Dual Use of Copper-Modified TiO2 Nanotube Arrays as Material for Photocatalytic NH3 Degradation and Relative Humidity Sensing
Abstract
:1. Introduction
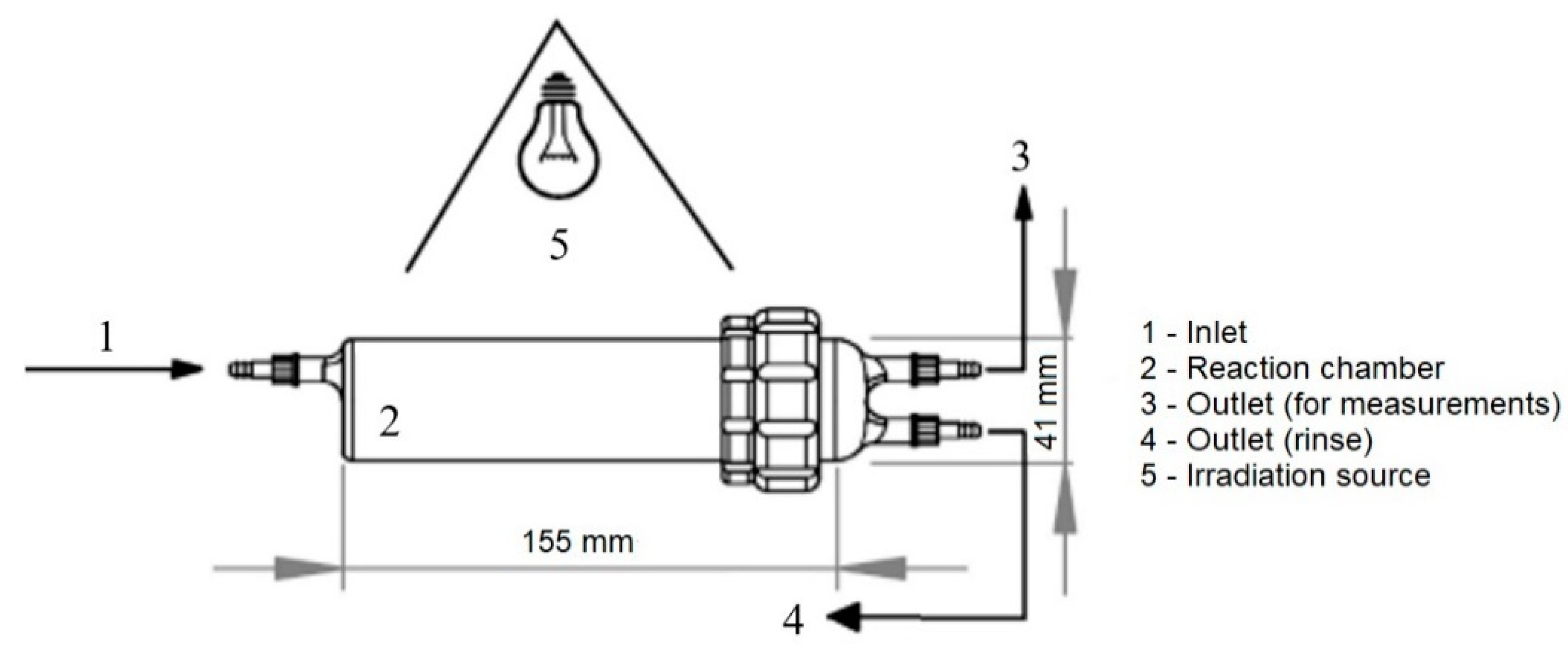
2. Materials and Methods
2.1. Synthesis of Cu-Modified TiNT
2.2. Structural, Electrical, and Morphological Characterization
3. Results
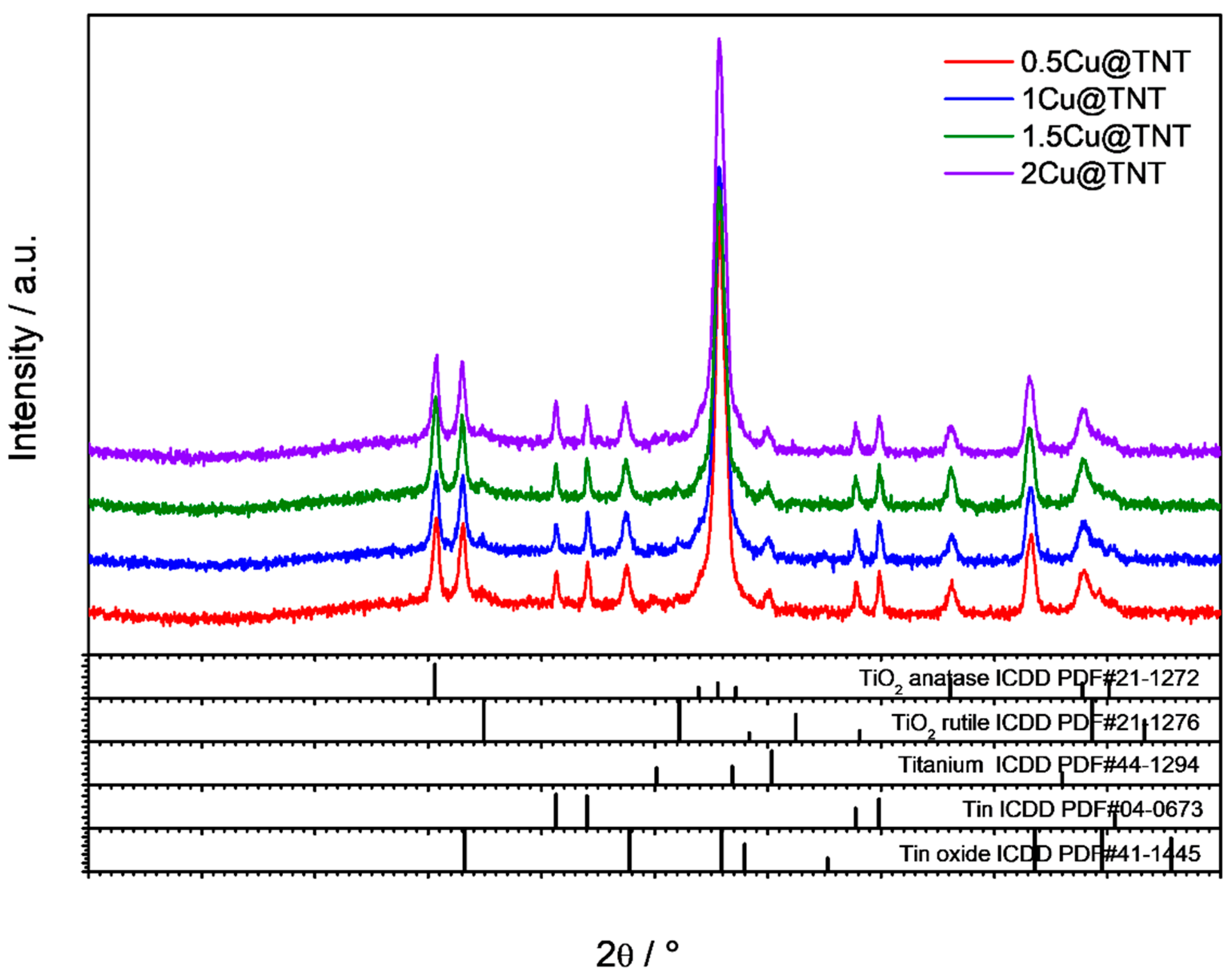
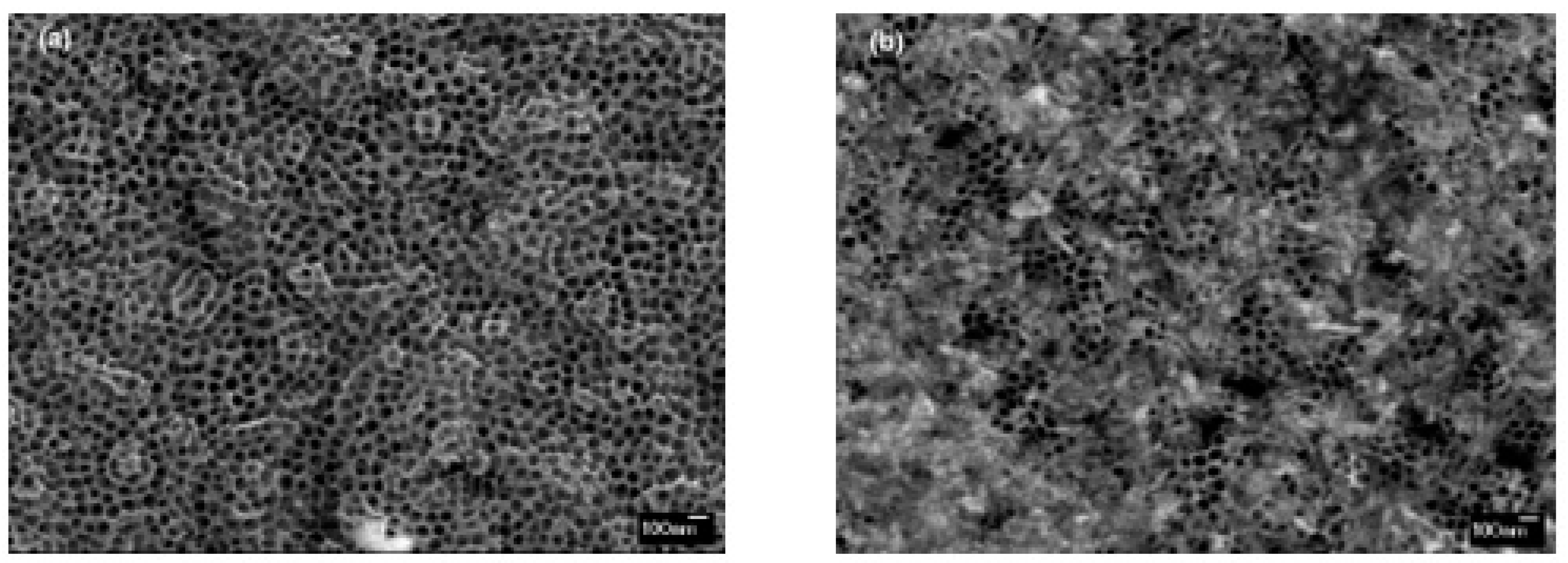
3.1. Structural and Morphological Properties
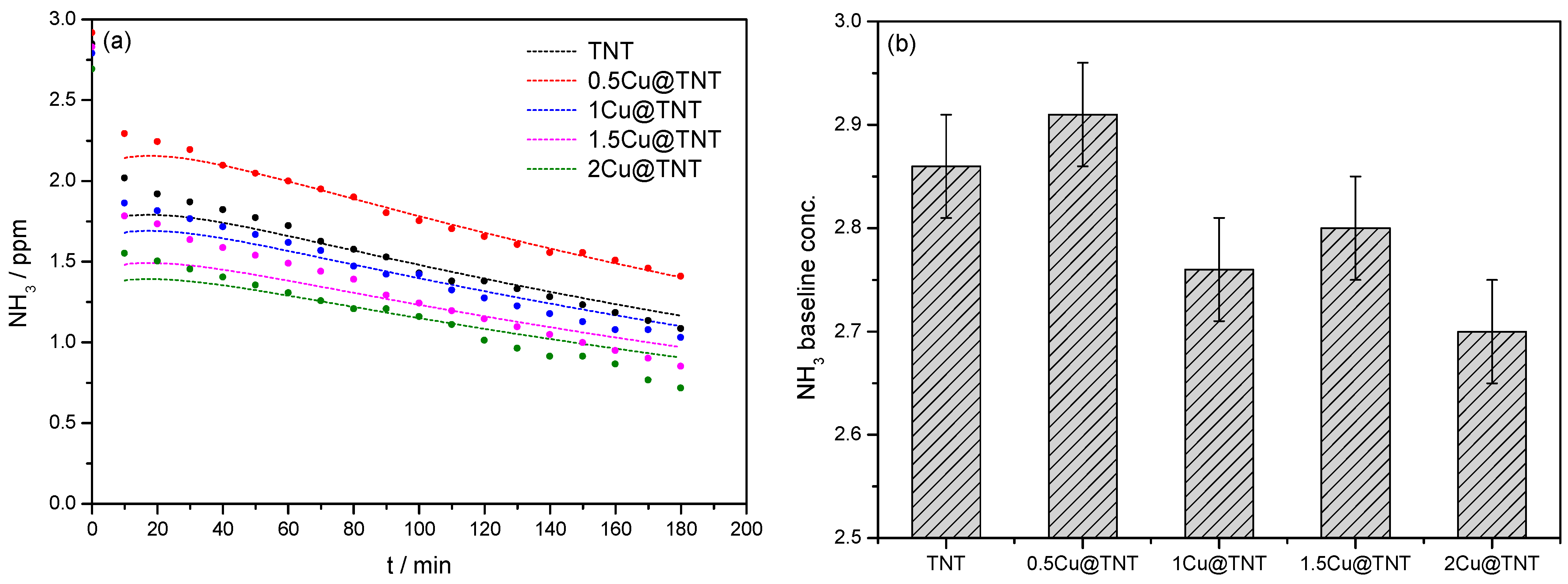
3.2. Results and Kinetic Study of Ammonia Photocatalytic Oxidation
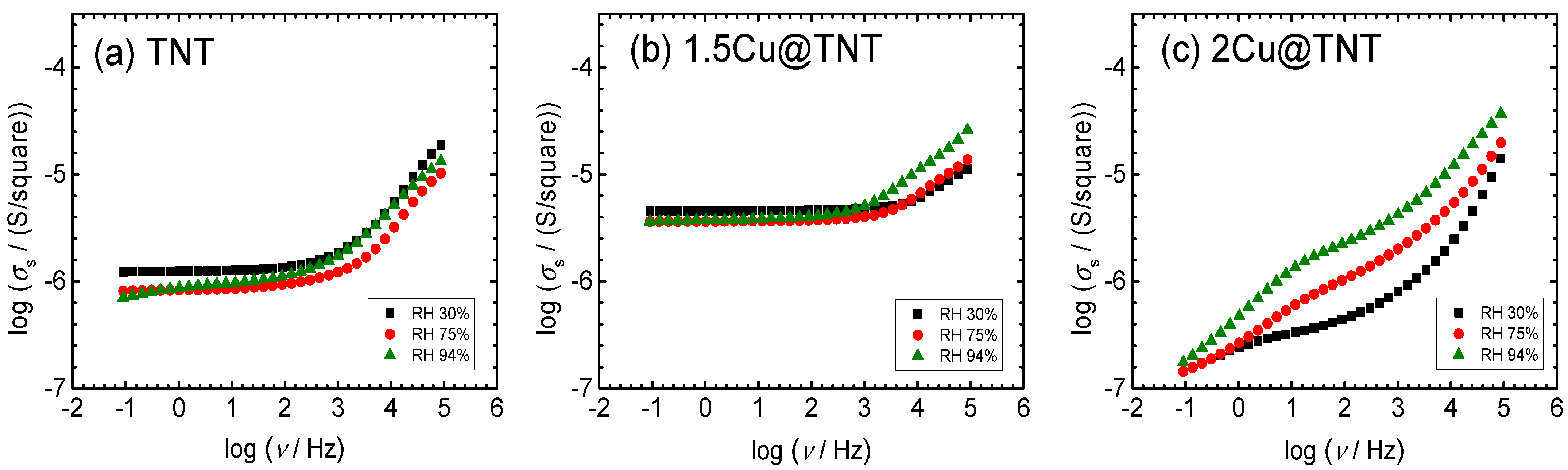
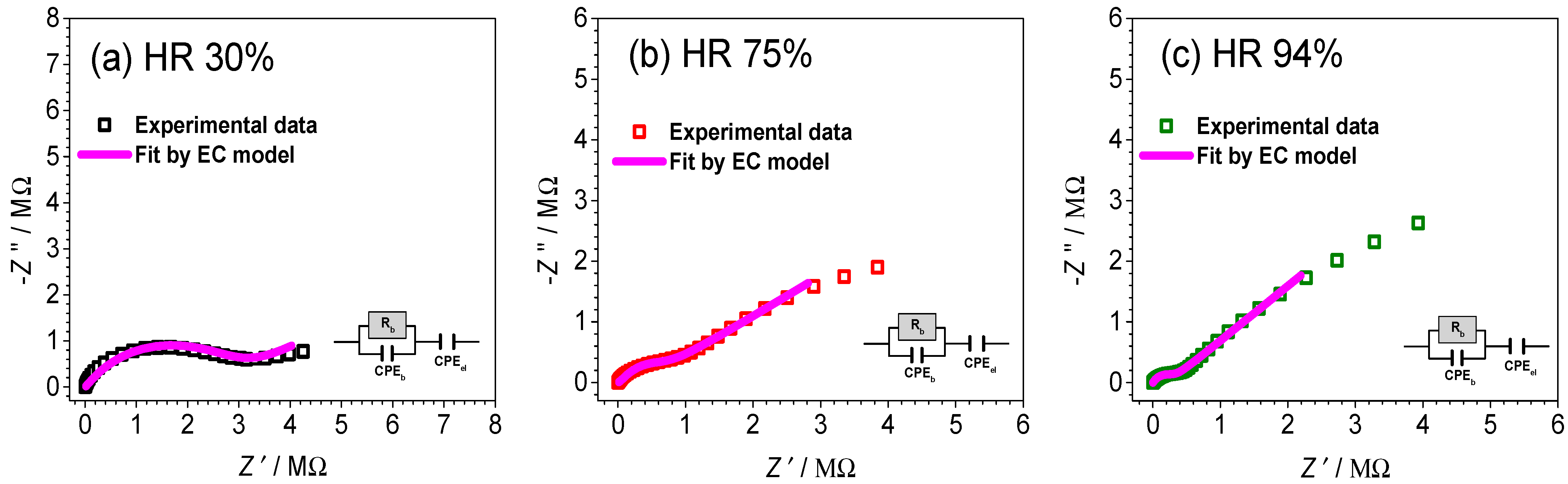
3.3. Impedance Spectroscopy and DC Conductivity under Controlled Humidity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Obata, K.; Kishishita, K.; Okemoto, A.; Taniya, K.; Ichihashi, Y.; Nishiyama, S. Photocatalytic decomposition of NH3 over TiO2 catalysts doped with Fe. Appl. Catal. B 2014, 160, 200–203. [Google Scholar] [CrossRef]
- Kisslinger, R.; Askar, A.M.; Thakur, U.K.; Riddell, S.; Dahunsi, D.; Zhang, Y.; Zeng, S.; Goswami, A.; Shankar, K. Preferentially oriented TiO2 nanotube arrays on non-native substrates and their improved performance as electron transporting layer in halide perovskite solar cells. Nanotechnology 2019, 30, 204003. [Google Scholar] [CrossRef] [PubMed]
- Capelli, I.; Fort, A.; Grasso, A.L.; Panzardi, E.; Mugnaini, M.; Vignoli, V. RH sensing by means of TiO2 nanoparticles: A comparison among different sensing techniques based on modeling and chemical/physical interpretation. Chemosensors 2020, 8, 89. [Google Scholar] [CrossRef]
- Nyankson, E.; Agyei-Tuffour, B.; Asare, J.; Annan, E.; Rwenyagila, E.R.; Konadu, D.S.; Yaya, A.; Dodoo-Arhin, D. Nanostructured TiO2 and their energy applications—A review. ARPN J. Eng. Appl. Sci. 2013, 8, 17. [Google Scholar]
- Zhang, J.; Shi, C.; Chen, J.; Wang, Y.; Li, M. Preparation of ultra-thin and high-quality WO3 compact layers and comparison of WO3 and TiO2 compact layer thickness in planar perovskite solar cells. J. Solid State Chem. 2016, 238, 223–228. [Google Scholar] [CrossRef]
- Farahani, E.; Mohammadpour, R. Fabrication of flexible self-powered humidity sensor based on super-hydrophilic titanium oxide nanotube arrays. Sci. Rep. 2020, 10, 13032. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, Y.Z.; Huang, S.S.; Ren, S.T.; Li, P.; Li, J.J. Resistive and capacitive response of nitrogen-doped TiO2 nanotubes film humidity sensor. Nanotechnology 2011, 22, 025501. [Google Scholar] [CrossRef]
- Mohan, L.; Dennis, C.; Padmapriya, N.; Anandan, C.; Rajendran, N. Effect of electrolyte temperature and anodization time on formation of TiO2 nanotubes for biomedical applications. Mater. Today Commun. 2020, 23, 101103. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z. Anodic formation of ordered TiO2 nanotube arrays: Effects of electrolyte temperature and anodization potential. J. Phys. Chem. C 2009, 113, 4026–4030. [Google Scholar] [CrossRef]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of nano-titanium dioxide (TiO2-NP) through various routes of exposure: A review. Biol. Trace Elem. Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Farsinezhad, S.; Mohammadpour, A.; Dalrymple, A.N.; Geisinger, J.; Kar, P.; Brett, M.J.; Shankar, K. Transparent anodic TiO2 nanotube arrays on plastic substrates for disposable biosensors and flexible electronics. J. Nanosci. Nanotech. 2013, 13, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Li, X.; Nowotny, J. Photocatalytical properties of TiO2 nanotubes. Solid State Phenom. 2010, 162, 295–328. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Liu, B.; Zeng, W.; Wang, Y. Plasmonic photocatalysis properties of au nanoparticles precipitated anatase/rutile mixed TiO2 nanotubes. Nanoscale 2013, 5, 9739. [Google Scholar] [CrossRef]
- Čižmar, T.; Panžić, I.; Salamon, K.; Grčić, I.; Radetić, L.; Marčec, J.; Gajović, A. Low-cost synthesis of Cu-modified immobilized nanoporous TiO2 for photocatalytic degradation of 1H-Benzotriazole. Catalysts 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Čižmar, T.; Panžić, I.; Capan, I.; Gajović, A. Nanostructured TiO2 photocatalyst modified with Cu for improved imidacloprid degradation. Appl. Surf. Sci. 2021, 569, 151026. [Google Scholar] [CrossRef]
- Hejazi, S.; Mohajernia, S.; Wu, Y.; Andryskova, P.; Zoppellaro, G.; Hwang, I.; Tomanec, O.; Zboril, R.; Schmuki, P. Intrinsic Cu nanoparticle decoration of TiO2 nanotubes: A platform for efficient noble metal free photocatalytic H2 production. Electrochem. Commun. 2019, 98, 82–86. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Ghonchegi, Z. Fabrication and characterization of copper doped TiO2 nanotube arrays by in situ electrochemical method as efficient visible-light photocatalyst. Ceram. Int. 2015, 41, 8735–8741. [Google Scholar] [CrossRef]
- Zaki, S.M.; Sreekantan, S. Preparation and characterization of Cu loaded TiO2 nanotube arrays and their photocatalytic activity. Adv. Mater. Res. 2011, 364, 377–381. [Google Scholar] [CrossRef]
- Zhu, J.; Jian, Y.; Long, D.; Wang, H.; Zeng, Y.; Li, J.; Xiao, R.; Pu, S. Degradation of ammonia gas by Cu2O/{001}TiO2 and its mechanistic analysis. RSC Adv. 2021, 11, 3695–3702. [Google Scholar] [CrossRef]
- Wu, H.; Ma, J.; Li, Y.; Zhang, C.; He, H. Photocatalytic oxidation of gaseous ammonia over fluorinated TiO2 with exposed (001) Facets. Appl. Catal. B 2014, 152, 82–87. [Google Scholar] [CrossRef]
- Sopyan, I. Kinetic analysis on photocatalytic degradation of gaseous acetaldehyde, ammonia and hydrogen sulfide on Nanosized porous TiO2 films. Sci. Technol. Adv. Mater. 2007, 8, 33–39. [Google Scholar] [CrossRef]
- Kočí, K.; Reli, M.; Troppová, I.; Prostějovský, T.; Žebrák, R. Degradation of ammonia from gas stream by advanced oxidation processes. J. Environ. Sci. Health. Part A 2019, 55, 433–437. [Google Scholar] [CrossRef]
- Yuzawa, H.; Mori, T.; Itoh, H.; Yoshida, H. Reaction mechanism of ammonia decomposition to nitrogen and hydrogen over metal loaded titanium oxide photocatalyst. J. Phys. Chem. C 2012, 116, 4126–4136. [Google Scholar] [CrossRef]
- Grčić, I.; Marčec, J.; Radetić, L.; Radovan, A.M.; Melnjak, I.; Jajčinović, I.; Brnardić, I. Ammonia and methane oxidation on TiO2 supported on glass fiber mesh under artificial solar irradiation. Environ. Sci. Pollut. Res. 2020, 28, 18354–18367. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, Y.; Hu, L.; Cao, J.; Gao, F.; Liu, Y.; Wang, L. Improved and excellent CO sensing properties of Cu-doped TiO2 nanofibers. Chin. Sci. Bull. 2010, 55, 228–232. [Google Scholar] [CrossRef]
- Lupan, O.; Santos-Carballal, D.; Ababii, N.; Magariu, N.; Hansen, S.; Vahl, A.; Zimoch, L.; Hoppe, M.; Pauporté, T.; Galstyan, V.; et al. TiO2/Cu2O/CuO multi-nanolayers as sensors for H2 and volatile organic compounds: An experimental and theoretical investigation. ACS Appl. Mater. Interfaces 2021, 13, 32363–32380. [Google Scholar] [CrossRef] [PubMed]
- Vathani, A.M. Synthesis and electrochemical studies on Cu-TiO2 thin films deposited by spray pyrolysis technique for sensing uric acid. Int. J. Nano Dimens. 2019, 10, 230–241. [Google Scholar]
- Singh, A.K.; Patil, S.B.; Nakate, U.T.; Gurav, K.V. Effect of Pd and Au sensitization of bath deposited flowerlike TiO2 thin films on CO sensing and photocatalytic properties. J. Chem. 2013, 2013, 370578. [Google Scholar] [CrossRef] [Green Version]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; ud Dowla Biswas, M.R.; Oh, W.C.; Alshahateet, S.F.; Fatimah, I.; Mohammad, F.; Al-Lohedan, H.A.; Paiman, S.; et al. Enhanced gas sensing and photocatalytic activity of reduced graphene oxide loaded TiO2 nanoparticles. Chem. Phys. Lett. 2021, 780, 138897. [Google Scholar] [CrossRef]
- Zou, R.; He, G.; Xu, K.; Liu, Q.; Zhang, Z.; Hu, J. ZnO Nanorods on reduced graphene sheets with excellent field emission, gas sensor and photocatalytic properties. J. Mater. Chem. A 2013, 1, 8445. [Google Scholar] [CrossRef]
- Cao, Q.-L.; Yu, Q.; Han, C.; Dong, G.-Y.; Fu, L. Sensing and photocatalytic properties of two Zinc(II) coordination polymers containing Bis(Benzimidazole) ligands. Polyhedron 2021, 203, 115237. [Google Scholar] [CrossRef]
- Ding, Q.; Pan, Y.; Luo, Y.; Zhou, M.; Guan, Y.; Li, B.; Trivedi, M.; Kumar, A.; Liu, J. Photocatalytic and ferric ion sensing properties of a new three-dimensional metal–organic framework based on cuboctahedral secondary building units. ACS Omega 2019, 4, 10775–10783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Photocatalytic oxidation of trimethylamine and isovaleraldehyde in an annular reactor: Influence of the mass transfer and the relative humidity. J. Photochem. Photobiol. A Chem. 2012, 236, 61–69. [Google Scholar] [CrossRef]
- Tepe, O.; Dursun, A.Y. Combined effects of external mass transfer and biodegradation rates on removal of phenol by immobilized Ralstonia eutropha in a packed bed reactor. J. Hazard. Mater. 2008, 151, 9–16. [Google Scholar] [CrossRef]
- Grčić, I.; Papić, S.; Brnardić, I. Photocatalytic activity of TiO2 thin films: Kinetic and efficiency study. Int. J. Chem. React. Eng. 2017, 16, 20160153. [Google Scholar] [CrossRef]
- Coronado, J.M.; Hernandez-Alonso, M.D. Design of advanced photocatalytic materials for energy and environmental applications. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications, 1st ed.; Coronado, J.M., Fresno, F., Hernández-Alonso, M.D., Portela, R., Eds.; Springer: London, UK, 2013; pp. 5–35. [Google Scholar]
- Lasek, J.; Yu, Y.-H.; Wu, J.C.S. Removal of NOx by photocatalytic processes. J. Photochem. Photobiol. C Photochem. 2013, 14, 29–52. [Google Scholar] [CrossRef]
- Garlisi, C.; Lai, C.Y.; George, L.; Chiesa, M.; Palmisano, G. Relating photoelectrochemistry and wettability of sputtered Cu-and N-doped TiO2 thin films via an integrated approach. J. Phys. Chem. C. 2018, 122, 12369–12376. [Google Scholar] [CrossRef]
- Farzaneh, A.; Mohammadzadeh, A.; Esrafili, M.D.; Mermer, O. Experimental and theoretical study of TiO2 based nanostructured semiconducting humidity sensor. Ceram. Int. 2019, 45, 8362–8369. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Kytin, V.; Dittrich, T.H.; Bisquert, J. Effect of humidity on the Ac conductivity of nanoporous TiO2. J. Appl. Phys. 2003, 94, 5261–5264. [Google Scholar] [CrossRef]
| Element | Atomic % | |||
|---|---|---|---|---|
| 0.5Cu@TNT | 1Cu@TNT | 1.5Cu@TNT | 2Cu@TNT | |
| O | 55.4 ± 0.4 | 54.2 ± 0.5 | 53.4 ± 0.4 | 52.1 ± 0.3 |
| Ti | 44.3 ± 0.2 | 45.2 ± 0.3 | 45.5 ± 0.2 | 46.3 ± 0.4 |
| Cu | 0.3 ± 0.2 | 0.6 ± 0.3 | 1.1 ± 0.3 | 1.6 ± 0.2 |
| Photocatalyst | kapp, s−1 |
|---|---|
| TNT | 0.00035 |
| 0.5Cu@TNT | 0.00024 |
| 1Cu@TNT | 0.00038 |
| 1.5Cu@TNT | 0.00044 |
| 2Cu@TNT | 0.00047 |
| Model Parameter | Photocatalyst | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TNT | 0.5Cu@TNT | 1Cu@TNT | 1.5Cu@TNT | 2Cu@TNT | ||||||
| km, NH3, cm min−1 | 0.5693 | |||||||||
| kp,int, NH3, cm1.5 mW−0.5 min−1 | 3.59 × 10−6 | 3.53 × 10−6 | 3.56 × 10−6 | 3.57 × 10−6 | 3.60 × 10−6 | |||||
| NH3, cm2 min−1 | 0.18 | |||||||||
| μUVA, cm−1 | 4.95 × 104 | 1.45 × 104 | ||||||||
| μUVB, cm−1 | 1.33 × 105 | 6.30 × 104 | ||||||||
| Pvis, mW cm−3 | 0 | 0 | 50,000 | 120,000 | 165,000 | |||||
| I0@photocatalytic surface, mW cm−2 | I0, UVA = 0.571 I0, UVB = 0.678 | |||||||||
| as, cm2 g−1 | 76,923.07 | |||||||||
| Sample | Relative Humidity (%) | Ra/(Ω) ± 0.5% | σDCb/(Ω cm)−1 ± 0.5% |
|---|---|---|---|
| TNT | 30 | 7.94 × 105 | 1.26 × 10−6 |
| 1.5Cu@TNT | 30 | 2.19 × 105 | 4.57 × 10−6 |
| 2Cu@TNT | 30 75 94 | 2.67 × 106 3.70 × 105 2.39 × 105 | 3.57 × 10−7 2.70 × 10−6 4.18 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čižmar, T.; Grčić, I.; Bohač, M.; Razum, M.; Pavić, L.; Gajović, A. Dual Use of Copper-Modified TiO2 Nanotube Arrays as Material for Photocatalytic NH3 Degradation and Relative Humidity Sensing. Coatings 2021, 11, 1500. https://doi.org/10.3390/coatings11121500
Čižmar T, Grčić I, Bohač M, Razum M, Pavić L, Gajović A. Dual Use of Copper-Modified TiO2 Nanotube Arrays as Material for Photocatalytic NH3 Degradation and Relative Humidity Sensing. Coatings. 2021; 11(12):1500. https://doi.org/10.3390/coatings11121500
Chicago/Turabian StyleČižmar, Tihana, Ivana Grčić, Mario Bohač, Marta Razum, Luka Pavić, and Andreja Gajović. 2021. "Dual Use of Copper-Modified TiO2 Nanotube Arrays as Material for Photocatalytic NH3 Degradation and Relative Humidity Sensing" Coatings 11, no. 12: 1500. https://doi.org/10.3390/coatings11121500
APA StyleČižmar, T., Grčić, I., Bohač, M., Razum, M., Pavić, L., & Gajović, A. (2021). Dual Use of Copper-Modified TiO2 Nanotube Arrays as Material for Photocatalytic NH3 Degradation and Relative Humidity Sensing. Coatings, 11(12), 1500. https://doi.org/10.3390/coatings11121500











