Polyphenol-Rich Purified Bioactive Fraction Isolated from Terminalia catappa L.: UHPLC-MS/MS-Based Metabolite Identification and Evaluation of Their Antimicrobial Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Isolation
2.2. Microbial Cultures and Growth Media
2.3. Antimicrobial Assays
2.4. Time Kill Assay
2.5. HRLCMS-Based Metabolite Profiling of TCAF2
2.6. Total Flavonoid Content (TFC) Determination by Aluminium Chloride Method
3. Results
3.1. Inhibitory Effect of Purified Fraction on Human Pathogenic Bacteria and Fungi Assessed by Minimum Inhibitory and Minimum Bactericidal Concentration
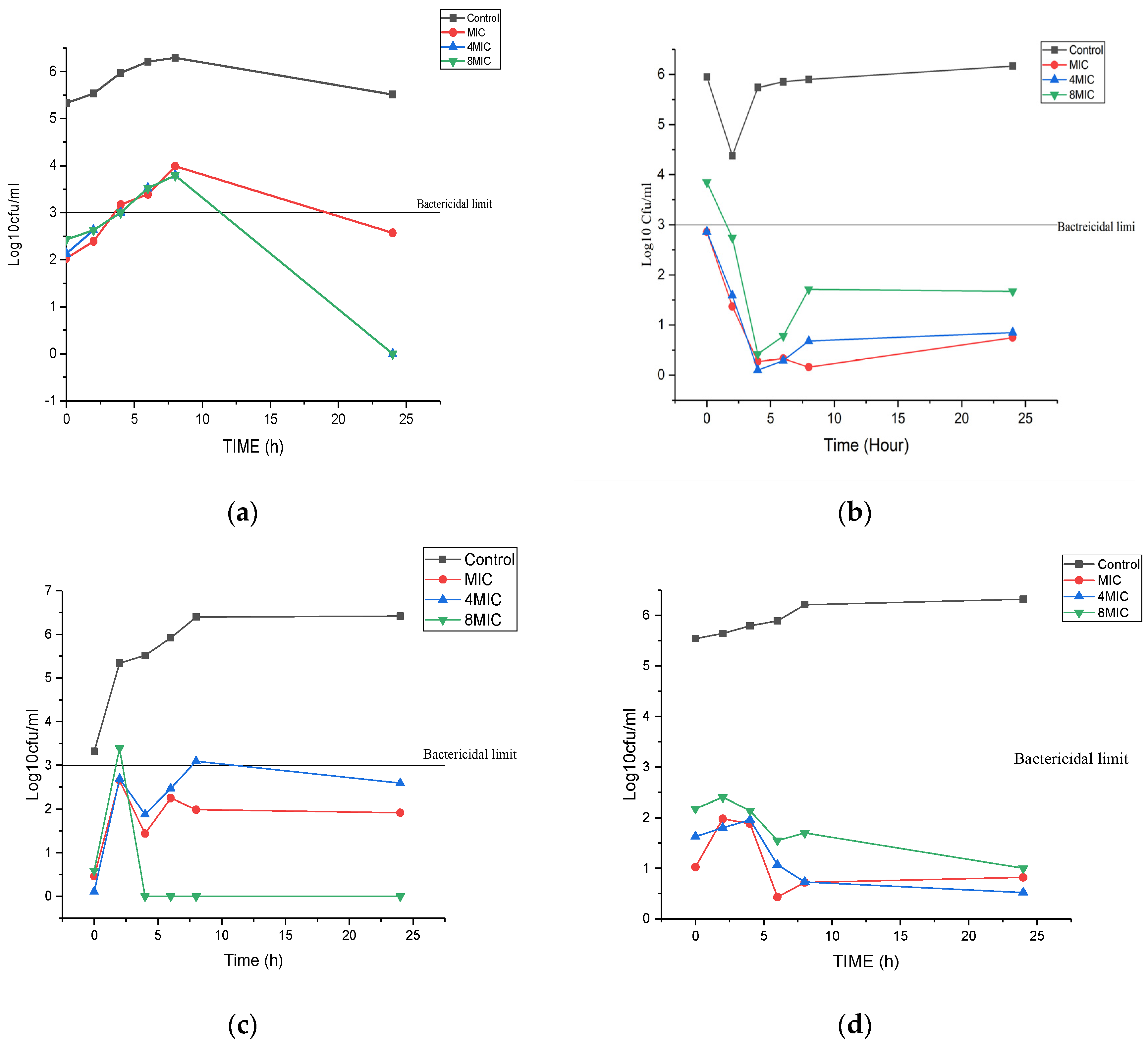
3.2. Time Kill Assay
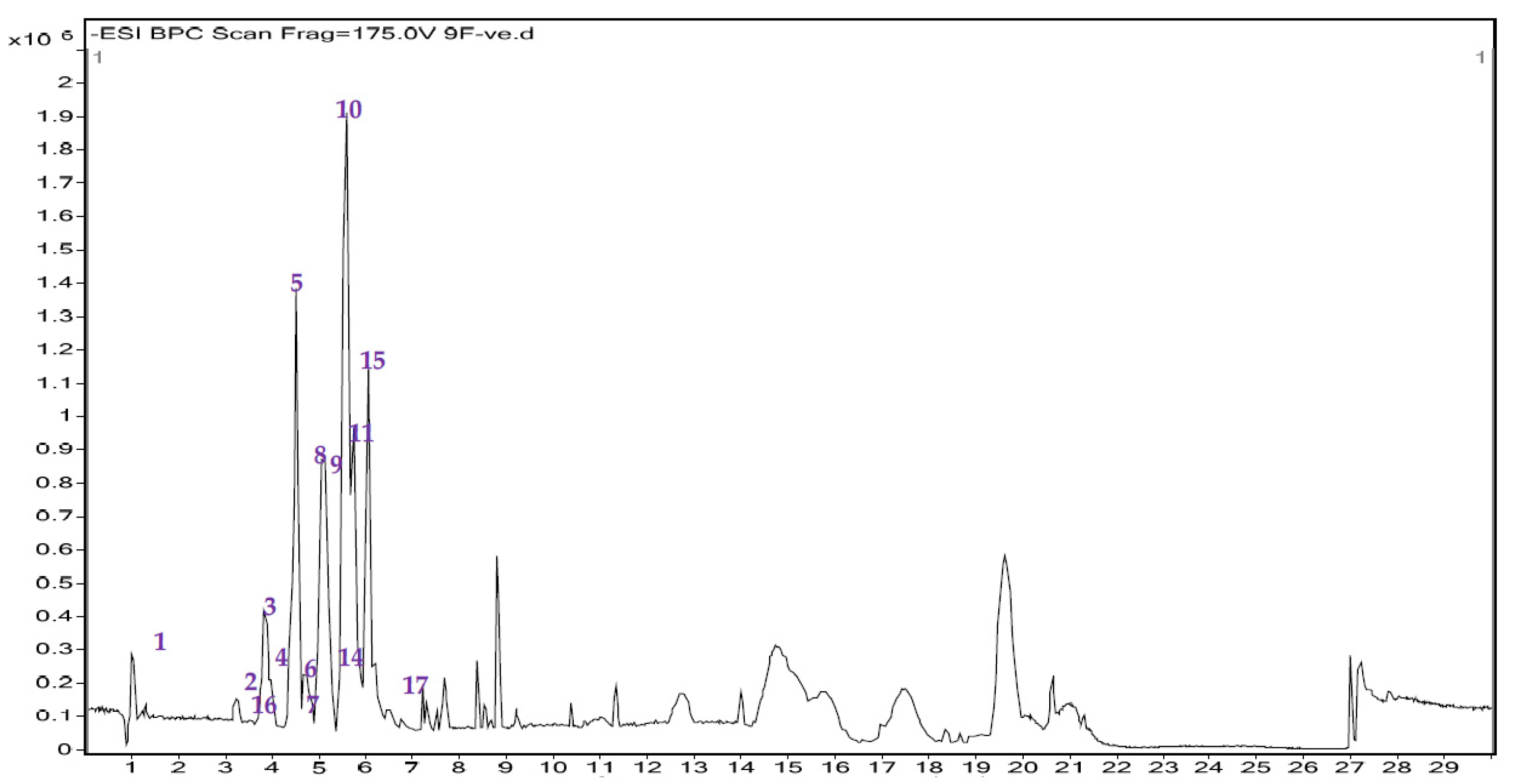
3.3. UHPLC-MS/MS Analysis and Total Flavonoid Content Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdissa, D.; Geleta, G.; Abdissa, N. Phytochemical investigation of Aloe pulcherima roots and evaluation for its antibacterial and antiplasmodial activities. PLoS ONE 2017, 12, e0173882. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 15, 911. [Google Scholar] [CrossRef]
- Tan, L.; Zhou, Z.; Lin, X.; Li, J.; Zheng, Y.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Feng, X.; et al. Overcoming multi-drug resistant MRSA using conventional aminoglycoside antibiotics. Adv. Sci. 2020, 7, 1902070. [Google Scholar] [CrossRef] [PubMed]
- Kali, A. Antibiotics and bioactive natural products in treatment of Methicillin resistant Staphylococcus aureus: A brief review. Phcog. Rev. 2015, 9, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Rueda, R.Y.R. Natural plant products used agaisnt Methicillin-resistant Staphylococcus aureus. In Fighting Multi Drug Resistance, 1st ed.; Rai, M., Kon, M.V., Eds.; Academic Press: London, UK, 2013; p. 11. [Google Scholar]
- Spengler, G.; Kincses, A.; Gajdacs, M.; Amarlal, L. New roads leading to old destinations: Efflux pumps as targets to reverse multi-drug resistance in bacteria. Molecules 2017, 22, 468. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.; Kemmerly, S.A. Infection control and prevention. A review of hospital acquired infections and the economic implications. Ochsner J. 2009, 9, 7–31. [Google Scholar]
- Stone, P.W. Economic burden of healthcare-associated infections: An American perspective. Expert Rev. Pharm. Outcomes Res. 2009, 9, 417–422. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimcirbial reistance. A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Serwecinska, L. Antimicrobials and antibiotic resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Marquez, L.; Quave, C.L. Prevalence and therapeutic challenges of fungal drug resistance: Role for plants in drug discovery. Antibiotis 2020, 9, 150. [Google Scholar] [CrossRef]
- Geratti, V.R.; Oda, F.; Sorrechia, R.; Kapp, B.R.; Serraphim, C.M.; Weckwerth, A.C.V.B.; Chorlli, M.; De Silva, P.B.; Eloy, J.O.; Kogan, M.J.; et al. Poly-e-caprolactone nanoparticles loaded with 4-nerolidylcatechol (4-NC) for growth inhibition of Microsporum Canis. Antibiotics 2020, 9, 894. [Google Scholar] [CrossRef]
- Abdel-aziz, M.M.; Al-omar, M.S.; Mohammed, H.A.; Emam, T.M. Invitro and exvivo antibiofilm activity of a lipopeptide biosurfactant porduced by the entomopahtogenic Beauveria bassiana strain against Microsporum Canis. Microorganisms 2020, 8, 232. [Google Scholar] [CrossRef]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multi-drug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef]
- Roana, J.; Mandras, N.; Scalas, D.; Campagna, P.; Tullio, V. Antifungal activity of Melaleuca alternifolia essential oil (TTO) and its synergy with itraconazole and ketoconazole against Trichophyton rubrum. Molecules 2021, 26, 461. [Google Scholar] [CrossRef]
- Andrade, G.; Orlando, H.C.S.; Scorzoni, L.; Pedroso, R.S.; Abrao, F.; Carvalho, M.T.M.; Veneziani, R.C.L.; Ambrosio, S.R.; Bastos, J.K.; Mendes-Giannini, M.J.S.; et al. Brazilian copaifra species: Antifungal activity agisnt clinicaly relevant Candida species, cellular target and invivo toxicity. J. Fungi 2020, 6, 153. [Google Scholar] [CrossRef]
- Touil, H.F.Z.; Boucherit, K.; Boucherit-otmani, Z.; Kohder, G.; Madkour, M.; Soliman, S.S.M. Optimum inhibition of amphotericin- B-resistant Candida albicans strain in single and mixed spices biofilms by Candida and non-Candida terpenoids. Biomolecules 2020, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshaly, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mehcanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 1028. [Google Scholar] [CrossRef]
- Panda, S.K.; Das, R.; Lavingne, R.; Luyten, W. Indian medicinal plant extracts to control multi-drug resistant Staphylococcus aureus including in biofilms. S. Afr. J. Bot. 2020, 128, 83–291. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Dussart, F. Selected fungal natural products with antimicrobial properties. Molecules 2020, 25, 911. [Google Scholar] [CrossRef] [PubMed]
- Atef, N.M.; Shanab, S.M.; Negm, S.I.; Abbas, Y.A. Evaluation of antimicrobial activities of some plant extracts against antibiotic susceptible and resistant bacterial stains causing wound infection. Bull. Natl. Res. Cent. 2019, 43, 144. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simoes, M. Plants as sources of new antimicrobials and resistance modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Anand, U.; Herrera, N.J.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics. Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Upadhyaya, I.; Johny, A.K.; Venkatanarayanan, K. Combating pathogenic microorganisms using plant derived antimicrobials. A minireview of the mechanistic basis. BioMed Res. Int. 2014, 2014, 761741. [Google Scholar] [CrossRef] [PubMed]
- Manilal, A.; Sabu, K.R.; Shewangizaw, M.; Akilu, A.; Seid, M.; Merdekios, B.; Tsegaye, B. Invitro antibacterial activity of medicinal plants against biofilm forming methicillin-resistant Staphylococcus aureus: Efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon 2020, 6, e03303. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, V.; Chen, X.; Hanna, G.; Mayasari, D.; Hamman, M.T. Role of symbiosis in the discovery of novel antibiotics. J. Antibiot. 2020, 73, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Mojoumouo, M.S.; Sharma, J.R.; Sibuyi, N.R.S.; Tincho, M.B.; Boyom, F.F.; Meyer, M. Synthesis of biogenic gold nanoparticles form Terminalia mentaly extracts and evaluation of their invitro cytotoxic effects in cancer cells. Molecules 2020, 25, 4469. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, K.J. Monocyclic phenolic acids; hydroxy and polyhydroxybenzoic acids: Occurrence and recent bioactivity studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Salares, E.F.O. Balala, L.M. Phytochemical screening and antimicrobial activity of Terminalia catappa L. leaf extract against potential pathogens of animals. J. Sci. Eng. Technol. 2018, 6, 15–26. [Google Scholar]
- Lee, D.Y.; Kim, H.W.; Yang, H.; Sung, S.H. Hydrolysable tannins from fruits of Terminalia chebula Retz and their alpha-glucosidase inhibitory activities. Phytochemistry 2017, 137, 109–116. [Google Scholar] [CrossRef]
- Venkatalakshmi, P.; Vadivel, V.; Brindha, P. Phytopharmacological significance of Terminalia catappa L.: An updated review. Int. J. Res. Ayurveda Pharm. 2016, 7, 130–137. [Google Scholar]
- Nagappa, A.; Thakur desai, P.A.; Venkat rao, N.; Singh, J. Antidiabetic activity of Terminalia catappa fruits. J. Ethnopharmacol. 2003, 88, 45–50. [Google Scholar] [CrossRef]
- Fan, Y.M.; Xu, L.Z.; Gao, J.; Wang, Y.; Tang, X.H.; Zhao, Y.N.; Zhang, Z.X. Phytochemical and antiinflamatory studies on Terminalia catappa. Fitoterapia 2004, 75, 253–260. [Google Scholar] [CrossRef]
- Tercas, A.G.; Monteiro, A.S.; Moffa, E.B.; Dos Santos, J.R.A.; De Sousa, E.M.; Pinto, A.R.B.; Da Silva Costa, P.; Borges, A.C.R.; Torres, L.M.B.; Fillo, A.K.D.; et al. Phytochemical characterization of Terminalia catappa L. extracts and their antifungal activity against Candida species. Front. Microbiol. 2017, 8, 595. [Google Scholar] [CrossRef]
- Rathnasooriya, W.D.; Dharmasir, I.M.G. Effects of Terminalia catappa seeds on sexual behaviour and fertility of male rats. Asian J. Aandrol. 2000, 2, 213–219. [Google Scholar]
- Pandya, N.B.; Tigari, P.; Dupadahalli, K.; Kamurthy, H.; Nadendra, R.R. Antitumor and antioxidant status of Terminalia catappa against Ehrilich ascites carcinoma in swiss albino mice. Indian J. Pharmacol. 2013, 45, 464–469. [Google Scholar] [PubMed]
- Das, G.; Kim, D.Y.; Gutierrez-Grijalva, E.P.; Heredia, J.B.; Nissapatorn, V.; Mitsuwan, W.; Pereira, M.L.; Nawaz, M.; Siyadatpanah, A.; Norouzi, R.; et al. Plants of the genus Terminalia: An insights on its biological potentials, pre-clinical and clinical studies. Front. Pharmacol. 2020, 11, 561248. [Google Scholar] [CrossRef] [PubMed]
- Qaiyumi, S. Macro and Microdilution methods of antimicrobial susceptibility testing. In Antimicrobial Susceptibility Testing Protocols, 9th ed.; Schwalbe, R., Steele-moore, L., Goodwin, C., Eds.; CRC Press: London, UK, 2012; pp. 75–79. [Google Scholar]
- Espinel-Ingroff, S.; Canton, E. Antifungal susceptibility testing for filamentous fungi. In Antimicrobial Susceptibility Testing Protocols, 9th ed.; Schwalbe, R., Steele-moore, L., Goodwin, C., Eds.; CRC Press: London, UK, 2012; pp. 209–241. [Google Scholar]
- Joray, M.B.; Rollan, M.D.R.; Ruiz, G.M.; Palacios, S.M.; Carpinella, M.C. Antibacterial activity of extracts form plants of central Argentina- Isolation of active principle from Achyrcline satureioides. Planta Med. 2011, 77, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Neupane, P.; Lamichhane, J. Estimation of total phenolic content, total flavonoid content and antioxidant capacities of five medicinal plants from Nepal. Vegetos 2020, 33, 360–366. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of gallic acid and ellagic acid derivatives in different plant parts of T.arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod.Commun. 2016, 11, 239–244. [Google Scholar] [PubMed]
- Sobeh, M.; Mahmoud, M.F.; Hasan, R.A.; Abd elfattah, M.A.O.; Osman, S.; Rashid, H.O.; El-Shazly, A.M.; Wink, M. Chemical composition, antioxidant and hepatoprotective activity of methanol extract from leaves of T. bellirica and T. sericea (Combretaceae). PeerJ 2019, 7, e6322. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ueda, N.; Shinohara, H.; Nonaka, G.I.; Kouno, I. Four new C-glycosidic ellagitannins, castacrenins D-G from Japanese chestnut wood (Castanea crenata SIEB. et Zucc). Chem. Pharm. Bull. 1999, 45, 1751–1755. [Google Scholar] [CrossRef]
- Linares, I.B.; Lopez, M.H.; Catalan, E.B.; Roman, D.A.; Alvarez, I.G.; Bermejo, M.; Gutierrez, A.F.; Micol, V.; Carretero, A.S. Permeability study of polyphenols derived from a phenolic-enriched Hibiscus sabdariffa extract by UHPLC-EI-Qq-TOF-MS. Int. J. Mol. Sci. 2015, 16, 18396–18411. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, Q.; Liang, W.; Zhou, K.; Jian, P.; She, G.; Zhang, L. A comprehensive review of the structural elucidation of tannins from Terminalia Linn. Evid. Based Complement. Altern. Med. 2019, 2019, 8623909. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrapole time of flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef] [PubMed]
- Wyerpkowski, C.C.; Da costa, D.L.M.G.; Sihorin, A.P.; Vileas, W.; De Grandis, R.A.; Resende, F.A.; Varanda, E.A.; Santos, L.C.D. Characterization and quantification of the compounds of the ethanolic extract from Caesalpania ferrea stem bark and evaluation of their mutagenic activity. Molecules 2014, 19, 16039–16057. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, A.H.; Wu, H.; Pan, Y.; Wang, K.; Jin, Y.; Zhang, C. Characterization and quantification by LC-MS/MS of the chemical components of the heating products of the flavonoid extract in pollen typhae for transformation rule exploration. Molecules 2015, 20, 18352–18366. [Google Scholar] [CrossRef]
- Zhang, X.R.; Kaunda, J.S.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. The Genus Terminalia (Combretaceae): An Ethnopharmacological Phytochemical and Pharamacological review. Nat. Prod. Bioprospect. 2019, 9, 357–392. [Google Scholar] [CrossRef] [PubMed]
- Tadic, V.; Arsic, I.; Zvezdanovic, J.; Zugic, A.; Cvetkovic, D.; Pavkov, S. The estimation of the traditionally used yarrow (Achllea millefolium L. Asteraceae) oil extracts with antiinflamatory potential in topical applications. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Johnson, J.V.; Talcott, S.T. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J. Agric. Food Chem. 2005, 53, 6003–6010. [Google Scholar] [CrossRef]
- Lee, H.B.; Kim, L.K.; Park, S.S.; Bang, S.G.; Kim, T.G.; Chung, D.W. Isolation and anti-inflammatory effect of Astragalin synthesized by enzymatic hydrolysis of tea seed extract. J. Sci. Food Agric. 2011, 91, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, J.P.; Xu, S.P.; Wang, J.H.; Liu, X. Separation and determination of secoisolariciresinol diglucoside oligomers and their hydrolysates in the flax seed extract by high-performance liquid chromatography. J. Chromatogr. A 2018, 1185, 223–232. [Google Scholar] [CrossRef]
- Parveen, I.; Winters, A.; Threadgile, M.D.; Hauk, B.; Morris, P. Extraction structural characterization and evaluation of hydroxycinnamate esters of orchard grass (Bactyis glomerata) as substrates for polyphenol oxidase. Phytochemistry 2018, 69, 2799–2806. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, H.; Xu, X.; Xue, X.; Lin, M.; Xu, S.; Lin, H.; Gao, Y.; Zhang, H.; Li, X. Compound identification in semen cuscate by ultra-high performance liquid chromatography (UPLC’s) coupled to electrospray ionization mass spectrometry. Molecules 2018, 23, 1199. [Google Scholar] [CrossRef]
- Mackenbrock, U.; Vogelsang, R.; Barx, W. Isoflavone and pterocarpan malonylglucosides and ß-1,3-glucan and chitin-hydrolases are vacuolar constituents in Chick pea (Cicer arientinum L.). Z. Nat. 1992, 47, 815–822. [Google Scholar]
- Hasanuddin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Patil, B.M.; Chand, J.; Naaz, Y. Anthraquinone derivatives as an immune booster and their therapeutic option against COVID-19. Nat. Prod. Bioprospect. 2020, 10, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Takahashi, S.; Taked, S.; Hatta, H. Distribution of Flavan-3-ol species in ripe strawberry fruit revealed by matrix-assisted laser desorption/ionization-mass spectrometry imaging. Molecules 2020, 25, 103. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Nee, C.W.; Mordi, M.N.; Ramanathan, S. Evaluation of phenolic content and antioxidant property of hydrolyzed extracts of Terminalia catappa L. leaf. Asian J. Plant Sci. 2010, 9, 479–485. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of phenolic compounds form medicinal plants (Hops and Juniper berries) and their antioxidant activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflamatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef]
- Reti, L. Cactus alkaloids and some related compounds. In Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1950; Volume 6, pp. 242–289. [Google Scholar]
- Lee, X.W.; Basri, D.F.; Ghazali, A.R. Bactericidal effect of pterostilbene alone and in combination with gentamycin against human pathogenic bacteria. Molecules 2017, 22, 463. [Google Scholar] [CrossRef]
- Cui, Z.H.; He, H.L.; Wu, S.B.; Dong, C.L.; Lu, S.Y.; Shan, T.J.; Fang, L.X.; Liao, X.P.; Liu, Y.H.; Sun, J. Rapid screening of essential oils as substances which enhance antibiotic activity using a modified well diffusion method. Molecules 2021, 10, 463. [Google Scholar]
- Suekep, A.J.; Fan, M.; Sarker, S.D.; Kuete, V.; Guo, M.Q. Plukentetia huayllabambana fruits: Analysis of bioactive compounds antibacterial activity and relative action mechanims. Molecules 2020, 9, 1111. [Google Scholar]
- Asong, J.A.; Amoo, S.O.; Mcgaw, L.J.; Nkadimeng, S.M.; Aremu, A.O.; Otang-Mbeng, W. Antimicrobial activity, antioxidant potential, Cytotoxicity and phytochemical profiling of four plants locally used against skin diseases. Plants 2019, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Ravi, L.; Jindam, D.; Kumaresan, S.; Selvaraj, V.; Rddy, J. Anti-methicillin Staphylococcus aureus potential of phytochemicals in Terminalia catappa and their proposed in silico mechanism of action. Asian J. Pharm. Clin. Res. 2001, 12, 133–137. [Google Scholar]
- Mbengui, R.D.; Guessennd, W.K.; M’boh, G.M.; Golly, J.K.; Okou, C.O.; Nguessan, J.D.; Dosso, M.; Djaman, J.A. Phytochemical screening and study of comparitive antibacterial activity of aqueous and alcoholic extracts of leaves and bark of terminalia catappa on multiresistant strains. J. Appl. Biosci. 2012, 65, 5040–5048. [Google Scholar]
- Thikshani, S.; Gas, P.; Mohammad, A.A.; Balamuthu, K.; Nimsha, W.S. Invitro antibacterial activity of selected underutilized plants and cytotoxic property of Terminalia catappa. Int. J. Pharm. Pharm. Sci. 2017, 9, 218–225. [Google Scholar] [CrossRef]
- Cock, I.E. The medicinal properties and phytochemistry of plants of genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, S.; Rajan, S.; Thirunalasundari, T.; Jeeva, S. Antifungal activity of Ocimum sanctum Linn. (lamiaceae) on clinically isoalted dermatophytic fungi. Asian Pac. J. Trop. Med. 2011, 4, 654–657. [Google Scholar] [CrossRef]
- Masoko, O.; Eloff, J.N. The diversity of antifungal compounds of six South African Terminalia species (combretaceaae) determined by bioautography. Afr. J. Biotechnol. 2005, 4, 1425–1431. [Google Scholar]
- Eloff, J.N.; Katerere, D.R.; McGraw, L.J. The biological activity and chemistry of the southern African Combretaceae. J. Ethnopharmacol. 2008, 119, 686–699. [Google Scholar] [CrossRef]
- Fyhrquist, P.; Mwasumli, L.; Haeggstrom, C.A.; Vourela, H.; Hiltunen, R.; Vuorela, P. Antifungal activity of selected species of Terminalia, Pteleopsis and Combretum (Combretaceae) collected in Tanzania. Pharm. Biol. 2008, 42, 308–317. [Google Scholar] [CrossRef]
- Tasneem, M.I.F.; Narsegowda, P.N. Antimicrobial activity of different varieties of Terminalia catappa leaves. Int. J. Pharm. Sci. Res. 2018, 9, 4430–4435. [Google Scholar]
- Sakender, H.; Akhilesh, B.; Koteshwara, A.R. Evaluation of antifungal potential of selected medicinal plants against human pathogenic fungi. Int. J. Green Pharm. 2015, 2, 110–117. [Google Scholar]
- Olajuyigbe, O.O.; Afolayan, A.J. Invitro antibacterial and time kill evaluate of Erythrina caffra Thunb. extract against bacteria associated with diarrhea. Sci. World J. 2012, 2012, 738314. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, M.; Kumar, S.; Yadav, J.P. Bactericidal effect of Acacia nilotica: Invitro antibacterial and time kill kinetic studies. Int. J. Curr. Res. 2015, 11, 22289–22294. [Google Scholar]
- Buzgaia, N.; Awin, T.; Elabbar, F.; Abdusalam, K.; Lee, S.O.; Rukayadi, Y.; Abas, A.; Shaari, K. Antibacterialactivity of Arbutus pavarii Pamp against Methicillin resistant staphylococcus aureus (MRSA) and UHPLC-MS/MS profile of bioactive fraction. Plants 2020, 9, 1539. [Google Scholar] [CrossRef]
- Ngouana, T.K.; Mbouna, C.D.J.; Kuipou, R.M.T.; Tchuemogne, M.A.T.; Zeuko, E.M.; Ngouana, V.; Mallie, M.; Baertout, S.; Boyom, F.F. Potent and synergistic extract combination from Terminalia catappa, Terminalia mentaly and Monodora tenuifolia against pathogenic yeasts. Medicines 2015, 2, 220–235. [Google Scholar] [CrossRef]
- Raju, K.S.R.; Kadian, N.; Taneja, I.; Wahajuddin, M. Phytochemical analysis of isoflavonoids using liquid chromatography coupled with tandem mass spectrometry. Phytochem. Rev. 2015, 14, 469–498. [Google Scholar] [CrossRef]
- Chibane, L.B.; Degraeve, P.; Ferhour, H.; Bouakila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Sahloul, R.B.; Fredj, R.B.; Boughalleb, N.; Shiraa, J.; Saguem, S.; Hilbert, J.L.; Trotin, F.; Ammar, S.; Bouzid, S.; Skhiri, F.H. Phenolic composition and antioxidant and antimicrobial activity of extracts obtained from Crataegus azarolus L. Var. Aronia (Willd.) Batti. Ovaries Calli. J. Bot. 2014, 1–11. [Google Scholar] [CrossRef][Green Version]
- Szmechtyk, M.E.; Nowak, A.; Czyzowdla, A. Plant extract rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef]
- Ohara, R.; Perico, L.L.; Rodrigues, V.P.; Bueno, G.; Zanatta, A.C.; Santos, L.C.D.; Vilegas, W.; Constatino, F.B.; Justulin, L.A.; Lima, A.H. Terminalia catappa L. infusion accelerates the healing process of gastric ischemia reperfusion injury in rats. J.Ethnopharmacol. 2020, 256, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khamenehb, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure- activity relationship: An updated review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Osanga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial activity of new class of phosphorylated and modified flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ozarowski, M.; Karpinski, T.M. Antibacterial acivity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med. 2010, 9, 109. [Google Scholar] [CrossRef]
- Karaca, C.H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and E. coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytr, O.; Beyatli, A.; Yeskaliyev, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Jean-de-Dieu, T.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazaabadiokov, L. Antimicrobial activity of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Su, Y.; Ma, L.; Wen, Y.; Wang, H.; Zhang, S. Studies of the invitro antibacterial activities of several polyphenols agaisnt clinical isoaltes of Methicillin resistant Staphylococcus aureus. Molecules 2014, 19, 12630–12639. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Hamed, A.A.; Hassan, M.H.; Belbhari, L.; Rateb, M.E.; Sayed, A.M. Flavonoids as potential anti-MRSA agents through modulation of PBP2a: A computational andxperimetnal study. Antibiotics 2020, 9, 562. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Gontijo, A.V.L.; Salvador, M.J.; Tanaka, M.H.; Brighenti, F.L.; Delbem, A.C.B.; Delbem, A.C.B.; Koga-ito, C.Y. Effects of acetone fraction from Buchenavia toemntosa aqueous extracts and gallic acid on Candida albicans biofilms and virulence factors. Front. Microbiol. 2018, 19, 647. [Google Scholar] [CrossRef]
- Djouossi, S.S.I.; Tamakou, J.D.; Ngnokam, D.; Kuiate, J.R.; Tapandjou, L.A.; Harakat, D.; Voutquenne-Nazabadioko, L. Antimicrobial and antioxidant flavonoids from the leaves of Oncoba spinosa Forssk. (Salicaceae). BMC Complement. Altern. Med. 2015, 15, 134. [Google Scholar] [CrossRef]
- Al Aboody, L.S.; Mickymaray, S. Antifungal efficacy and mechanisms of flavanoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, H.T.; Zhang, M.; Wang, D.; Yang, C.R.; Zhang, Y.J. Termintomenins F and G two new lignan glucosides from Terminalia chebula var. tomentella (kurz) C.B. Clarke. Nat. Prod. Bioprospect. 2021, 11, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.S.A.; El-toumy, S.A.A.; Moharram, F.A.; Shalaly, N.M.M.; Ahmed, A.A.E. Pharmacologically active ellagitannis from Terminalia Myriocarpa. Planta Med. 2002, 68, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Flonso- calleja, C.; Aamarlal, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of phenolic profile of Castanea sativa Mill. By-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antibacterial activities of ellagitannins against Clostridiales perfringens Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef]
- Elendran, S.M.; Muniyandy, S.; Lee, W.W.; Palaniswamy, U.D. Permeability of the ellagitannin geraniin and its metabolites in a human colon adenocarcinoma caco-2 cell culture model. Food Funct. 2019, 10, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Cosarca, S.; Lucia-Muntean, D. A critical review on phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [PubMed]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Akiyama, H.; Fujii, K.; Yamasakio, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef]
- Ajiboye, A.E.; Babatunde, S.K.; Adedayo, M.R.; Adetumbi, M.A.; Ahuwon, I.B.; Ajasegun, T.A. Antibacterial potency and phytochemical screening of the bark of Terminalia catappa against some clinical isolates. Int. J. Phytomed. 2016, 8, 193–201. [Google Scholar]
- Chinemezu, A.P.; Ngozi, A.K.; Cemaluk, A.C.; Uchechi, E.E. Determination of some phytoconstituents present in Terminalia catappa endocarp flour and biochemical evaluation of its ethanol extract on hepatic indices in male winstar rats. J. Pharmacol. Toxicol. 2018, 13, 27–36. [Google Scholar]
- Anato, M.; Ketema, T. Antiplasmodial activity of Combretum Molle (Combretaceae) (zwoo) seed extract in swiss albino mice. BMC Res. Notes. 2018, 11, 312. [Google Scholar] [CrossRef]
- Wande, O.M.; Babatunde, S.B. Invitro screening of ten Combretaceous plants for antimalarial activities applying the inhibition of beta-hematin formation. Int. J. Biochem. Sci. 2017, 11, 2971–2981. [Google Scholar]
- Feng, S.X.; Feng, M.Q.; Huan, K.Y.; Yu, B.; Hang, G.Y.; Li, W.W.; Dong, Q.A. Isolation of active compounds from methanol extract of Toddalia asiatica against Ichtyophthirius multifilis in gold fish. Vet. Parasitol. 2014, 199, 25–254. [Google Scholar]

| Test Bacteria | MIC (mg/mL) | MBC (mg/mL) | Gentamycin (mg/mL) |
|---|---|---|---|
| S. aureus (MTCC 7443) | 0.195 | 12.25 | 0.0019 |
| S. typhi (MTCC 733) | 0.097 | 0.78 | 0.0019 |
| Clinical Isolates | MIC (mg/mL) | MBC (mg/mL) | Ciprofloxacin (mg/mL) |
|---|---|---|---|
| S. aureus | 0.097 | 0.390 | 0.0019 |
| P. vulgaris | 0.781 | 25 | 0.0019 |
| MRSA 1503 MRSA1007 | 0.097 0.390 | 25 6.25 | 0.0019 0.0019 |
| Dermatophytes | MIC (mg/mL) | MFC (mg/mL) | Amphotericin (mg/mL) | Miconazole (mg/mL) |
|---|---|---|---|---|
| M. gypseum (MTCC 2830) | 0.195 | 0.390 | ND | 0.0019 |
| M. canis (MTCC 2820) | 0.097 | 0.390 | ND | 0.0019 |
| T. rubrum (MTCC 296) | 0.097 | 0.097 | ND | 0.0019 |
| C. albicans (MTCC 183) | 0.097 | 0.097 | 0.0019 | ND |
| T. asahii (MTCC 6179) | 1.56 | 1.56 | 0.015 | ND |
| Sl. No | Rt (Min) | Identified Compound | Class of Compound | Formula | [M − H]− | MS/MS Fragment Ions | Mass | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.108 | 1-O-Galloyl fructose | Gallotannin | C13H16O10 | 331.06 | 211.02,271.045, 169.012, 125.22 | 332.03 | [42] |
| 2 | 3.267 | Punicacortein D | Gallotannin | C48H28O30 | 541.02 | 300.996 | 1084.06 | [43] |
| 3 | 3.935 | 2,6-Digalloyl glucose | Gallotannin | C20H20O14 | 483.076 | 169, 271.044 | 484.03 | [43] |
| 4 | 3.985 | Castacrenin D | Ellagitannin | C48H30O30 | 541.02 | 781.05,601.99, 300.99 | 1804.06 | [43] |
| 5 | 4.432 | Punicacortein B | Gallotannin | C27H22O18 | 541.024 | 300.91 | 634.07 | [43] |
| 6 | 4.606 | 2-O-Ferruloylhydroxyxitric acid | Phenolic acid | C16H16O11 | 383.06 | 384.06 | - | [44] |
| 7 | 4.78 | 1,3,6,Trigalloyl glucose | Galloyl glucose | C27H24O18 | 635.087 | 483.0,635.08,300.993, 161.01 | 636.09 | [45] |
| 8 | 5.069 | Kaempferol 3-O-β-D-galactoside | Flavone glycoside | C21H20O11 | 447.092 | 447.089, 285.03 | 448.1 | [46] |
| 9 | 5.249 | Gallic acid 3-O-(6-galloylglucoside | Galloylglucoside | C20H20O14 | 483.069 | 129, 169, 313 | 484.07 | [47,48] |
| 10 | 5.483 | Ellagic acid | Phenolic acid | C14H6O8 | 300.99 | 229.012, 257.008 | 484.07 | [48] |
| 11 | 5.54 | Rutin | Flavonol | C27H30O16 | 609.14 | 271.024, 301.0345, 609.14,150.99, 300.264 | 610.152 | [49] |
| 12 | 5.621 | Apigenin 7-glucoside | Flavone glucoside | C21H20O10 | 431.09 | 283, 269 | 432.10 | [50,51] |
| 13 | 5.773 | Myricetin 7-rhamnoside | Flavonoid glucoside | C21H20O12 | 463.08 | 301.033, 272.0277, 255.0285 | 564.09 | [52] |
| 14 | 5.903 | Astragalin 7-rhamnoside | Dihydroxyflavone Galactoside | C27H22O12 | 593.14 | 557.24, 284.03, 164.83 | 594.15 | [53] |
| 15 | 6.062 | Melitric acid A | Phenolic acid | C27H22O12 | 583.10 | 431.09, 583.11, 311.0, 169.01 | 538.10 | [50] |
| 16 | 3.935 | Methyltrihydroxy benzoate | Phenolic acid | C8H8O5 | 183.02 | 183.02, 124.01, 293.12 | 184.03 | [50] |
| 17 | 7.251 | (8R,8′R)-Secoisorlariciresinol 9-glucoside | Lignan glucoside | C26H36O11 | 524.22 | 523.21, 300.99, 169.01, 446.97 | 524.225 | [54] |
| Sl. No | Rt (Min) | Identified Compound | Class of Compound | Molecular Formula | [M−H]− M/Z | MS/MS Fragment Ions | Mass | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.288 | 2-O-Ferruloyl hydroxycitric aicd | Phenolic acid | C16H16O11 | 385.07 | 385.07, 247.02, 468.11, 177.05 | 384.07 | [55] |
| 2 | 5.1 | Kaempferol 7-O glucoside | Flavanol glucoside | C21H20O11 | 449.10 | 450.11, 299.05, 165.01, 595.16 | 448.10 | [50] |
| 3 | 5.4 | Cynaroside | Glycosyloxyflavone | C21H20O11 | 449.10 | 449.11, 329.06, 191.08 | 448.10 | [56] |
| 4 | 5.59 | Cicerin 7-(6-malonyl glucoside) | Isoflavonoid-o-glycoside | C26H26O15 | 601.12 | 564.17, 433.11, 329.06, 300.99 | 578.13 | [57] |
| 5 | 5.59 | 6-C-Fucosyl luteolin | Flavonoid C-glycosides | C21H20O10 | 433.11 | 433.11, 313.07, 165.01, 235.05 | 432.10 | [58] |
| 6 | 5.652 | Hyperoside | Tetrahydroxy flavone | C21H20O12 | 465.10 | 303.05, 229.04, 433.11 | 464.09 | [50] |
| 7 | 5.685 | Nogalonic acid methyl ester | Dihydroxy anthraquinone | C21H16O18 | 397.09 | 433.11, 323.96, 283.06, 303.05 | 396.08 | [59] |
| 8 | 6.13 | Gambiriin C | Condensed tannin | C30H26O11 | 585.12 | 585.12, 586.12, 313.07, 287.05, 283.06 | 562.13 | [60] |
| 9 | 11.48 | 3-Oxo-12,18-ursadien-28-oic acid | Triterpenoid | C30H44O3 | 452.33 | 224.12 | 452.33 | [48,61] |
| 10 | 6.156 | (3S,4S)-3-Hydroxytetradecane- 1,3,4-tricarboxylic acid | Phenolic acid | C17H30O7 | 369.19 | 369.188, 540.24 | 346.20 | Pubchem id: 5460247 |
| 11 | 6.202 | Kaempferol | Tetrahydroxyflavone | C15H10O6 | 287.05 | 287.05, 449.10, 585.12, 586.12 | 286.04 | [49] |
| 12 | 6.541 | Quinidinone | Alkaloid | C20H22N2O2 | 323.7 | 192.08, 193.08, 432.10, 323.17, 324.17 | 322.16 | Pubchem id: 84497 |
| 13 | 7.684 | 5,6,7,3,4′-Pentahydroxy isoflavone | Flavone | C15H10O7 | 303.05 | 303.05, 305.05 | 302.04 | [62] |
| 14 | 7.75 | Maculosidine | Alkaloid | C14H13NO4 | 260.09 | 199.06, 227.05, 228.06 | 259.08 | [63] |
| 15 | 26.71 | Anhalonidine | Alkaloid | C12H17NO3 | 224.12 | 224.12, 165.005, 222.11, 230.14 | 223.12 | [64] |
| 16 | 4.99 | Hemipic acid | Benzoic acid | C10H10O6 | 249.04 | 249.05, 207.02 | 226.05 | Chemspider id: 61516 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowmya, T.N.; Raveesha, K.A. Polyphenol-Rich Purified Bioactive Fraction Isolated from Terminalia catappa L.: UHPLC-MS/MS-Based Metabolite Identification and Evaluation of Their Antimicrobial Potential. Coatings 2021, 11, 1210. https://doi.org/10.3390/coatings11101210
Sowmya TN, Raveesha KA. Polyphenol-Rich Purified Bioactive Fraction Isolated from Terminalia catappa L.: UHPLC-MS/MS-Based Metabolite Identification and Evaluation of Their Antimicrobial Potential. Coatings. 2021; 11(10):1210. https://doi.org/10.3390/coatings11101210
Chicago/Turabian StyleSowmya, Tumakuru Nataraj, and Koteshwar Anandrao Raveesha. 2021. "Polyphenol-Rich Purified Bioactive Fraction Isolated from Terminalia catappa L.: UHPLC-MS/MS-Based Metabolite Identification and Evaluation of Their Antimicrobial Potential" Coatings 11, no. 10: 1210. https://doi.org/10.3390/coatings11101210
APA StyleSowmya, T. N., & Raveesha, K. A. (2021). Polyphenol-Rich Purified Bioactive Fraction Isolated from Terminalia catappa L.: UHPLC-MS/MS-Based Metabolite Identification and Evaluation of Their Antimicrobial Potential. Coatings, 11(10), 1210. https://doi.org/10.3390/coatings11101210






