Durable Superhydrophobic Coating for Efficient Microplastic Removal
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Fabrication
2.2. Characterization Techniques
2.3. Durability Test
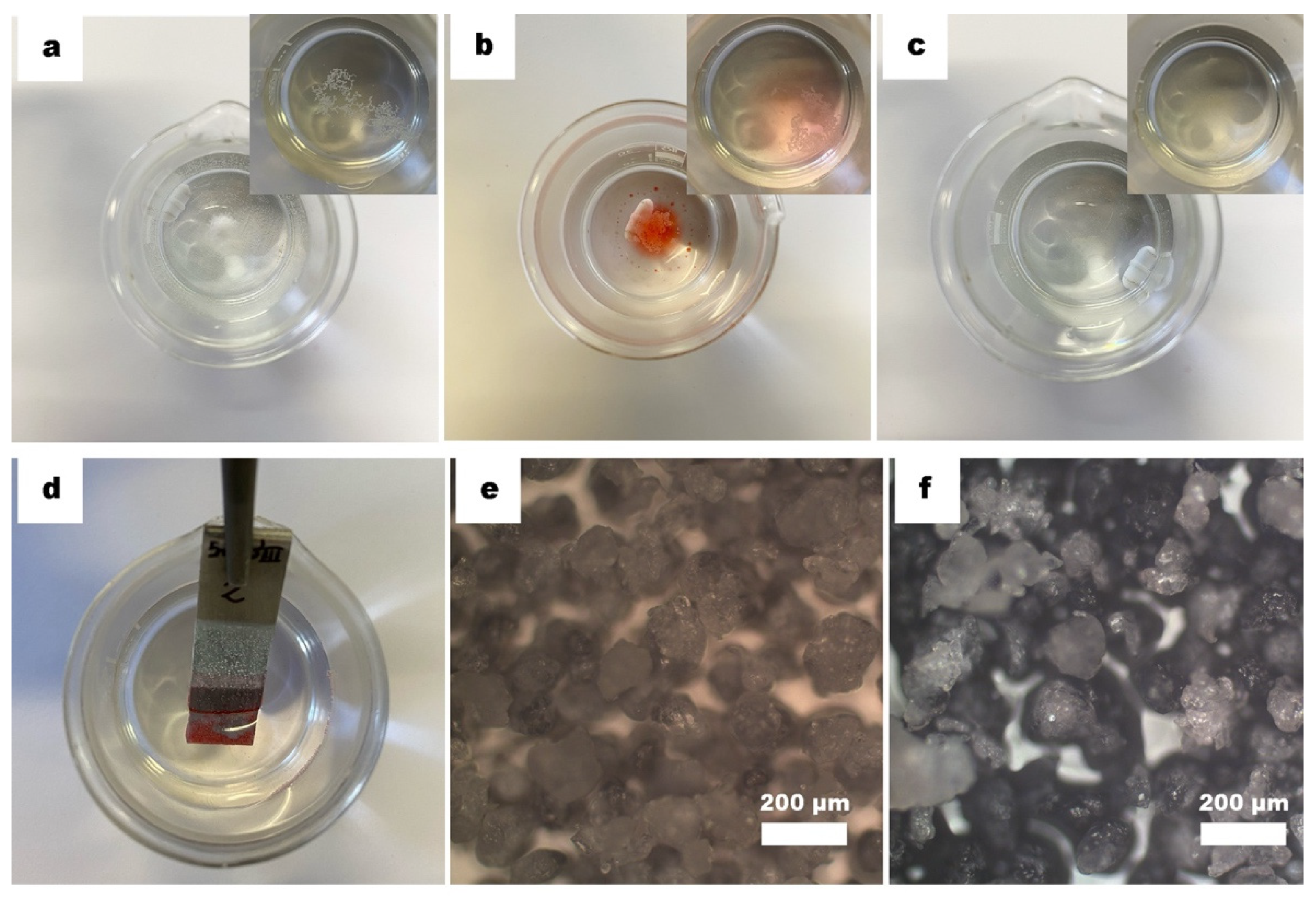
2.4. Microplastics
3. Results and Discussion
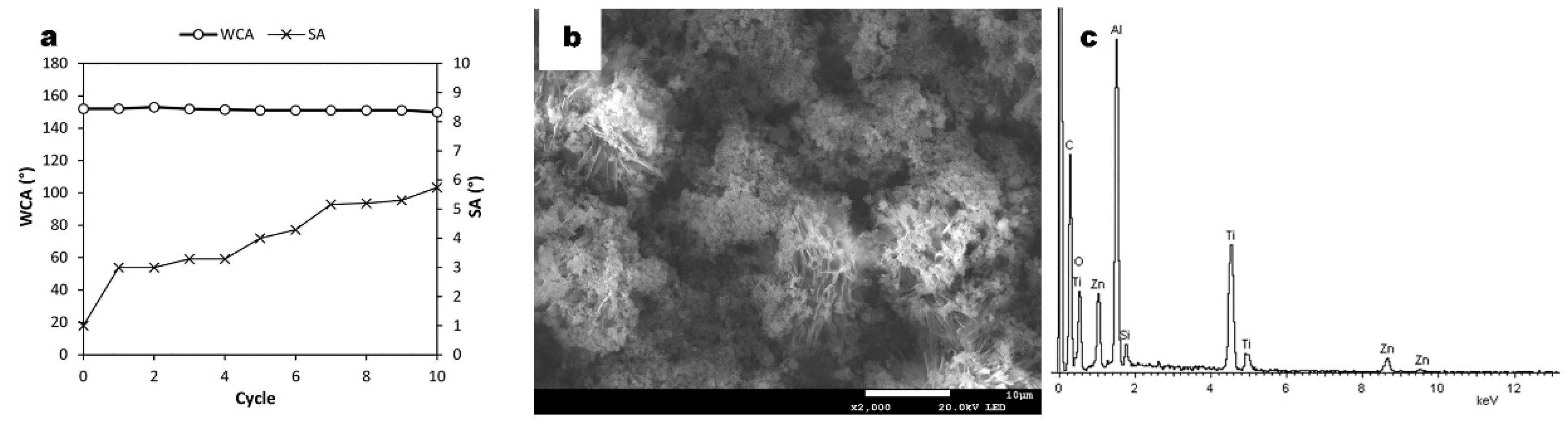
3.1. Morphology
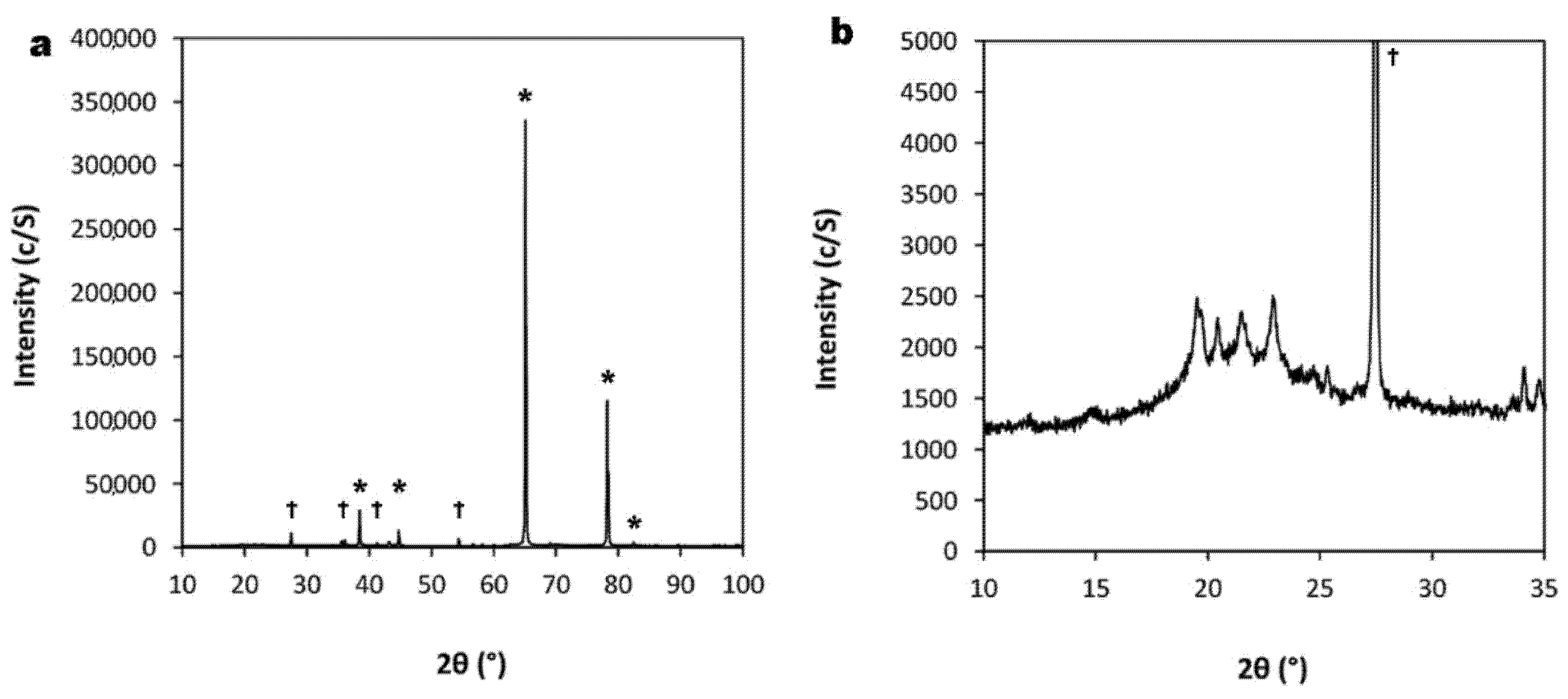
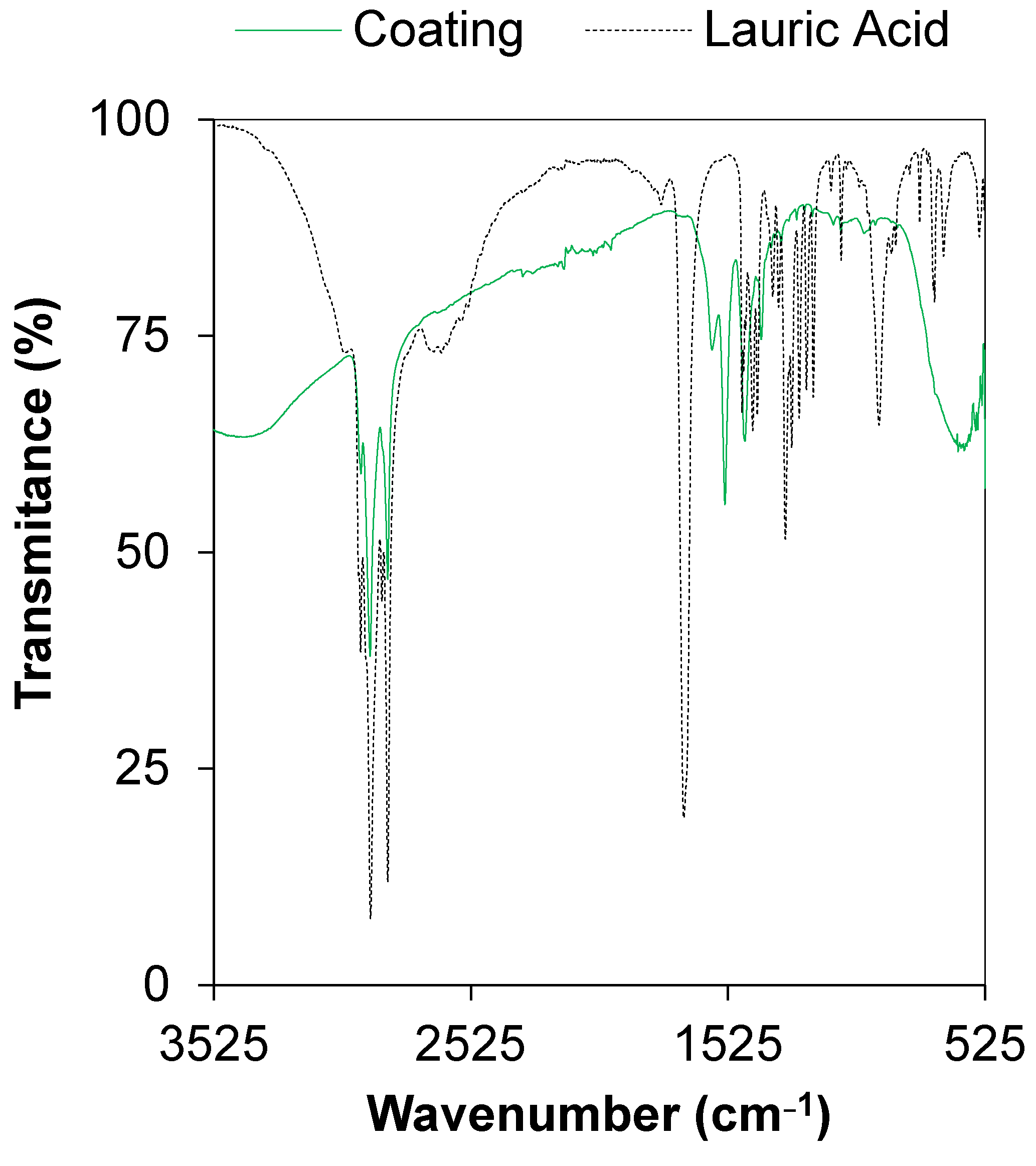
3.2. Chemical Composition
3.3. Wetting Properties
3.4. Durability
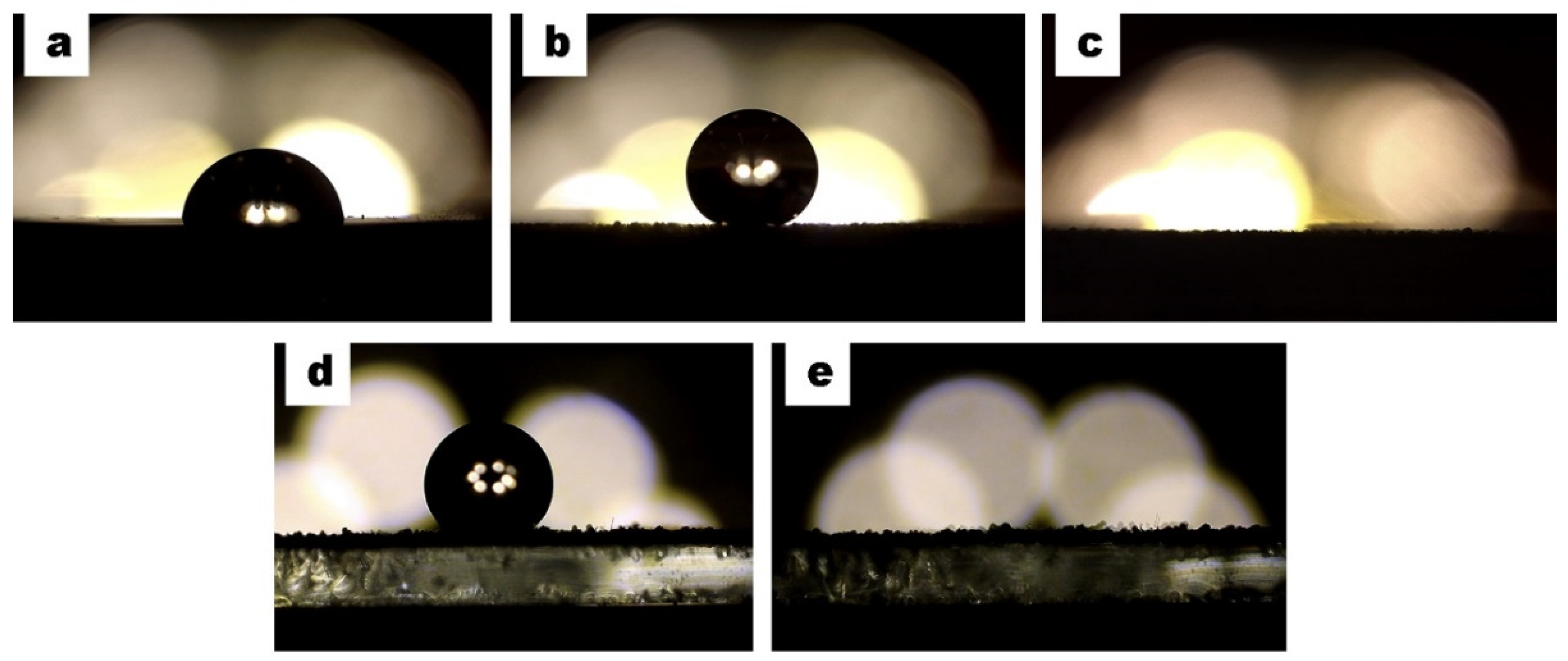
3.5. Microplastics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, R.; Lu, G.; Yan, Z.; Jiang, R.; Bao, X.; Lu, P. A review of the influences of microplastics on toxicity and transgenerational effects of pharmaceutical and personal care products in aquatic environment. Sci. Total Environ. 2020, 732, 139222. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Teles, M.; Balasch, J.C.; Oliveira, M.; Sardans, J.; Peñuelas, J. Insights into nanoplastics effects on human health. Sci. Bull. 2020, 65, 1966–1969. [Google Scholar] [CrossRef]
- Estahbanati, M.R.K.; Kiendrebeogo, M.; Mostafazadeh, A.K.; Drogui, P.; Tyagi, R.D. Treatment processes for microplastics and nanoplastics in waters: State-of-the-art review. Mar. Pollut. Bull. 2021, 168, 112374. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes—Origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef]
- da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, C.; Huang, Z. Sedimentation of nanoplastics from water with Ca/Al dual flocculants: Characterization, interface reaction, effects of pH and ion ratios. Chemosphere 2020, 252, 126450. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired interfaces with superwettability: From materials to chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/Water separation with selective superantiwetting/superwetting surface materials. Angew. Chemie Int. Ed. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhang, D.; Cao, N.; Yang, L.; Ju, H.; Boukherroub, R.; Lin, X.; Li, H.; Jin, Y. Mussel-inspired superhydrophobic surfaces on 316L stainless steel with enhanced corrosion resistance. Metall. Mater. Trans. A 2020, 51, 909–919. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Liu, Y.; Wen, Y.; Jiang, J.; Shang, W.; Peng, N. Corrosion resistance and forming mechanism of the lauric acid/graphene composite films on aluminum alloy by electrodeposition. Adv. Eng. Mater. 2021, 2001540. [Google Scholar] [CrossRef]
- Baig, U.; Matin, A.; Gondal, M.A.; Zubair, S.M. Facile fabrication of superhydrophobic, superoleophilic photocatalytic membrane for efficient oil-water separation and removal of hazardous organic pollutants. J. Clean. Prod. 2019, 208, 904–915. [Google Scholar] [CrossRef]
- Ge, B.; Tang, S.; Zhang, H.; Li, W.; Wang, M.; Ren, G.; Zhang, Z. Water vapor recovery device designed with interface local heating principle and its application in clean water production. J. Mater. Chem. A 2021, 9, 7967–7976. [Google Scholar] [CrossRef]
- Luo, J.; Wang, L.; Huang, X.; Li, B.; Guo, Z.; Song, X.; Lin, L.; Tang, L.-C.; Xue, H.; Gao, J. Mechanically durable, highly conductive, and anticorrosive composite fabrics with excellent self-cleaning performance for high-efficiency electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2019, 11, 10883–10894. [Google Scholar] [CrossRef]
- Wang, M.; Zi, Y.; Zhu, J.; Huang, W.; Zhang, Z.; Zhang, H. Construction of super-hydrophobic PDMS@MOF@Cu mesh for reduced drag, anti-fouling and self-cleaning towards marine vehicle applications. Chem. Eng. J. 2021, 417, 129265. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhao, X.; Hu, X.; Yang, L.; Wang, N.; Li, Y.; Hou, B. Biomimetic one step fabrication of manganese stearate superhydrophobic surface as an efficient barrier against marine corrosion and Chlorella vulgaris-induced biofouling. Chem. Eng. J. 2016, 306, 441–451. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, C.; Tu, J. Robust slippery coating with superior corrosion resistance and anti-icing performance for AZ31B Mg alloy protection. ACS Appl. Mater. Interfaces 2017, 9, 11247–11257. [Google Scholar] [CrossRef]
- Rico, V.; Mora, J.; García, P.; Agüero, A.; Borrás, A.; González-Elipe, A.R.; López-Santos, C. Robust anti-icing superhydrophobic aluminum alloy surfaces by grafting fluorocarbon molecular chains. Appl. Mater. Today 2020, 21, 100815. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, B.; Wang, Z.; Li, B. Fabrication of omniphobic PVDF composite membrane with dual-scale hierarchical structure via chemical bonding for robust membrane distillation. J. Memb. Sci. 2021, 622, 119038. [Google Scholar] [CrossRef]
- Abdu, B.; Munirasu, S.; Kallem, P.; Hasan, S.W.; Banat, F. Investigating the effect of various foulants on the performance of intrinsically superhydrophobic polyvinylidene fluoride membranes for direct contact membrane distillation. Sep. Purif. Technol. 2020, 252, 117416. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Shen, J.; Guo, S. Optimized microporous structure of ePTFE membranes by controlling the particle size of PTFE fine powders for achieving high oil-water separation performances. J. Memb. Sci. 2021, 629, 119294. [Google Scholar] [CrossRef]
- Yu, T.; Lu, S.; Xu, W.; Boukherroub, R. Preparation of superhydrophobic/superoleophilic copper coated titanium mesh with excellent ice-phobic and water-oil separation performance. Appl. Surf. Sci. 2019, 476, 353–362. [Google Scholar] [CrossRef]
- Ko, T.-J.; Hwang, J.-H.; Davis, D.; Shawkat, M.S.; Han, S.S.; Rodriguez, K.L.; Oh, K.H.; Lee, W.H.; Jung, Y. Superhydrophobic MoS2-based multifunctional sponge for recovery and detection of spilled oil. Curr. Appl. Phys. 2020, 20, 344–351. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, T.; Xia, Q.; Yu, X.; Wang, Y. Constructing superhydrophobic ZIF-8 layer with bud-like surface morphology on PDMS composite membrane for highly efficient ethanol/water separation. J. Environ. Chem. Eng. 2021, 9, 104977. [Google Scholar] [CrossRef]
- Kamelian, F.S.; Mohammadi, T.; Naeimpoor, F. Fast, facile and scalable fabrication of novel microporous silicalite-1/PDMS mixed matrix membranes for efficient ethanol separation by pervaporation. Sep. Purif. Technol. 2019, 229, 115820. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, D.; Sun, L.; Zhao, Y.; Tiwari, M.K.; Carmalt, C.J.; Parkin, I.P.; Lu, Y. Super-durable, non-fluorinated superhydrophobic free-standing items. J. Mater. Chem. A 2018, 6, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Chatzigrigoriou, A.; Karapanagiotis, I.; Poulios, I. Superhydrophobic coatings based on siloxane resin and calcium hydroxide nanoparticles for marble protection. Coatings 2020, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Mai, Z.; Shu, X.; Chen, D.; Liu, M.; Xu, W. Superhydrophobic/superoleophilic cotton fabrics treated with hybrid coatings for oil/water separation. Adv. Compos. Hybrid Mater. 2019, 2, 254–265. [Google Scholar] [CrossRef]
- Saji, V.S. Electrophoretic-deposited superhydrophobic coatings. Chem.—Asian J. 2021, 16, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Gadermann, M.; Preston, T.; Troster, C.; Signorell, R. Characterization of palmitic and lauric acid aerosols from rapid expansion of supercritical CO2 solutions. Mol. Phys. 2008, 106, 945–953. [Google Scholar] [CrossRef]
- Jiesheng, L.; Yuanyuan, Y.; Xiang, H. Research on the preparation and properties of lauric acid/expanded perlite phase change materials. Energy Build. 2016, 110, 108–111. [Google Scholar] [CrossRef]
- Nelson, P.N.; Taylor, R.A. Powder X-ray diffraction, infrared and 13C NMR spectroscopic studies of the homologous series of some solid-state zinc(II) and sodium(I) n-alkanoates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 800–806. [Google Scholar] [CrossRef]
- Estruga, M.; Domingo, C.; Ayllón, J.A. Solution-processable ZnO nanoparticles obtained by low-temperature solventless synthesis. J. Mater. Chem. 2011, 21, 4408. [Google Scholar] [CrossRef]
- Hermans, J.J.; Keune, K.; van Loon, A.; Iedema, P.D. An infrared spectroscopic study of the nature of zinc carboxylates in oil paintings. J. Anal. At. Spectrom. 2015, 30, 1600–1608. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Shih, K.; Gao, Y.; Li, F.; Wei, L. Dechlorinating transformation of propachlor through nucleophilic substitution by dithionite on the surface of alumina. J. Soils Sediments 2012, 12, 724–733. [Google Scholar] [CrossRef] [Green Version]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction—Pyrolytic method for TiO2 polymorphs production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Sohrabi, S.; Keshavarz Moraveji, M.; Iranshahi, D. Morphological and structural insights into high aspect ratio lauric acid/TiO2 nanowires: A low-temperature synthesis. Ceram. Int. 2021, 47, 9424–9436. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, S.; Wang, W.; Wang, Y.; Ju, H.; Zhu, J. Temperature-induced structural evolution of Sm nanoparticles on Al2O3 thin film: An in-situ investigation using SRPES, XPS and STM. Appl. Surf. Sci. 2016, 1–6. [Google Scholar] [CrossRef]
- Men, S.; Jiang, X.; Xiang, X.; Sun, G.; Yan, Y.; Lyu, Z.; Jin, Y. Synthesis of cellulose long-chain esters in 1-butyl-3-methylimidazolium Acetate: Structure-property relations. Polym. Sci. Ser. B 2018, 60, 349–353. [Google Scholar] [CrossRef]
- Xing, J.-H.; Xia, Z.-B.; Hu, J.-F.; Zhang, Y.-H.; Zhong, L. Growth and crystallization of titanium oxide films at different anodization modes. J. Electrochem. Soc. 2013, 160, C239–C246. [Google Scholar] [CrossRef]
- Gautier, P.; Vallee, A.; Sinito, C.; Etcheberry, A.; Simon, N. Effect of growth temperature on the electrodeposition of zinc oxide layers on diamond surfaces. Diam. Relat. Mater. 2016, 62, 1–6. [Google Scholar] [CrossRef]
- Liu, T.; Fei, X.; Hu, L.; Zhang, H.; Li, Y.; Duo, S. Effect of substrate surface pretreatment and annealing treatment on morphology, structure, optical and electrical properties of sputtered ZnO films. Superlattices Microstruct. 2015, 83, 604–617. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Milionis, A.; Loth, E.; Bayer, I.S. Recent advances in the mechanical durability of superhydrophobic materials. Adv. Colloid Interface Sci. 2016, 229, 57–79. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically durable superhydrophobic surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, V.; Khonsari, M.M. On the degradation of superhydrophobic surfaces: A review. Wear 2017, 372–373, 145–157. [Google Scholar] [CrossRef]
- Li, M.; Bian, C.; Yang, G.; Qiang, X. Facile fabrication of water-based and non- fluorinated superhydrophobic sponge for efficient separation of immiscible oil/water mixture and water-in-oil emulsion. Chem. Eng. J. 2019, 368, 350–358. [Google Scholar] [CrossRef]
- Liu, W.; Cui, M.; Shen, Y.; Zhu, G.; Luo, L.; Li, M.; Li, J. Waste cigarette filter as nanofibrous membranes for on-demand immiscible oil/water mixtures and emulsions separation. J. Colloid Interface Sci. 2019, 549, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Thasma Subramanian, B.; Alla, J.P.; Essomba, J.S.; Nishter, N.F. Non-fluorinated superhydrophobic spray coatings for oil-water separation applications: An eco-friendly approach. J. Clean. Prod. 2020, 256, 120693. [Google Scholar] [CrossRef]
- Rius-Ayra, O.; Llorca-Isern, N. A robust and anticorrosion non-fluorinated superhydrophobic aluminium surface for microplastic removal. Sci. Total Environ. 2021, 760, 144090. [Google Scholar] [CrossRef] [PubMed]
- Rius-Ayra, O.; Biserova-Tahchieva, A.; LLorca-Isern, N. Surface-functionalised materials for microplastic removal. Mar. Pollut. Bull. 2021, 167, 112335. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rius-Ayra, O.; Biserova-Tahchieva, A.; Llorca-Isern, N. Durable Superhydrophobic Coating for Efficient Microplastic Removal. Coatings 2021, 11, 1258. https://doi.org/10.3390/coatings11101258
Rius-Ayra O, Biserova-Tahchieva A, Llorca-Isern N. Durable Superhydrophobic Coating for Efficient Microplastic Removal. Coatings. 2021; 11(10):1258. https://doi.org/10.3390/coatings11101258
Chicago/Turabian StyleRius-Ayra, Oriol, Alisiya Biserova-Tahchieva, and Nuria Llorca-Isern. 2021. "Durable Superhydrophobic Coating for Efficient Microplastic Removal" Coatings 11, no. 10: 1258. https://doi.org/10.3390/coatings11101258
APA StyleRius-Ayra, O., Biserova-Tahchieva, A., & Llorca-Isern, N. (2021). Durable Superhydrophobic Coating for Efficient Microplastic Removal. Coatings, 11(10), 1258. https://doi.org/10.3390/coatings11101258







