Mechanism of Polyurethane Binder Curing Reaction and Evaluation of Polyurethane Mixture Properties
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
2.1.1. Raw Material
2.1.2. Mixture Composition and Production
2.2. Methodology
2.2.1. Fourier Transform Infrared Spectroscopy Test for PU Binder
2.2.2. Basic Performance Test Method for the Mixtures
- (1)
- Marshall specimen height test
- (2)
- Splitting tensile test
- (3)
- Rutting test
- (4)
- Low-temperature bending test
- (5)
- Freeze–thaw splitting test
- (6)
- Four-point bending fatigue test
3. Results and Discussion
3.1. Curing Reaction Mechanism of the PU Binder
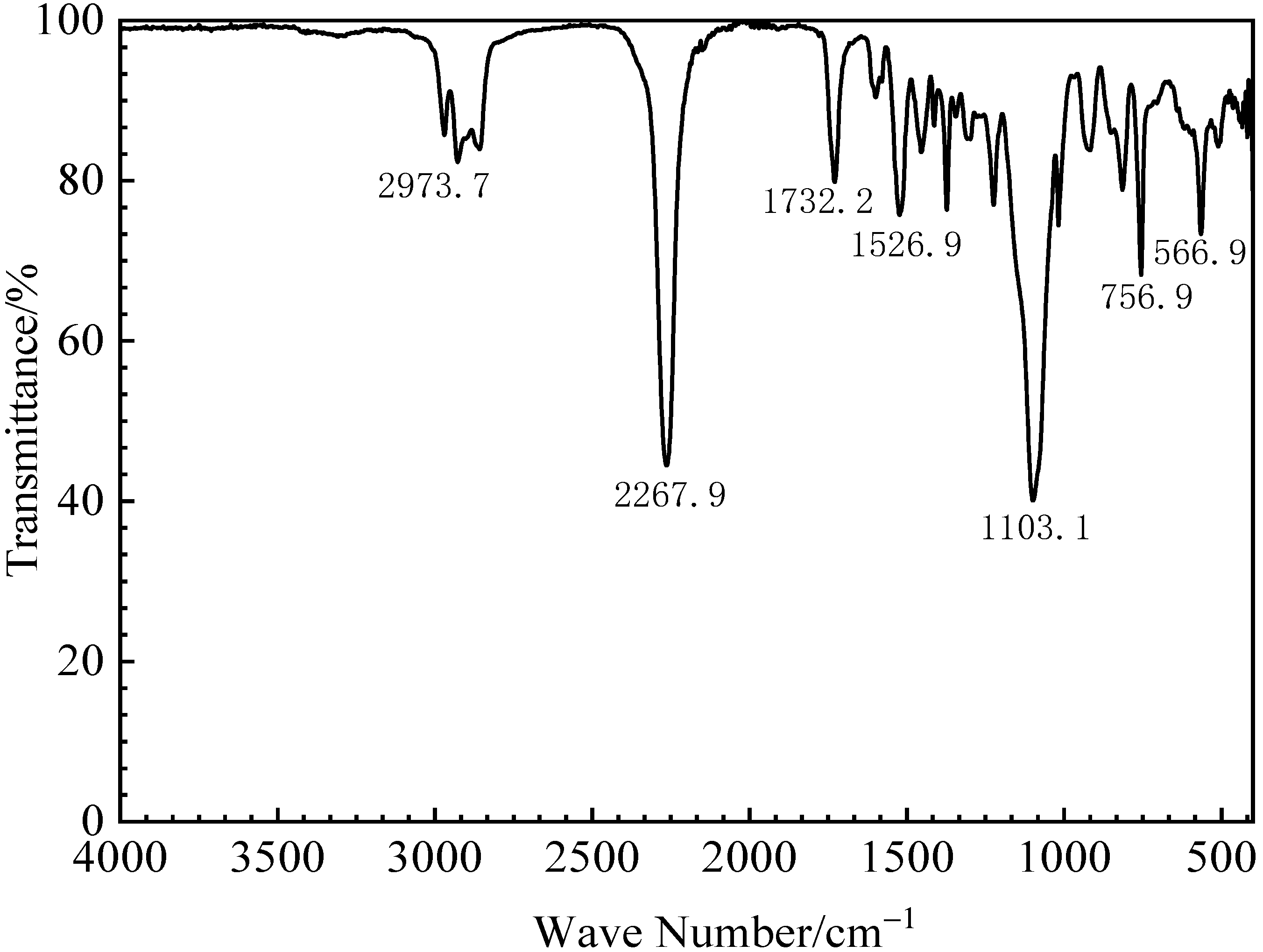
3.1.1. Fourier Transform Infrared Spectroscopy Analysis of the Initial PU Binder
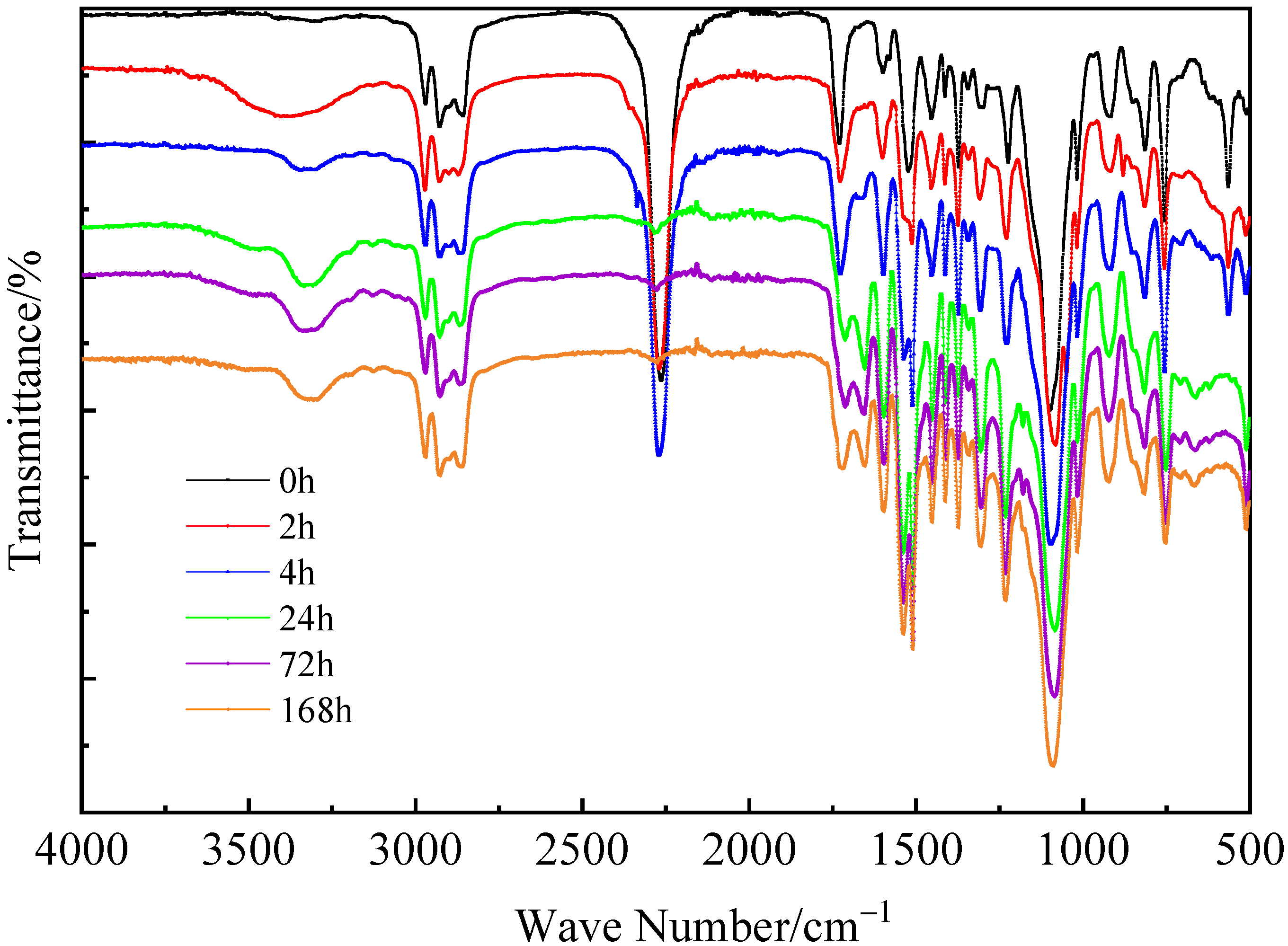
3.1.2. Reaction Characteristics of PU Binder during Curing
3.1.3. Main Group Changes of the PU Binder during Curing
3.2. Volume Characteristics and Strength Formation Law of the PU Mixture
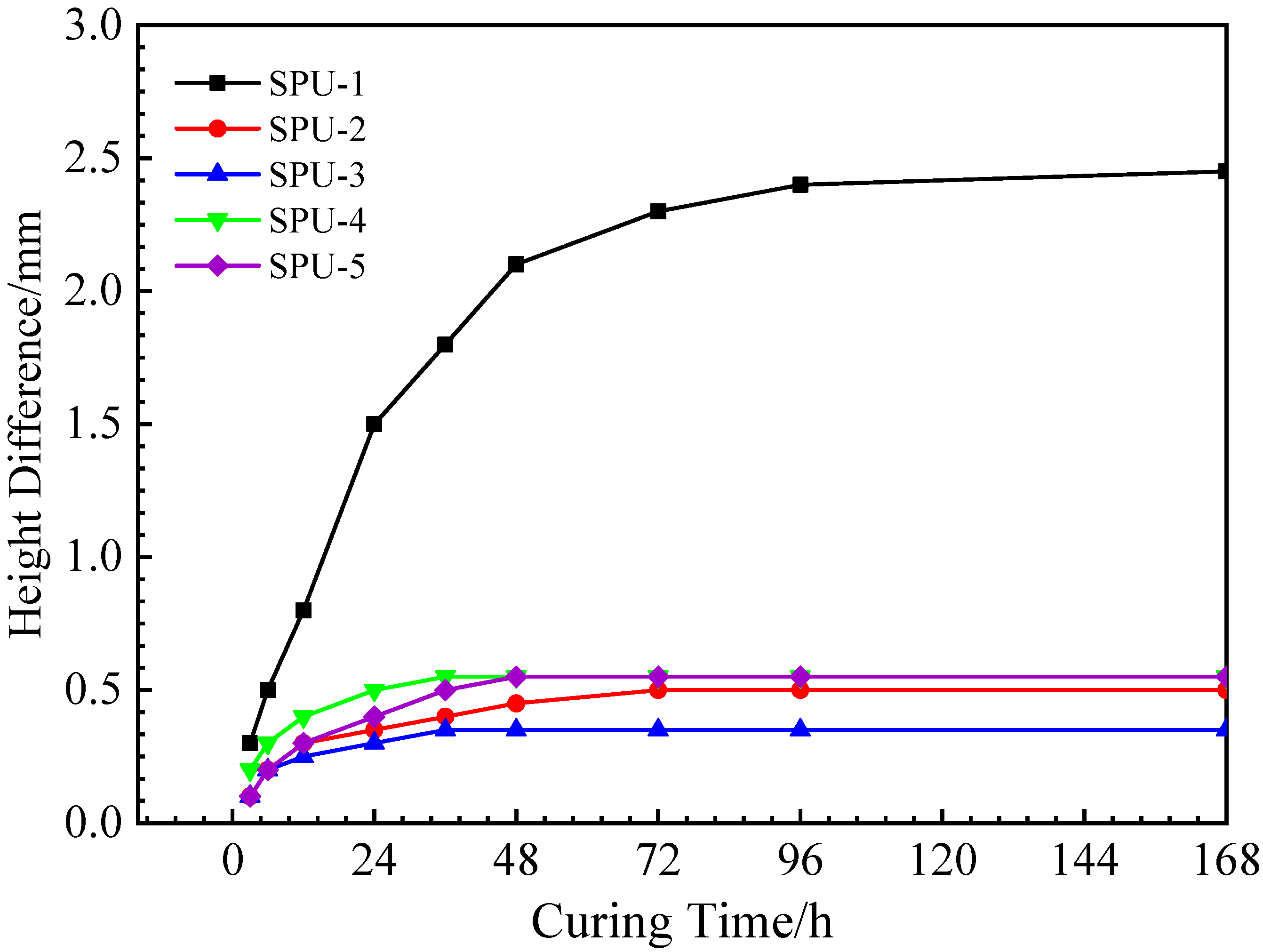
3.2.1. Volume Characteristics of the PU Mixture during Curing
3.2.2. Permissible Stacking Time of the PU Mixture
3.2.3. Strength Formation Law of the PU Mixture
3.3. Basic Performance of the PU Mixture
3.3.1. Temperature Stability of the PU Mixture
3.3.2. Water Stability of the PU Mixture
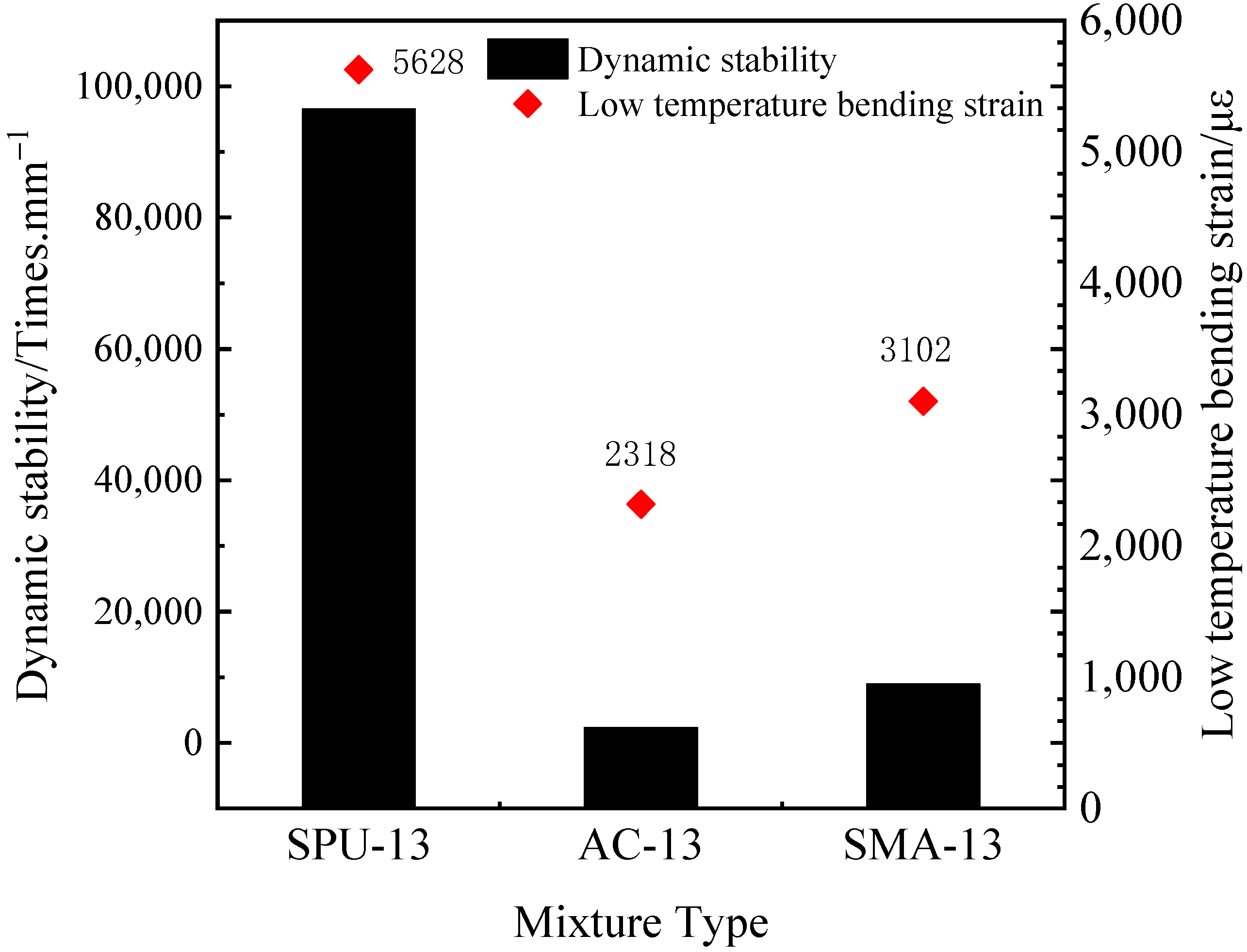
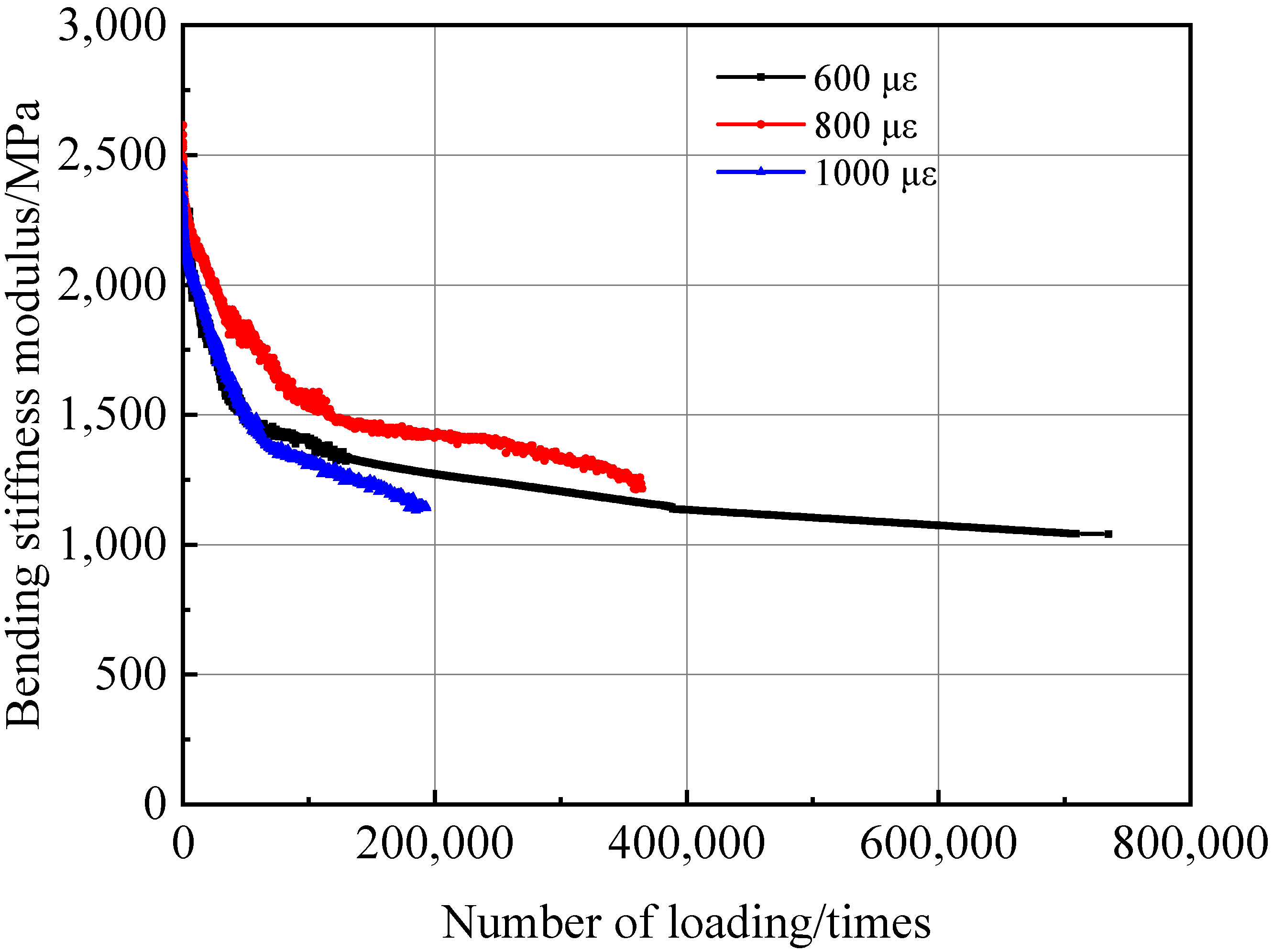
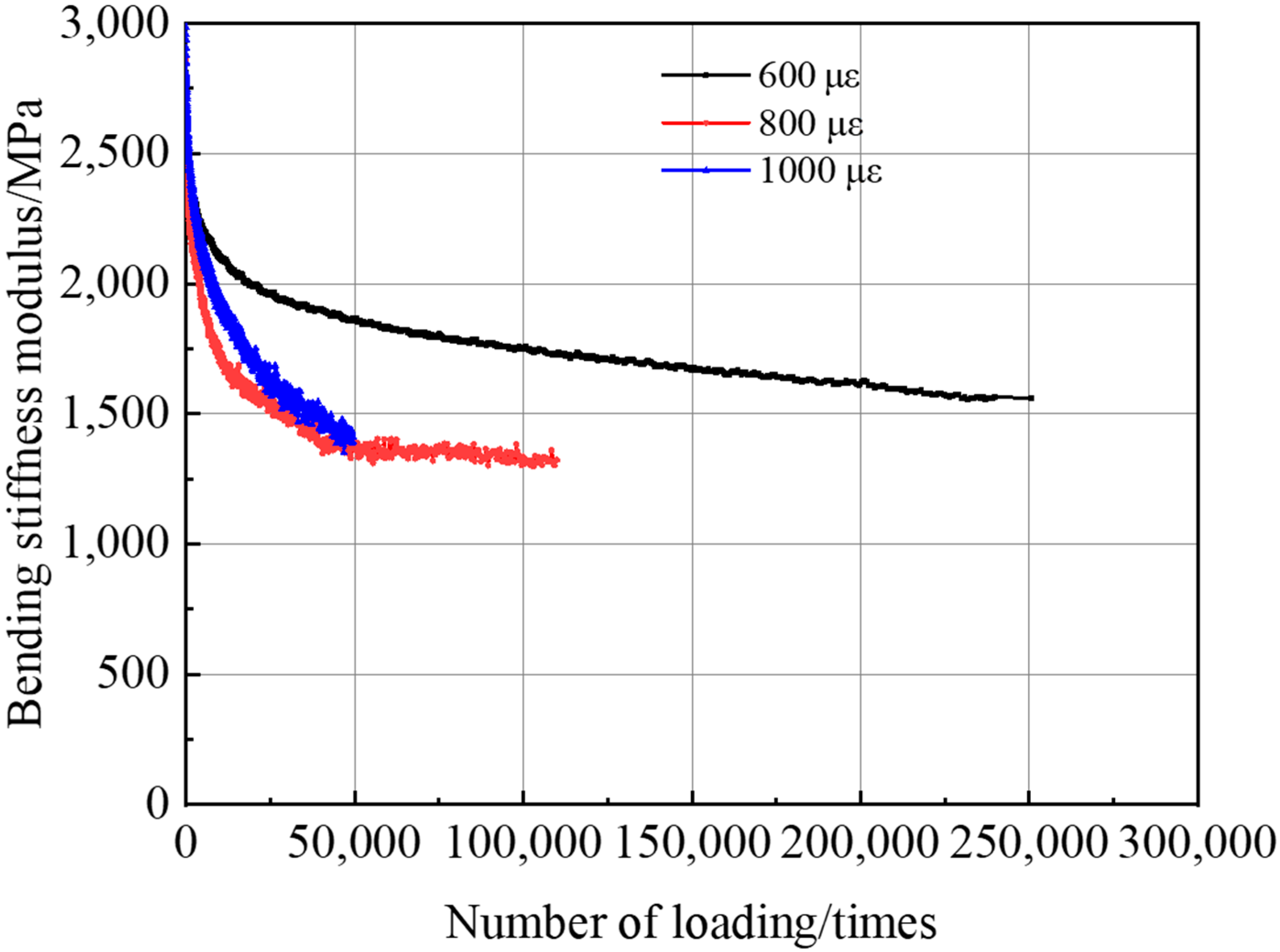
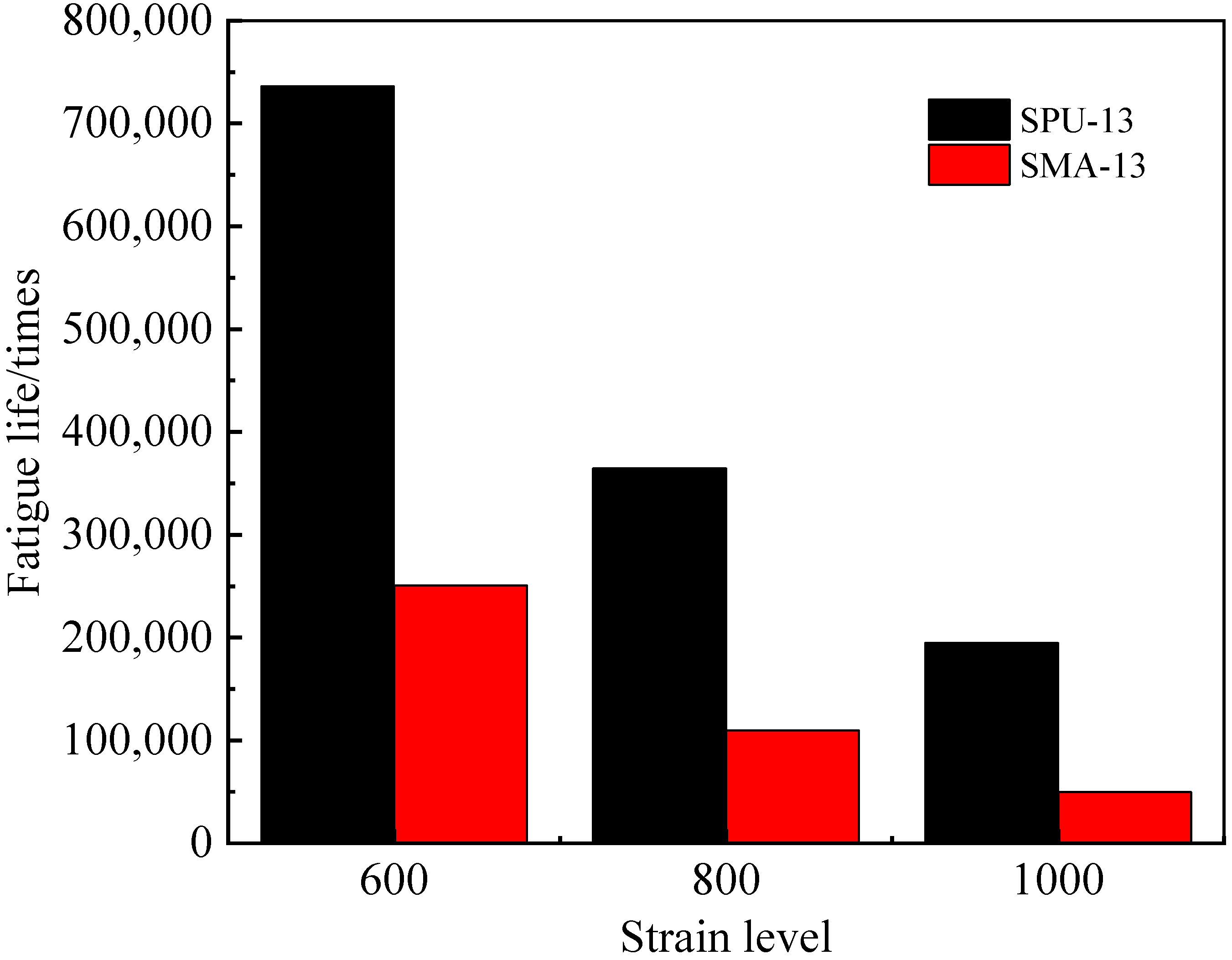
3.3.3. Fatigue Resistance of the PU Mixture
4. Conclusions
- The internal hydrogen bond of PU was formed in the first 24 h, and then the reaction slowed down. The linear polymer chain increased with the progress of the reaction in the first 72 h, and then the reaction speed slowed down because some unreacted NCO groups were wrapped in the adhesive system, blocking the contact between-NCO groups and water.
- It was recommended to stack the PU mixture for 4 h before compaction under conditions of 50% humidity and 15–40 °C temperature. Furthermore, it is recommended that the PU mixture be cured for 2 days before being opened to traffic.
- The PU mixture has excellent high and low temperature stability and fatigue stability. Though the freeze–thaw stability is not good, the splitting strength of the PU mixture before after freeze–thaw can meet the requirements of pavement materials. Thus, the PU mixture can be applied in pavement materials to prolong the service life of pavement and reduce energy consumption and emission.
- The freeze-thaw damage mechanism and its enhancement mechanism are still unclear. More meso and micro tests should be carried out to improve the freeze-thaw stability of PU mixture in the future so to apply it to more complex working conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, F.; Yao, S.; Wang, J.; Wei, J.; Amirkhanian, S. Physical and chemical properties of plasma treated crumb rubbers and high temperature characteristics of their rubberised asphalt binders. Road Mater. Pavement Des. 2020, 21, 587–606. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, J.; Yuan, D.; Lu, H.; Xu, S.; Huang, Y. Design and experiment of thermoelectric asphalt pavements with power-generation and temperature-reduction functions. Energy Build. 2018, 169, 39–47. [Google Scholar] [CrossRef]
- Guo, T.; Fu, H.; Wang, C.; Chen, H.; Chen, Q.; Wang, Q.; Chen, Y.; Li, Z.; Chen, A. Road Performance and Emission Reduction Effect of Graphene/Tourmaline-Composite-Modified Asphalt. Sustainability 2021, 13, 8932. [Google Scholar] [CrossRef]
- Cong, L.; Guo, G.; Yu, M.; Yang, F.; Tan, L. The energy consumption and emission of polyurethane pavement construction based on life cycle assessment. J. Clean. Prod. 2020, 256, 120395. [Google Scholar] [CrossRef]
- Wang, H.M.; Li, R.K.; Wang, X.; Ling, T.Q.; Zhou, G. Strength and road performance for porous polyurethane mixture. China J. Highw. Transp. 2014, 27, 24–31. (In Chinese) [Google Scholar]
- Chen, J.; Yin, X.; Wang, H.; Ding, Y. Evaluation of durability and functional performance of porous polyurethane mixture in porous pavement. J. Clean. Prod. 2018, 188, 12–19. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Wang, H.; Xie, P.; Huang, W. Experimental study on anti-icing and deicing performance of polyurethane concrete as road surface layer. Constr. Build. Mater. 2018, 161, 598–605. [Google Scholar] [CrossRef]
- Chen, J.; Yao, C.; Wang, H.; Huang, W.; Ma, X.; Qian, J. Interface Shear Performance between Porous Polyurethane Mixture and Asphalt Sublayer. Appl. Sci. 2018, 8, 623. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Schacht, A.; Leng, Z.; Leng, C.; Kollmann, J.; Oeser, M. Effects of material composition on mechanical and acoustic performance of poroelastic road surface (PERS). Constr. Build. Mater. 2017, 135, 352–360. [Google Scholar] [CrossRef]
- Wang, D.; Liu, P.; Leng, Z.; Leng, C.; Lu, G.; Buch, M.; Oeser, M. Suitability of PoroElastic Road Surface (PERS) for urban roads in cold regions: Mechanical and functional performance assessment. J. Clean. Prod. 2017, 165, 1340–1350. [Google Scholar] [CrossRef]
- Lu, G.; Renken, L.; Li, T.; Wang, D.; Li, H.; Oeser, M. Experimental study on the polyurethane-bound pervious mixtures in the application of permeable pavements. Constr. Build. Mater. 2019, 202, 838–850. [Google Scholar] [CrossRef]
- Li, T.; Lu, G.; Wang, D.; Hong, B.; Tan, Y.Q.; Oeser, M. Key properties of high-performance polyurethane bounded pervious mixture. China J. Highw. Transp. 2019, 32, 158–169. (In Chinese) [Google Scholar]
- Lu, G.; Liu, P.; Wang, Y.; Faßbender, S.; Wang, D.; Oeser, M. Development of a sustainable pervious pavement material using recycled ceramic aggregate and bio-based polyurethane binder. J. Clean. Prod. 2019, 220, 1052–1060. [Google Scholar] [CrossRef]
- Törzs, T.; Lu, G.; Monteiro, A.; Wang, D.; Grabe, J.; Oeser, M. Hydraulic properties of polyurethane-bound permeable pavement materials considering unsaturated flow. Constr. Build. Mater. 2019, 212, 422–430. [Google Scholar] [CrossRef]
- Lu, G.; Liu, P.; Törzs, T.; Wang, D.; Oeser, M.; Grabe, J. Numerical analysis for the influence of saturation on the base course of permeable pavement with a novel polyurethane binder. Constr. Build. Mater. 2020, 240, 117930. [Google Scholar] [CrossRef]
- Li, R.; Wang, H.; Zhou, G. Experimental study on the strength and influencing factors of multi-pore polyurethane gravel mixture. Chin. Foreign Road 2015, 35, 244–247. (In Chinese) [Google Scholar]
- Wang, H.; Hu, Q.; Li, R. Study on hydrothermal stability of permeable polyurethane mixture. Highw. Eng. 2014, 39, 246–250. (In Chinese) [Google Scholar]
- Li, R.; Wang, X.; Wang, H.; Zhou, G. Experimental study on glue dosage of permeable polyurethane pavement. Highw. Eng. 2015, 40, 105–108. (In Chinese) [Google Scholar]
- Cong, L.; Wang, T.; Tan, L.; Yuan, J.; Shi, J. Laboratory evaluation on performance of porous polyurethane mixtures and OGFC. Constr. Build. Mater. 2018, 169, 436–442. [Google Scholar] [CrossRef]
- Guo, G.; Cong, L.; Yang, F.; Tan, L. Application Progress of Polyurethane Material in Pavement Engineering. J. Highw. Transp. Res. Dev. 2020, 37, 1–9. (In Chinese) [Google Scholar]
- Cong, L.; Yang, F.; Guo, G.; Ren, M.; Shi, J.; Tan, L. The use of polyurethane for asphalt pavement engineering applications: A state-of-the-art review. Constr. Build. Mater. 2019, 225, 1012–1025. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, M.; Wang, Y.; Li, W.; Wang, Q. Mechanism analysis of snow and ice removal of new pavement material stabilized by polyurethane and rubber particles. Chin. Foreign Highw. 2015, 35, 258–261. (In Chinese) [Google Scholar]
- Zhang, D.; Ning, B.; Wu, J.; Chen, F. The Performance of Permeable Thin Layer Anti-Sliding Material. J. Chongqing Jiaotong Univ. (Nat. Sci.) 2019, 38, 61–67. (In Chinese) [Google Scholar] [CrossRef]
- Sun, M. Preparation of Polyurethane Binder and Composition Design and Performance Evaluation of its Mixture. Ph.D. Thesis, Chang’an University, Xian, China, 2019. (In Chinese). [Google Scholar]
- Min, S.; Bi, Y.; Zheng, M.; Chen, S.; Li, J. Evaluation of a Cold-Mixed High-Performance Polyurethane Mixture. Adv. Mater. Sci. Eng. 2019, 2019, 1507971. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Bi, Y.; Zheng, M.; Wang, J.; Wang, L. Performance of Polyurethane Mixtures with Skeleton-Interlocking Structure. J. Mater. Civ. Eng. 2020, 32, 04019358. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Guo, Y.; Ma, C.; Gao, D.; Peng, G. Determination of Polyurethane Concrete Compaction Timing Based on Penetration Resistance Test System. China J. Highw. Transp. 2021, 34, 226–235. (In Chinese) [Google Scholar]
- Kong, X.; Liu, G.; Curtis, J.M. Characterization of canola oil based polyurethane wood adhesives. Int. J. Adhes. Adhes. 2011, 31, 559–564. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Wang, T.; Zhang, X. Damping, thermal, and mechanical properties of polyurethane based on poly (tetramethylene glycol)/epoxy interpenetrating polymer networks: Effects of composition and isocyanate index. Appl. Phys. A 2011, 104, 375–382. [Google Scholar] [CrossRef]
- Xia, L.; Cao, D.; Zhang, H.; Guo, Y. Study on the classical and rheological properties of castor oil-polyurethane pre polymer (CPU) modified asphalt. Constr. Build. Mater. 2016, 112, 949–955. [Google Scholar] [CrossRef]
- Shen, D.; Shi, S.; Xu, T.; Huang, X.; Liao, G.; Chen, J. Development of shape memory polyurethane based sealant for concrete pavement. Constr. Build. Mater. 2018, 174, 474–483. [Google Scholar] [CrossRef]
- Hong, B.; Lu, G.; Gao, J.; Wang, C.; Wang, D. Anti-ultraviolet Aging Performance of Polyurethane Binders Used in Roads. China J. Highw. Transp. 2020, 33, 240–253. (In Chinese) [Google Scholar]
- Hong, B.; Lu, G.; Gao, J.; Wang, D. Evaluation of polyurethane dense graded concrete prepared using the vacuum assisted resin transfer molding technology. Constr. Build. Mater. 2021, 269, 121340. [Google Scholar] [CrossRef]
- Hong, B. Study on the Resistance to Water, Alkali and Salt Solutions of Pultruded Polyurethane-Based CFRP Plates. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2018. (In Chinese). [Google Scholar]
- JTG E20-2011. Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering; Ministry of Transport of the People’s Republic of China: Beijing, China, 2011. [Google Scholar]
- JTG F40-2004. Technical Specifications for Construction of Highway Asphalt Pavements; Ministry of Transport of the People’s Republic of China: Beijing, China, 2004. [Google Scholar]
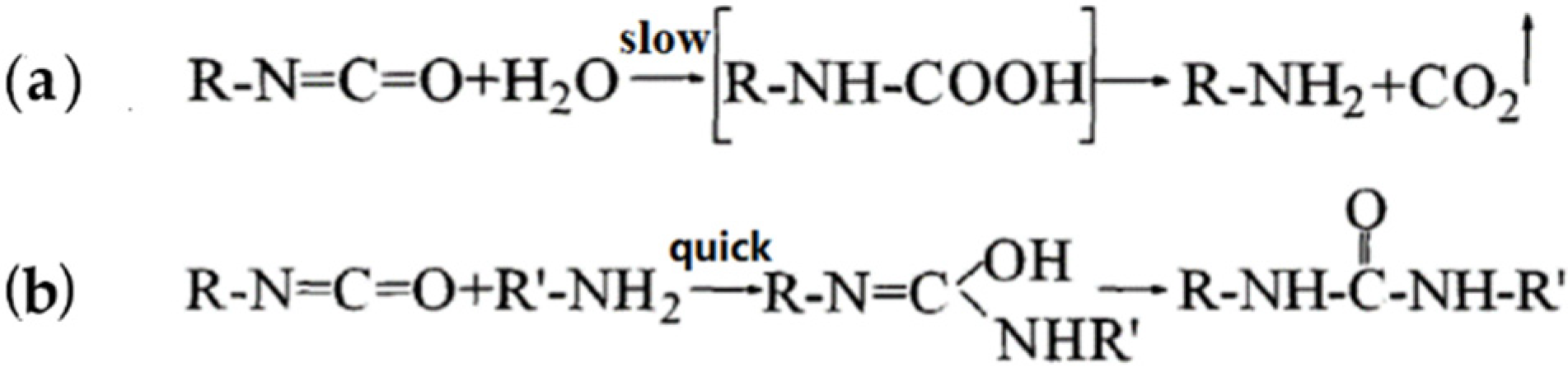





| Technical Indicators | Unit | Technical Requirement | |
|---|---|---|---|
| Surface drying time | min | 40 ± 10 | |
| Tensile strength | MPa | ≥15.0 | |
| Fracture elongation | % | ≥100 | |
| Molecular weight | / | 13,000–17,000 | |
| UV aging | Tensile strength | MPa | ≥9.0 |
| Fracture elongation | % | ≥40 | |
| Sieve size (mm) | Cumulative Passing Percentage of Each Sieve (%) | ||
|---|---|---|---|
| SMA-13 | AC-13 | SPU-13 | |
| 16 | 100 | 100 | 100 |
| 13.2 | 81.8 | 99.4 | 95.9 |
| 9.5 | 61.2 | 88.1 | 67.8 |
| 4.75 | 24.2 | 74.1 | 31 |
| 2.36 | 19.9 | 41.9 | 21.4 |
| 1.18 | 16.8 | 33.5 | 16.7 |
| 0.6 | 14.5 | 24.5 | 11.5 |
| 0.3 | 12.4 | 17.9 | 8.6 |
| 0.15 | 10.9 | 11.7 | 6.1 |
| 0.075 | 10.1 | 7.7 | 2.4 |
| Binder Content (%) | 5.9 | 4.8 | 4.4 |
| Temperature (°C) | Humidity (%) | Stacking Time (h) | Serial Number |
|---|---|---|---|
| 20 | 0.5 | 0 | SPU-1 |
| 20 | 0.5 | 4 | SPU-2 |
| 20 | 0.5 | 8 | SPU-3 |
| 20 | 0.95 | 4 | SPU-4 |
| 40 | 0.5 | 4 | SPU-5 |
| Wave Bands (cm−1) | Functional Group Category |
|---|---|
| 2973.7, 2927.9, 2857.7 | Telescopic vibration from –CH2 |
| 2267.9 | –N=C=O antisymmetric stretching vibration band |
| 1732.2 | C=O telescopic vibration from the –NHCOO functional group |
| 1599.0 | Bending vibration band from –NHCOO |
| 1526.9 | Bending vibration band from –NH |
| 1454.5 | Shear vibration from –CH2 |
| 1413.1 | –COO vibration band |
| 1372.7 | Symmetrical bending vibration from –CH3 |
| 1103.1 | Free ether oxygen characteristic band |
| 1017.7 | –C–C absorption band |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Bi, Y.; Zhuang, W.; Chen, S.; Zhao, P.; Pang, D.; Zhang, W. Mechanism of Polyurethane Binder Curing Reaction and Evaluation of Polyurethane Mixture Properties. Coatings 2021, 11, 1454. https://doi.org/10.3390/coatings11121454
Sun M, Bi Y, Zhuang W, Chen S, Zhao P, Pang D, Zhang W. Mechanism of Polyurethane Binder Curing Reaction and Evaluation of Polyurethane Mixture Properties. Coatings. 2021; 11(12):1454. https://doi.org/10.3390/coatings11121454
Chicago/Turabian StyleSun, Min, Yufeng Bi, Wei Zhuang, Sai Chen, Pinhui Zhao, Dezheng Pang, and Wensheng Zhang. 2021. "Mechanism of Polyurethane Binder Curing Reaction and Evaluation of Polyurethane Mixture Properties" Coatings 11, no. 12: 1454. https://doi.org/10.3390/coatings11121454
APA StyleSun, M., Bi, Y., Zhuang, W., Chen, S., Zhao, P., Pang, D., & Zhang, W. (2021). Mechanism of Polyurethane Binder Curing Reaction and Evaluation of Polyurethane Mixture Properties. Coatings, 11(12), 1454. https://doi.org/10.3390/coatings11121454






