Siyal, S.H.; Javed, M.S.; Ahmad, A.; Sajjad, M.; Batool, S.; Khan, A.J.; Najam, T. Free-standing 3D Co3O4@ NF micro-flowers composed of porous ultra-long nanowires as an advanced cathode material for supercapacitor. Curr. Appl. Phys. 2021, 31, 221–227. [Google Scholar] [CrossRef]
- Syah, R.; Ahmad, A.; Davarpanah, A.; Elveny, M.; Ramdan, D.; Albaqami, M.D.; Ouladsmane, M. Incorporation of Bi2O3 residuals with metallic bi as high performance electrocatalyst toward hydrogen evolution reaction. Catalysts 2021, 11, 1099. [Google Scholar] [CrossRef]
- Abbas, Q.; Javed, M.S.; Ahmad, A.; Siyal, S.H.; Asim, I.; Luque, R.; Tighezza, A.M. ZnO nano-flowers assembled on carbon fiber textile for high-performance supercapacitor’s electrode. Coatings 2021, 11, 1337. [Google Scholar] [CrossRef]
- Bibi, S.; Khan, A.; Khan, S.; Ahmad, A.; Sakhawat Shah, S.; Siddiq, M.; Al-Kahtani, A.A. Synthesis of Cr doped LiMnPO4 cathode materials and investigation of their dielectric properties. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Raees, A.; Jamal, M.A.; Ahmad, A.; Ahmad, I.; Saeed, M.; Habila, M.A.; Alomar, T.S. Synthesis and characterization of Ceria incorporated Nickel oxide nanocomposite for promising degradation of methylene blue via photocatalysis. Int. J. Environ. Sci. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Beena, V.; Rayar, S.L.; Ajitha, S.; Ahmad, A.; Iftikhar, F.J.; Abualnaja, K.M.; Ali, S. Photocatalytic dye degradation and biological activities of Cu-doped ZnSe nanoparticles and their insights. Water 2021, 13, 2561. [Google Scholar] [CrossRef]
- Mahmud, Z.H.; Kabir, M.H.; Ali, S.; Moniruzzaman, M.; Imran, K.M.; Nafiz, T.N.; Islam, M.S.; Hussain, A.; Hakim, S.A.I.; Worth, M.; et al. Extended-spectrum beta-lactamase-producing Escherichia coli in drinking water samples from a forcibly displaced, densely populated community setting in Bangladesh. Front. Public Health 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Ochien, G.; Atieno, L. Prevalence of Enterotoxigenic Escherichia coli among Children under Five Years in Siaya County, Western Kenya. Master’s Thesis, Maseno University, Kisumu, Kenya, 2021. [Google Scholar]
- Hassan, A.; Ojo, B.; Abdulrahman, A. Escherichia coli as a global pathogen. Achiev. J. Sci. Res. 2021, 3, 239–260. [Google Scholar]
- Marquezini, M.G.; da Costa, L.H.; Bromberg, R. Occurrence of the seven most common serotypes of Shiga toxin-producing Escherichia coli in beef cuts produced in meat-processing plants in the state of São Paulo, Brazil. J. Food Prot. 2021. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.G.; Motta, E.A.; Mass, A.P.; Fongaro, G.; Ramos, F.M.; Machado, M.S.; Bocchese, D.C.; Viancelli, A.; Michelon, W. Toxicity and enterobacteriaceae profile in water in different hydrological events: A case from south Brazil. Water Air Soil Pollut. 2021, 232, 1–12. [Google Scholar] [CrossRef]
- Bel, J.S.; Khaper, N.; Kurissery, S.; Leung, K.T. A novel comparison of virulence genes, biofilm-forming capacity, antibiotic resistance, and level of reactive oxygen species of sediment, sewage, and O157 E. coli. Water Air Soil Pollut. 2021, 232, 1–19. [Google Scholar] [CrossRef]
- Angulo-Zamudio, U.A.; Gutiérrez-Jiménez, J.; Monroy-Higuera, L.; Flores-Villaseñor, H.; Leon-Sicairos, N.; Velazquez-Roman, J.; Vidal, J.E.; Tapia-Pastrana, G.; Canizalez-Roman, A. Non-diarrheagenic and diarrheagenic E. coli carrying supplementary virulence genes (SVG) are associated with diarrhea in children from Mexico. Microb. Pathog. 2021, 157, 104994. [Google Scholar] [CrossRef]
- Ravi, M.; Ngeleka, M.; Kim, S.-H.; Gyles, C.; Berthiaume, F.; Mourez, M.; Middleton, D.; Simko, E. Contribution of AIDA-I to the pathogenicity of a porcine diarrheagenic Escherichia coli and to intestinal colonization through biofilm formation in pigs. Vet. Microbiol. 2007, 120, 308–319. [Google Scholar] [CrossRef]
- Corzo-Ariyama, H.A.; García-Heredia, A.; Heredia, N.; García, S.; León, J.; Jaykus, L.; Solís-Soto, L. Phylogroups, pathotypes, biofilm formation and antimicrobial resistance of Escherichia coli isolates in farms and packing facilities of tomato, jalapeño pepper and cantaloupe from Northern Mexico. Int. J. Food Microbiol. 2019, 290, 96–104. [Google Scholar] [CrossRef]
- Safadi, R.A.; Abu-Ali, G.S.; Sloup, R.E.; Rudrik, J.T.; Waters, C.M.; Eaton, K.A.; Manning, S.D. Correlation between in vivo biofilm formation and virulence gene expression in Escherichia coli O104: H4. PLoS ONE 2012, 7, e41628. [Google Scholar] [CrossRef] [PubMed]
- Sukkua, K.; Rattanachuay, P.; Sukhumungoon, P. Ex vivo adherence to murine ileal, biofilm formation ability and presence of adherence-associated of human and animal diarrheagenic Escherichia coli. Southeast Asian J. Trop. Med. Public Health 2016, 47, 40. [Google Scholar]
- Al-Gallas, N.; Annabi, T.A.; Bahri, O.; Boudabous, A. Isolation and characterization of shiga toxin-producing Escherichia coli from meat and dairy products. Food Microbiol. 2002, 19, 389–398. [Google Scholar] [CrossRef]
- Chandran, A.; Mazumder, A. Occurrence of diarrheagenic virulence genes and genetic diversity in Escherichia coli isolates from fecal material of various avian hosts in British Columbia, Canada. Appl. Environ. Microbiol. 2014, 80, 1933–1940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, S.-W.; Hsu, B.-M.; Su, Y.-J.; Ji, D.-D.; Lin, W.-C.; Chen, J.-L.; Shih, F.-C.; Kao, P.-M.; Chiu, Y.-C. Occurrence of diarrheagenic Escherichia coli genes in raw water of water treatment plants. Environ. Sci. Pollut. Res. 2012, 19, 2776–2783. [Google Scholar] [CrossRef]
- Chandran, A.; Mazumder, A. Prevalence of diarrhea-associated virulence genes and genetic diversity in Escherichia coli isolates from fecal material of various animal hosts. Appl. Environ. Microbiol. 2013, 79, 7371–7380. [Google Scholar] [CrossRef]
- Wakimoto, N.; Nishi, J.; Sheikh, J.; Nataro, J.P.; Sarantuya, J.; Iwashita, M.; Manago, K.; Tokuda, K.; Yoshinaga, M.; Kawano, Y. Quantitative biofilm assay using a microtiter plate to screen for enteroaggregative Escherichia coli. Am. J. Trop. Med. Hyg. 2004, 71, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Moglad, E.H.; Jalil Adam, O.A.E.; Alnosh, M.M.; Altayb, H.N. Retracted: Detection of virulence genes of diarrheagenic Escherichia coli strains from drinking water in Khartoum State. J. Water Health 2020. [Google Scholar] [CrossRef]
| Pathotype | Clinical Manifestation | Pathology | Susceptible Population |
|---|---|---|---|
| ETEC | Watery diarrhea | Not any definite change | Children in developing areas, travelers to risky areas |
| EIEC | Bacillary diarrhea | Disrupted mucosa with inflammation of large intestine | Common in developing countries, affect all ages |
| EPEC | Gastroentritis | Attaching and effacing lesions | Infants and travelers to risky areas |
| EHEC | Bloody diarrhea | Attaching and effacing lesions in intestine followed by necrosis | Inhabitants of industrialized areas |
| EAEC | Persistent diarrhea | Inflammatory responses in intestine, cytotoxicity of enterocytes | Children in developing areas and travelers to those areas |
| Pathotype | Target Gene | Primer Sequence (5′–3′) | Size of Product (kb) | References |
|---|---|---|---|---|
| EHEC | stx1 | F:ACTTCTCGACTGCAAAGACGTATG R:ACAAATTATCCCCTGAGCCACTATC | 132 | [34] |
| EHEC | stx2 | F:TTCCGGAATGCAAATCAG TC R:CGATACTCCGGAAGCACATTG | 264 | [35] |
| EPEC | eaeA | F:CCGATTCCTCTGGTGACGA R:CCACGGTTTATCAAACTGATAACG | 105 | [36] |
| ETEC | sth | F:TTCACCTTTCCCTCAGGATG R:CTATTCATGCTTTCAGGACCA | 120 | [36] |
| Sample | eaeA (EPEC) | stx1 (EHEC) | stx2 (EHEC) | sth (ETEC) |
|---|---|---|---|---|
| 1 | - | - | - | - |
| 2 | - | - | - | - |
| 3 | - | - | - | - |
| 4 | + | + | + | - |
| 5 | - | + | + | + |
| 6 | - | - | - | - |
| 7 | - | - | - | - |
| 8 | + | + | + | + |
| 9 | - | - | - | - |
| 10 | - | - | - | - |
| 11 | - | - | - | - |
| 12 | + | + | + | + |
| 13 | - | - | - | - |
| 14 | - | - | - | - |
| 15 | - | - | - | - |
| 16 | - | - | - | - |
| 17 | - | - | - | - |
| 18 | - | - | - | - |
| 19 | - | - | - | - |
| 20 | - | - | - | - |
| 21 | + | + | + | + |
| 22 | + | + | + | + |
| 23 | - | - | - | - |
| 24 | - | - | - | - |
| 25 | - | - | - | - |
| 26 | + | - | - | + |
| 27 | - | - | - | - |
| 28 | - | - | - | - |
| 29 | + | + | + | + |
| 30 | - | + | + | - |
| 31 | + | - | - | + |
| 32 | - | - | - | - |
| 33 | - | - | - | - |
| 34 | - | - | - | - |
| 35 | + | - | - | + |
| Samples | OD570 | Strength of Adhesion |
|---|---|---|
| 1 | 0.2 | Strongly adherent |
| 2 | 0.15 | Moderately adherent |
| 3 | 0.1 | Weakly adherent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, S.; Tabassum, S.; Aslam, S.; Sillanpaa, M.; Al-Qahtani, W.H.; Ali, S. Detection of Virulence Genes and Biofilm Forming Capacity of Diarrheagenic E. coli Isolated from Different Water Sources. Coatings 2021, 11, 1544. https://doi.org/10.3390/coatings11121544
Tariq S, Tabassum S, Aslam S, Sillanpaa M, Al-Qahtani WH, Ali S. Detection of Virulence Genes and Biofilm Forming Capacity of Diarrheagenic E. coli Isolated from Different Water Sources. Coatings. 2021; 11(12):1544. https://doi.org/10.3390/coatings11121544
Chicago/Turabian StyleTariq, Sadaf, Sobia Tabassum, Sadia Aslam, Mika Sillanpaa, Wahidah H. Al-Qahtani, and Shafaqat Ali. 2021. "Detection of Virulence Genes and Biofilm Forming Capacity of Diarrheagenic E. coli Isolated from Different Water Sources" Coatings 11, no. 12: 1544. https://doi.org/10.3390/coatings11121544
APA StyleTariq, S., Tabassum, S., Aslam, S., Sillanpaa, M., Al-Qahtani, W. H., & Ali, S. (2021). Detection of Virulence Genes and Biofilm Forming Capacity of Diarrheagenic E. coli Isolated from Different Water Sources. Coatings, 11(12), 1544. https://doi.org/10.3390/coatings11121544







