Abstract
Fusobacterium nucleatum (F. nucleatum) is a gram-negative obligate anaerobe bacterium that threatens human periodontal health. It can cause many oral diseases, including periodontitis, gingivitis and peri-implantitis, and even some diseases such as colorectal cancer are related to it. This paper aims to develop a novel and simple surface modification method for anti-Fusobacterium nucleatum on titanium, i.e., the material for implants. In this study, different concentrations (0.0–1.0%) of PHMB were dip-coated on the titanium surface. The surface properties were examined with the aid of Scanning electron microscopy, Energy-dispersive X-ray spectroscopy, Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy, and the antibacterial property against F. nucleatum was investigated using colony-forming unit. It was found that the PHMB successfully formed a self-assembled coating on the titanium surface and the PHMB-coated titanium had a strong capability of inhibiting F. nucleatum. Even though differences were found among the several concentrations, PHMB exhibited promising results as a simple coating strategy for dental implants.
1. Introduction
With the development of dentistry and material science, dental implants are an increasingly popular option for oral rehabilitation [1]. The concept of the modern dental implant as well as the concept of osseointegration was first introduced by Per-Ingvar Brånemark in the 1960s. He demonstrated that the implant surface made of titanium could be combine permanently with surrounding bone tissue [2,3]. This direct interface of arranged, living bone tissue directly adhered to the implant surface without any intermediate layer of tissue, is defined as osseointegration [4]. This characteristic is considered the cornerstone of modern implantology and the single most important criterion for implant success. Factors promoting osseointegration induce implant design, materials and surface topography—aspects commonly determined by surface treatment and coatings [2].
Although titanium is considered an ideal material for implant dentistry and other implantable medical devices [5], implant failure still occurs [6]. One of the major reasons for failure is infections caused by bacteria, such as peri-implantitis [7]. Among the many bacteria that can cause infection on titanium implantable medical devices, Fusobacterium nucleatum (F. nucleatum) is common one [8]. It acts as a keystone colonizer for periodontal diseases such as peri-implant mucositis (the stage before peri-implantitis) [9,10]. In addition, it also causes miscarriage [11], esophageal cancer [12], colon cancer [13], etc. When considering coating strategies to modify the surface, UV-photofunctionalization has been proposed to increase the hydrophilicity of titanium without influencing the surface topography [14]. This strategy has been shown to reduce bacterial attachment and subsequent biofilm formation. Nevertheless, some bacteria can still attach to hydrophilic surfaces, altering the biofilm in the function of the implant’s surface [14,15].
Polyhexamethylene biguanide (PHMB) is a polymer with the biocidal activity which is widely used as a preservative and as a broad-spectrum antiseptic. It is used as an antiseptic in the medical field in wound therapy [16], on skin and mucosa [17], and in eye disinfection [18,19,20,21,22,23]. In dentistry, PHMB is currently commercially available in mouthrinses and oral gels at various percentages up to 2% w/w [24].
PHMB is a strong antibacterial agent that interacts with acidic, negatively charged phospholipids in the membrane creating physical shifts, increasing permeability and causing the loss of integrity, ultimately killing the organism [25,26,27,28]. Muller and Cramer [29] reported a strong antiseptic effect with lower cytotoxicity. In addition, there is strong evidence that PHMB can enhance the wound healing process. Furthermore, PHMB is reported to be non-genotoxic, with the evidence of no increase in DNA fragmentation [30]. Koburger et al. compared the antiseptic effect of PHMB to chlorhexidine, triclosan or other antiseptics, and concluded that the efficacy of PHMB was higher than the rest when prolonged use or contact is needed [31]. Research is warranted for PHMB coatings of titanium surfaces as a potentially promising antibacterial surface coating method for dental implants.
This study aims to assess the antibacterial properties of PHMB-coated titanium. The study was designed to develop a novel surface modification strategy able to self-assembled on titanium surface structure that could prevent (re)infection on titanium made medical devices (e.g., peri-implantitis).
2. Materials and Methods
2.1. Materials and Equiment
Commercially pure grade 2 (cp-2) titanium specimens of 10.0 mm × 10.0 mm × 1.0 mm were supplied by Kobe Steel, Ltd., Tokyo, Japan. Polyhexamethylene biguanide (PHMB), 20% w/v, was supplied by Taizhou Sunny Chemical Co., Ltd., Taizhou, China.
A manual polisher with 220-, 320-, 500- and 1000-grits silicon carbide (SiC) abrasive paper was supplied by Lunn Major, Struers, Denmark. An ultrasonic cleaning machine was supplied by Decon FS200, Decon Ultrasonics Ltd.,Hove, UK. Ultra Violet (UV) light (365 nm) was supplied by Spectroline® CL-150, Spectronics Corporation, Melville, NY, USA. The Gamma ray machine was supplied by Shenzhen JPY Ion-tech. Co., Ltd., Shenzhen, China.
2.2. Surface Modification
The specimens were manually polished to remove superficial contaminants and the original oxidation layer, and to obtain a smooth surface. Subsequently, the samples were ultrasonically cleaned in the following sequence: acetone, 70% ethanol, de-ionized water, and then air-drying at room temperature. Then, the samples were treated with UV light (365 nm) for 30 min and randomly allocated into 4 groups. A fresh polyhexamethylene biguanide solution was freshly prepared at concentrations of 0.2%, 0.5% and 1.0% (w/v), and the analogous samples were designated respectively as Ti-0.2, Ti-0.5 and Ti-1.0. Afterwards, 108 Ti samples (n = 27 for each group, 3 for SEM/EDX, 3 for FTIR, 3 for EDX, 18 for the microbiological test) were randomly selected and immersed in the respective PHMB solutions at room temperature for 24 h immediately after the UV-treatment. Afterwards, the Ti samples were taken out and then dried at room temperature until both the water and solvent left on the surface of the samples evaporated. The titanium samples which were not immersed in PHMB were designated as Ti-UV as a positive control group. Each sample was placed in the cavity of a 24-well tissue culture plate. The plates were sterilized by Gamma ray with 25 kGy before use.
2.3. Morphological and Structural Surface Analysis
Scanning electron microscopy (SEM) (Hitachi SU-1510, Tokyo, Japan) was utilized to obtain surface images (magnification 500X, voltage 15.00 kV). Energy-dispersive X-ray spectroscopy (EDX) (IXRF systems, Inc., Austin, TX, USA) was used to measure the elemental composition of the titanium surface. A random sample chosen from each group—Ti-UV, Ti-0.2, Ti-0.5 and Ti-1.0—was examined with SEM. Fourier transform infrared spectroscopy (FTIR) was used to analyze the surface of the pretreated titanium samples. One random sample from each of the groups Ti-UV, Ti-0.2, Ti-0.5 and Ti-1.0 was examined with the FTIR spectrometer (PerkinElmer UATR TWO, Waltham, MA, USA). Meanwhile, the ultra-high vacuum chamber of an X-ray photoelectron spectrometer (X-ray Photoelectron Spectroscopy/ESCA, Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to analyze the elements of the obtained samples.
2.4. Microbiological Analysis
The anaerobic, gram-negative bacteria Fusobacterium nucleatum (F. nucleatum) ATCC 25586 was utilized as a test organism for this research. The bacteria suspension was adjusted to 107 colony-forming unit (CFU) per ml. A total of 1.0 mL of the bacteria suspension was syringed in each sample in the 24-well plates and incubated in the anaerobic incubator (Forma Anaerobic System, Thermo Fisher Scientific Inc., USA) for the formation of biofilm on the titanium surface for 4 and 7 days, similar to a previous study [32], using 9 samples for each group.
After incubation, the samples were carefully moved to the new 24-well plates, and each sample was washed with 1.0 mL of sterile PBS to remove the unattached bacterial cells. Then, the samples were transferred into a clean tube with 1 mL sterile mixture solution of Tryptic Soy broth and Yeast Extract and vortexed to ensure the attached bacterial cells were washed down in the solution. Serial dilutions were prepared in a 1.5 mL microcentrifuge tube (Eppendorf Tubes® 3810X, Eppendorf AG, Hamburg, Germany) and 0.05 mL of each dilution was plated in duplicate on a blood agar medium with a spiral plater (Autoplate®, Spiral Biotech Inc., Norwood, MA, USA). The petri dishes were incubated in the anaerobic incubator (Forma Anaerobic System, Thermo Fisher Scientific Inc., Waltham, MA, USA) at 37 °C for 3 days and the CFU of F. nucleatum was counted.
2.5. Statistical Analysis
The microbiological results were first analyzed with the Kolmogorov-Smirnov test for normality, and then analyzed using Kruskal Wallis and multiple Mann-Whitney U tests to examine the effects (day and PHMB concentration) and the group difference, respectively, at α = 0.05. The data were analyzed with the software SPSS v.20.0 (IBM Corp, New York City, NY, USA).
3. Results
3.1. Morphological and Structural Characterization
3.1.1. SEM and EDX Analysis
The morphology and elemental composition of the titanium surface were investigated separately. As can be seen from Figure 1, no visible difference was observed among the different surface images when comparing PHMB-coated titanium to the control or among the experimental groups coated with PHMB in different concentrations. In addition, it can be seen from the element content diagram (Figure 2), that the compositions of C, N, O, and Ti on the sample surfaces were similar. Although the percentage of titanium was the highest in Ti-UV, the differences between the samples were very small.
Figure 1.
Surface morphology (magnification 500X) of different samples: (a) Ti-UV; (b) Ti-0.2; (c) Ti-0.5; (d) Ti-1.0.
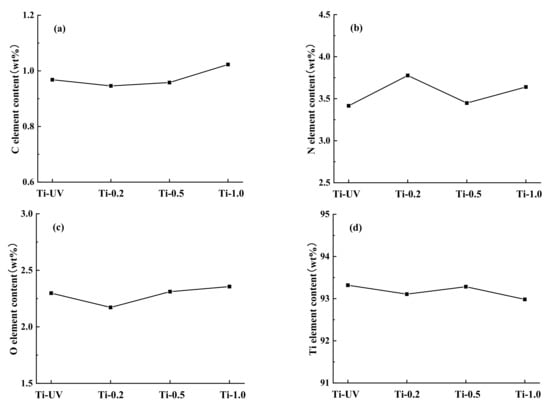
Figure 2.
Element content of different samples: (a) carbon; (b) nitrogen; (c) oxygen; (d) titanium.
3.1.2. FTIR Analysis
The surface chemical structure of the Ti and PHMB-coated Ti samples were analyzed by Fourier transform infrared spectroscopy. The results of the FTIR analysis showed that the Ti-0.2, Ti-0.5 and Ti-1.0 have a biguanide group (represented by a C=N bond) of PHMB together with an OH-N hydrogen bond on the surface of the titanium (Figure 3). The FTIR spectra of PHMB biocides showed biguanide group signals in the range of 1750–1550 cm−1, mainly consisting of a C=N (carbon–nitrogen bond) stretching band at 1630 cm−1 and N-H (nitrogen-hydrogen bond). The OH-N hydrogen bond was observed in the region of 3150–3450 cm−1, which should be formed by hydroxyl groups on the titanium surface and nitrogen from PHMB. This shows that PHMB was successfully coated on the titanium surface. In addition, the Ti-UV sample revealed none of these signals.
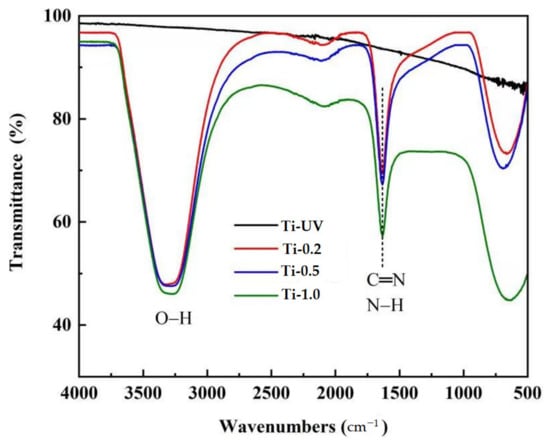
Figure 3.
Fourier transform infrared spectroscopy of different Ti samples.
3.1.3. XPS Analysis
As shown in Figure 4a–d, X-ray photoelectron spectroscopy (XPS) was used to analyze the top layers’ atomic percentage of all four groups after modification. The XPS analysis confirmed that the surface chemistry was altered by the PHMB treatments. Compared to the control group, the peak intensities of C1s and N1s increased while the Ti2p decreased with the increase of PHMB concentration (Figure 4b–d). Correspondingly, it is also shown in Figure 4e that the spectra of Ti2p decreased obviously by atomic percentage after the modification treatment; PHMB covers the surface of titanium, causing the reduction of surface atoms. Compared to Ti-UV, the Ti2P peak of the PHMB-modified Ti (458.7 eV) shifted slightly to the lower binding energy (band bending of ~0.04 eV).
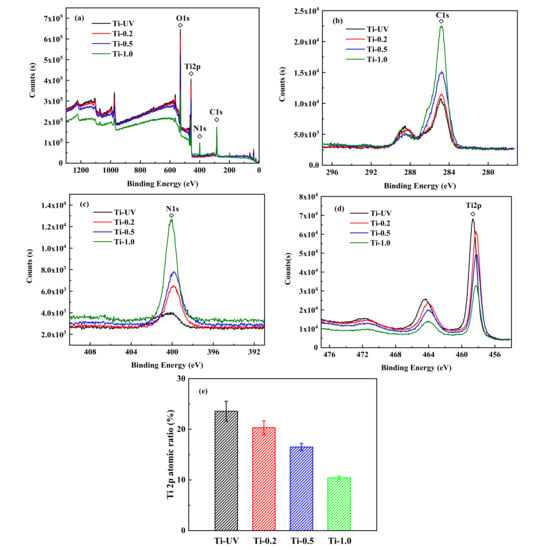
Figure 4.
XPS Spectra of Ti samples modified with different concentrations of PHMB (a), XPS Spectra of C1s (b), N1s (c), Ti2p (d), and variation of the surface atomic ratio of the Ti (e).
3.2. Antibacterial Effect
To evaluate the antibacterial effect of PHMB-coated titanium, CFU counts were conducted at the observation time points at days 4 and 7. All the data were converted to log10, and the normality test showed the data were not normally distributed (p = 0.014). The Kruskal-Wallis test showed a statistically significant difference in CFU counts between groups (p < 0.001); the coating (p < 0.001) was a significant effect, but not the days (p = 0.383).
The results of the CFU test are shown in Figure 5. According to the Mann-Whitney U test, when comparing the same group over different days, all groups had no statistical difference, except Ti-0.5, which had a statistically lower CFU count on day 7 than day 4 (p = 0.036). Increasing the concentration of PHMB reduced the CFU counts on the same day, whereas Ti-0.5 (p = 0.043) and Ti-1.0 (p = 0.012) had statistically lower CFU counts than Ti-UV on day 4, and both Ti-0.5 and Ti-1.0 had statistically lower CFU counts (p < 0.05) than both Ti-UV and Ti-0.2 on day 7. Overall, Ti-0.5 and Ti-1.0 exhibited a sustainable antibacterial effect up to 7 days.
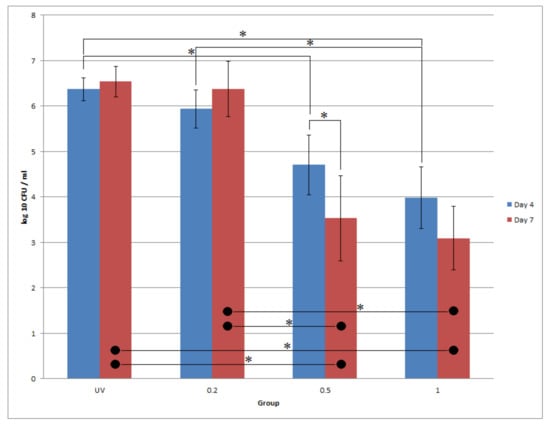
Figure 5.
log10 CFU values for different PHMB-Ti with different times. Blue bar: day 4; red bar: day 7. * denotes statistical significance of two groups at α = 0.05 with the Mann-Whitney U test.
4. Discussion
Despite titanium’s well-documented optimal characteristics [2,3], easy and novel strategies that can further improve bone healing and inhibit bacterial adherence should be investigated. In this study, PHMB-coated titanium was tested to explore its antibacterial properties.
Considering the surface analysis methods performed, the FTIR analysis demonstrated specific signals congruent with biguanide groups and typical a C=N bond at 1750–1550 cm−1 and 1630 cm−1, respectively [33], on the surface of Ti-0.2, Ti-0.5 and Ti-1.0. The OH-N hydrogen bond was observed in the region of 3150–3450 cm−1. From this study, it is possible to conclude that PHMB successfully coated the titanium surface. While the chemical mechanism remains unknown, this successful coating may be due to PHMB forms hydrogen bonding or physiochemical adsorption with UV-treated Ti. The guanidine group in PHMB has high activity, which can make the polymer become positively charged; it is easily absorbed by the bacteria, which are negatively charged, thereby inhibiting the division function of bacteria and causing them to lose their reproductive ability. The film formed by the polymer blocks the breathing channels of the bacteria, causing the bacteria to suffocate and die quickly (as shown in Figure 6).
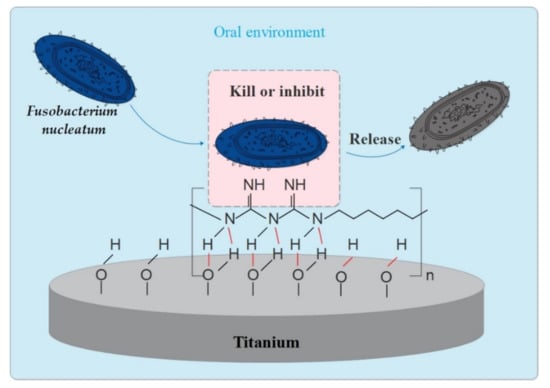
Figure 6.
Anti-F. nucleatum action model of PHMB-coated Ti for i) illustration of hydrogen bonding (red lines) or physiochemical adsorption between PHMB and UV-treated Ti; ii) the F. nucleatum was killed or inhibited by the Guanidine group on PHMB.
SEM and EDX showed no comparable difference between the PHMB-coated and UV-treated titanium either in the surface topography or the elemental composition. The negative results of SEM and EDX might be a result of the amount of PHMB coated on the surface, which might have been so low that it was difficult to detect using these methods. Nevertheless, this result also confirms the coating did not cause a significant change in the morphography of the titanium surface.
In order to evaluate the antibacterial properties of PHMB-coated implant surfaces, the CFU of F. nucleatum was assessed. This bacteria is a known periodontal pathogen and has also been strongly associated with peri-implantitis [34,35]. Previous studies addressing the antimicrobial properties of implants have similarly investigated the presence of this bacteria [36,37]. Indeed, peri-implantitis is a man-made infectious disease that is affecting 22% of the population [38]. This study provided and utilized a simple approach to decrease the occurrence of reinfection. We cannot not kill all the bacteria in oral condition; however, we should maintain a balance with oral microbes, and thus a localized antibacterial function on the titanium surface could be the way forward.
Mouthrinses containing PHMB showed higher efficacy of antibacterial effect in previous studies; there was a significant reduction of bacterial counts on the tooth surface with PHMB compared to the placebo after 4 h and after 5 days, indicating PHMB could be used in the prevention of plaque-related disease [39]. Another randomized clinical study showed similar results, with commercial 0.2% PHMB can be significantly more effective at inhibiting plaque re-growth and reducing bacterial count (for both tooth surface and mucosa) than the placebo rinse (10% ethanol, flavor) [40]. In this study, there was a reduction of the bacteria counts in the PHMB-coated titanium groups compared with the UV group using CFU counts, which is in accordance with previous observations relating to PHMB’s antibacterial efficacy [41]. A better antibacterial effect was exhibited with the increase of the PHMB concentration; Ti-0.5 and Ti-1.0 showed the best performance when compared with Ti-UV and Ti-0.2 in terms of anti-bacterial effect at specific durations. Nonetheless, it should be noted that this proof-of-concept study only showed the UV-treated titanium surface can be coated with various percentages of PHMB and retained for certain time, which could be a strategy for maintaining a low occurrence of F. nucleatum and possibly preventing re-infection on the titanium. This kind of easy self-assembled coating can also be applied to different titanium-based medical applications, such as orthodontics [42,43], dentofacial orthopaedics [44,45], and other implant medical devices [5] that are prone to infection. This variety of applications might need different concentrations for the optimal application of PHMB. Further studies are necessary.
There are two major limitations in this study that could be addressed in future research. First, future study should be focused on the antibacterial properties against other bacterial species that have a causative relation with peri-implantitis, as one species may not be enough to be clinically advantageous. Second, it is key to complement dental implant research comprehensively with osseointegration studies, residual activity, cytotoxicity and systematic risks in regard to surface-treated PHMB Ti implants so as to confirm the best concentration for this material. Clinical efficacy, the relationship between peri-implant mucositis, and the lifetime, e.g., dissolution of the coating, should also be further investigated.
5. Conclusions
In this study, the influence of PHMB coated titanium on the structure, surface properties, and antibacterial properties were investigated systematically. It was found that PHMB-coated titanium had a strong capability of inhibiting F. nucleatum. Moreover, the antibacterial capability of PHMB-coated titanium is dose-dependent. Even though differences were found among the several concentrations, PHMB exhibited promising results as a coating strategy for titanium dental implants.
Author Contributions
Conceptualization, J.Z. and J.K.-H.T.; methodology, J.Z., J.K.-H.T., A.H.S.D., and J.P.M.; formal analysis, J.Z. and S.J.; investigation, J.Z. and S.J.; resources, J.K.-H.T.; data curation, J.K.-H.T.; writing—original draft preparation, J.Z., S.J. and A.H.S.D.; writing—review and editing, Z.C., S.J. and J.K.-H.T.; visualization, J.Z. and S.J.; supervision, J.K.-H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available by corresponding author on request.
Acknowledgments
This publication was a part of the MSc Thesis by J.Z., submitted to the University of Faculty of Dentistry, the University of Hong Kong.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanif, A.; Qureshi, S.; Sheikh, Z.; Rashid, H. Complications in implant dentistry. Eur. J. Dent. 2017, 11, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branemark, P.I. Vital microscopy of bone marrow in rabbit. Scand. J. Clin. Lab. Investig. 1959, 11, 1–82. [Google Scholar]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet Neuronal Interact. 2009, 9, 61–71. [Google Scholar] [PubMed]
- Fukushima, A.; Mayanagi, G.; Nakajo, K.; Sasaki, K.; Takahashi, N. Microbiologically Induced Corrosive Properties of the Titanium Surface. J. Dent. Res. 2014, 93, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tsoi, J.K.; Tsang, P.C.; Park, Y.-J.; Song, H.-J.; Matinlinna, J.P. Candida albicans aspects of binary titanium alloys for biomedical applications. Regen. Biomater. 2020, 7, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Siddiqi, A.; Payne, A.G.; De Silva, R.K.; Duncan, W.J. Titanium allergy: Could it affect dental implant integration? Clin. Oral Implant. Res. 2011, 22, 673–680. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E.; Newman, M.G.; Nyman, S. Clinical and microbiologic findings that may contribute to dental implant failure. Int. J. Oral Maxillofac. Implant. 1990, 5, 31–38. [Google Scholar]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, L.F.; Watt, R.M.; Mattheos, N.; Si, M.S.; Lai, H.C.; Lang, N.P. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and peri-implant tissues. Clin. Oral Implant. Res. 2016, 27, 13–21. [Google Scholar] [CrossRef]
- Ghensi, P.; Manghi, P.; Zolfo, M.; Armanini, F.; Pasolli, E.; Bolzan, M.; Bertelle, A.; Dell’Acqua, F.; Dellasega, E.; Waldner, R.; et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Vander Haar, E.L.; So, J.; Gyamfi-Bannerman, C.; Han, Y.W. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe 2018, 50, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K.; Baba, Y.; Nakagawa, S.; Mima, K.; Miyake, K.; Nakamura, K.; Sawayama, H.; Kinoshita, K.; Ishimoto, T.; Iwatsuki, M.J. Human microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosis. Clin. Cancer Res. 2016, 22, 5574–5581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgård, J.E.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.J.F.i.c.; et al. Colon cancer-associated Fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front. Cell. Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- De Avila, E.D.; Lima, B.P.; Sekiya, T.; Torii, Y.; Ogawa, T.; Shi, W.; Lux, R. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials 2015, 67, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.G.; Seo, S.-J.; Lee, J.-H.; Kim, H.-W.J. On-site surface functionalization for titanium dental implant with nanotopography: Review and outlook. J. Nanomater. 2016, 2016, 3429532. [Google Scholar] [CrossRef] [Green Version]
- Hubner, N.O.; Kramer, A. Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Ski. Pharmacol. Physiol. 2010, 23, 17–27. [Google Scholar] [CrossRef]
- Fjeld, H.; Lingaas, E. Polyhexanide—Safety and efficacy as an antiseptic. Tidsskr Nor Legeforen 2016, 136, 707–711. [Google Scholar] [CrossRef]
- Fiscella, R.G.; Moshifar, M.; Messick, C.R.; Pendland, S.L.; Chandler, J.W.; Viana, M. Polyhexamethylene biguanide (PHMB) in the treatment of experimental Fusarium keratomycosis. Cornea 1997, 16, 447–449. [Google Scholar] [CrossRef]
- Lin, J.C.; Ward, T.P.; Belyea, D.A.; McEvoy, P.; Kramer, K.K. Treatment of Nocardia asteroides keratitis with polyhexamethylene biguanide. Ophthalmology 1997, 104, 1306–1311. [Google Scholar] [CrossRef]
- Azuara-Blanco, A.; Sadiq, A.S.; Hussain, M.; Lloyd, J.H.; Dua, H.S. Successful medical treatment of Acanthamoeba keratitis. Int. Ophthalmol. 1997, 21, 223–227. [Google Scholar] [CrossRef]
- Duguid, I.G.; Dart, J.K.; Morlet, N.; Allan, B.D.; Matheson, M.; Ficker, L.; Tuft, S. Outcome of acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology 1997, 104, 1587–1592. [Google Scholar] [CrossRef]
- Seal, D.J. Treatment of Acanthamoeba keratitis. Cornea 2003, 1, 205–208. [Google Scholar] [CrossRef]
- Walochnik, J.; Duchene, M.; Eibl, H.; Aspock, H. Treatment of Acanthamoeba keratitis: Possibilities, problems, and new approaches. Wien. Klin. Wochenschr. 2003, 115, 10–17. [Google Scholar]
- Souza, C.; Watanabe, E.; Borgheti-Cardoso, L.N.; Fantini, M.C.D.A.; Lara, M.G.J. Mucoadhesive system formed by liquid crystals for buccal administration of poly (hexamethylene biguanide) hydrochloride. J. Pharm. Sci. 2014, 103, 3914–3923. [Google Scholar] [CrossRef] [PubMed]
- Broxton, P.; Woodcock, P.M.; Heatley, F.; Gilbert, P. Interaction of some polyhexamethylene biguanides and membrane phospholipids in Escherichia coli. J. Appl. Bacteriol. 1984, 57, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Ledwith, A.; Bamford, C.H.; Hann, R.A. Interaction of a polymeric biguanide biocide with phospholipid membranes. Biochim. Biophys. Acta BBA Biomembr. 1984, 769, 57–66. [Google Scholar] [CrossRef]
- Ikeda, T.; Tazuke, S.; Bamford, C.; Ledwith, A. Spectroscopic studies on the interaction of polymeric in-chain biguanide biocide with phospholipid membranes as probed by 8-anilinonaphthalene-1-sulfonate. Bull. Chem. Soc. Jpn. 1985, 58, 705–709. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Tazuke, S.; Watanabe, M. Interaction of biologically active molecules with phospholipid membranes: I. Fluorescence depolarization studies on the effect of polymeric biocide bearing biguanide groups in the main chain. Biochim. Biophys. Acta Biomembr. 1983, 735, 380–386. [Google Scholar] [CrossRef]
- Muller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [Green Version]
- Creppy, E.E.; Diallo, A.; Moukha, S.; Eklu-Gadegbeku, C.; Cros, D. Study of epigenetic properties of Poly(HexaMethylene Biguanide) hydrochloride (PHMB). Int. J. Environ. Res. Public Health 2014, 11, 8069–8092. [Google Scholar] [CrossRef] [Green Version]
- Koburger, T.; Hübner, N.-O.; Braun, M.; Siebert, J.; Kramer, A.J. Standardized comparison of antiseptic efficacy of triclosan, PVP–iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother. 2010, 65, 1712–1719. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tsui, K.H.; Tsoi, J.K.H.; Green, D.W.; Jin, X.Z.; Deng, Y.Q.; Zhu, Y.M.; Li, X.G.; Fan, Z.; Cheung, G.S. A nanostructured anti-biofilm surface widens the efficacy against spindle-shaped and chain-forming rod-like bacteria. Nanoscale 2020, 12, 18864–18874. [Google Scholar] [CrossRef]
- Ashraf, S.; Akhtar, N.; Ghauri, M.A.; Rajoka, M.I.; Khalid, Z.M.; Hussain, I.J. Polyhexamethylene biguanide functionalized cationic silver nanoparticles for enhanced antimicrobial activity. Nanoscale Res. Lett. 2012, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-N.; Cho, E.; Kim, H.-S.; Kim, D.-S.; Jung, J.; Baek, J.-H.; Kyong Lim, Y.; Jo, E.; Chang, Y.-H.; Hwan Shin, J.J. Draft genome sequence of Fusobacterium nucleatum subsp. animalis ChDC F324, isolated from a human subgingival plaque in the republic of Korea. Genome Announc. 2013, 1, e01042-13. [Google Scholar] [CrossRef] [Green Version]
- Albandar, J.M.; Brown, L.J.; Löe, H.J. Putative periodontal pathogens in subgingival plaque of young adults with and without early-onset periodontitis. J. Periodontol. 1997, 68, 973–981. [Google Scholar] [CrossRef]
- Sbordone, L.; Barone, A.; Ramaglia, L.; Ciaglia, R.N.; Iacono, V.J. Antimicrobial susceptibility of periodontopathic bacteria associated with failing implants. J. Periodontol. 1995, 66, 69–74. [Google Scholar] [CrossRef]
- Suketa, N.; Sawase, T.; Kitaura, H.; Naito, M.; Baba, K.; Nakayama, K.; Wennerberg, A.; Atsuta, M.J.C. An antibacterial surface on dental implants, based on the photocatalytic bactericidal effect. Clin. Implant Dent. Relat. Res. 2005, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C.J.J. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Rosin, M.; Welk, A.; Kocher, T.; Majic-Todt, A.; Kramer, A.; Pitten, F.A. The effect of a polyhexamethylene biguanide mouthrinse compared to an essential oil rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque regrowth. J. Clin. Periodontol. 2002, 29, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Welk, A.; Splieth, C.H.; Schmidt-Martens, G.; Schwahn, C.; Kocher, T.; Kramer, A.; Rosin, M. The effect of a polyhexamethylene biguanide mouthrinse compared with a triclosan rinse and a chlorhexidine rinse on bacterial counts and 4-day plaque re-growth. J. Clin. Periodontol. 2005, 32, 499–505. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Y.; Wang, H.; Gao, Y.; Liu, X.; Wang, X.; Lin, T.J. Layer-by-layer assembly of antibacterial coating on interbonded 3D fibrous scaffolds and its cytocompatibility assessment. J. Biomed. Mater. Res. Part A 2012, 100, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, F.; Papoutsi, A.; Dianiskova, S.; Dalessandri, D.; Bonetti, S.; Tsoi, J.K.; Matinlinna, J.P.; Paganelli, C. Resistance to sliding in orthodontics: Misconception or method error? A systematic review and a proposal of a test protocol. Korean J. Orthod. 2018, 48, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Savoldi, F.; Visconti, L.; Dalessandri, D.; Bonetti, S.; Tsoi, J.; Matinlinna, J.; Paganelli, C. In vitro evaluation of the influence of velocity on sliding resistance of stainless steel arch wires in a self-ligating orthodontic bracket. Orthod. Craniofacial Res. 2017, 20, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, F.; Xu, B.; Tsoi, J.K.; Paganelli, C.; Matinlinna, J.P. Anatomical and mechanical properties of swine midpalatal suture in the premaxillary, maxillary, and palatine region. Sci. Rep. 2018, 8, 7073. [Google Scholar]
- Savoldi, F.; Tsoi, J.K.; Paganelli, C.; Matinlinna, J.P. Evaluation of rapid maxillary expansion through acoustic emission technique and relative soft tissue attenuation. J. Mech. Behav. Biomed. Mater. 2017, 65, 513–521. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).