Research on the Performance of Diamond-Like Carbon Coatings on Cutting Aluminum Alloy: Cutting Experiments and First-Principles Calculations
Abstract
1. Introduction
2. Experimental Details
2.1. Multilayer Films Deposition
2.2. Characterization Techniques
3. Results
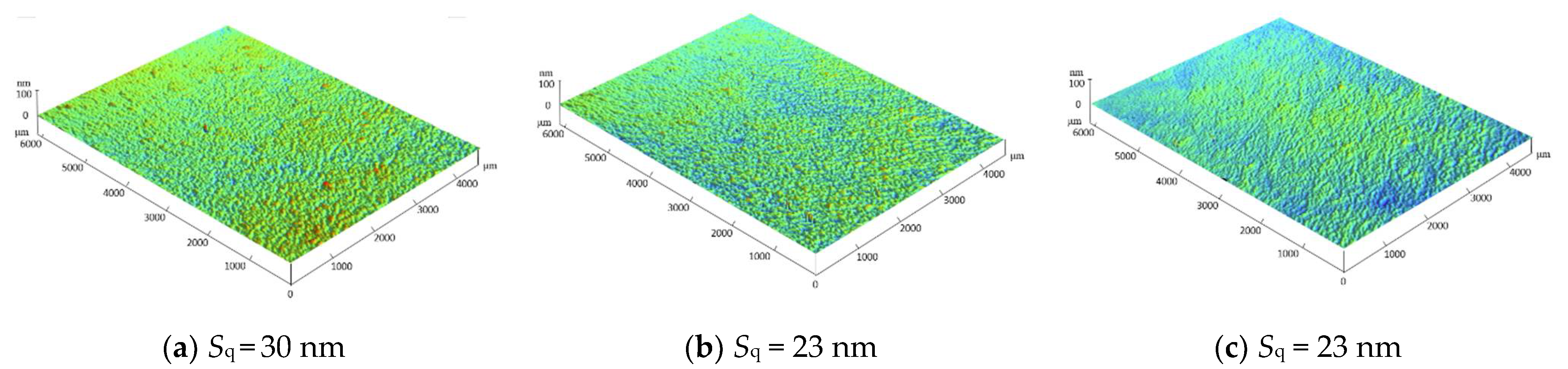
3.1. Morphology
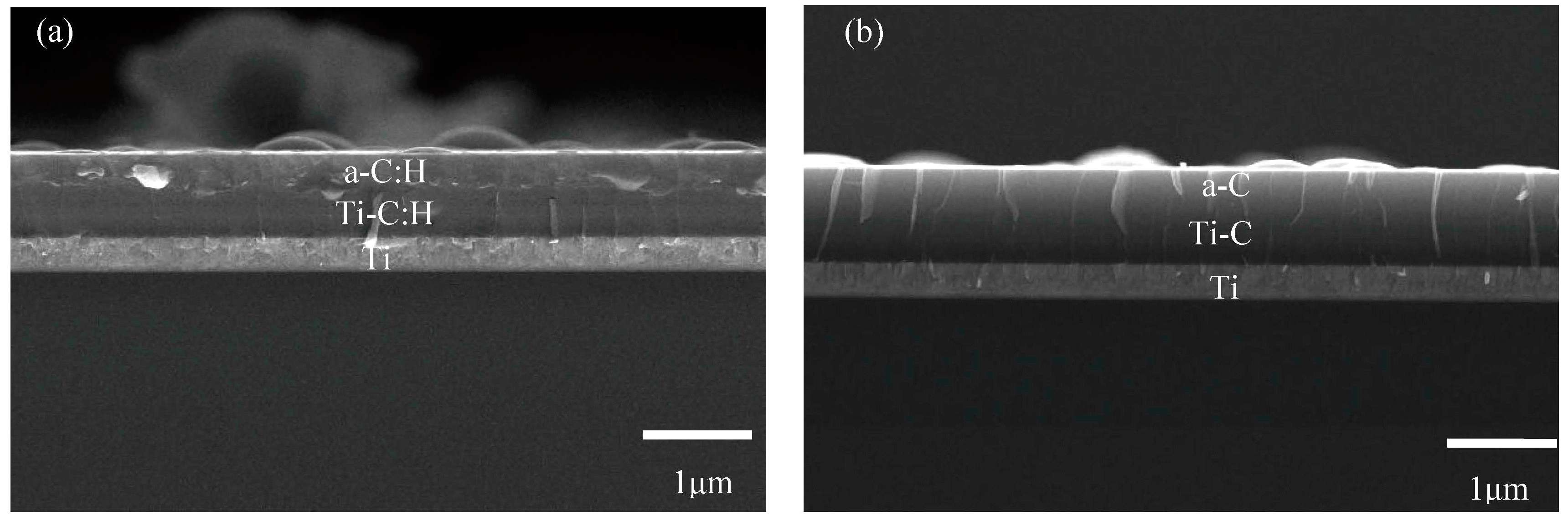
3.2. Cross-Section Topography
3.3. Microstructure
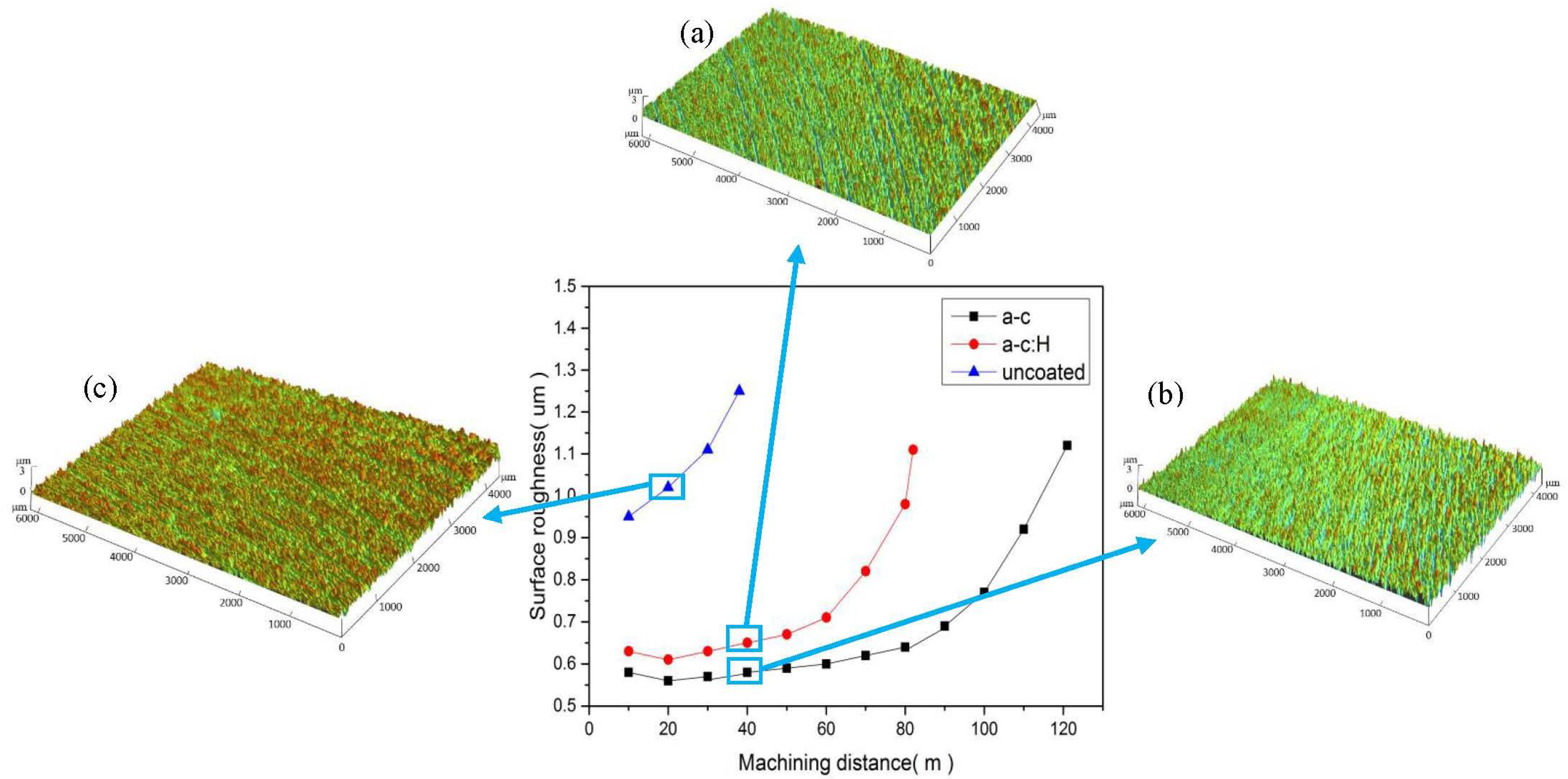
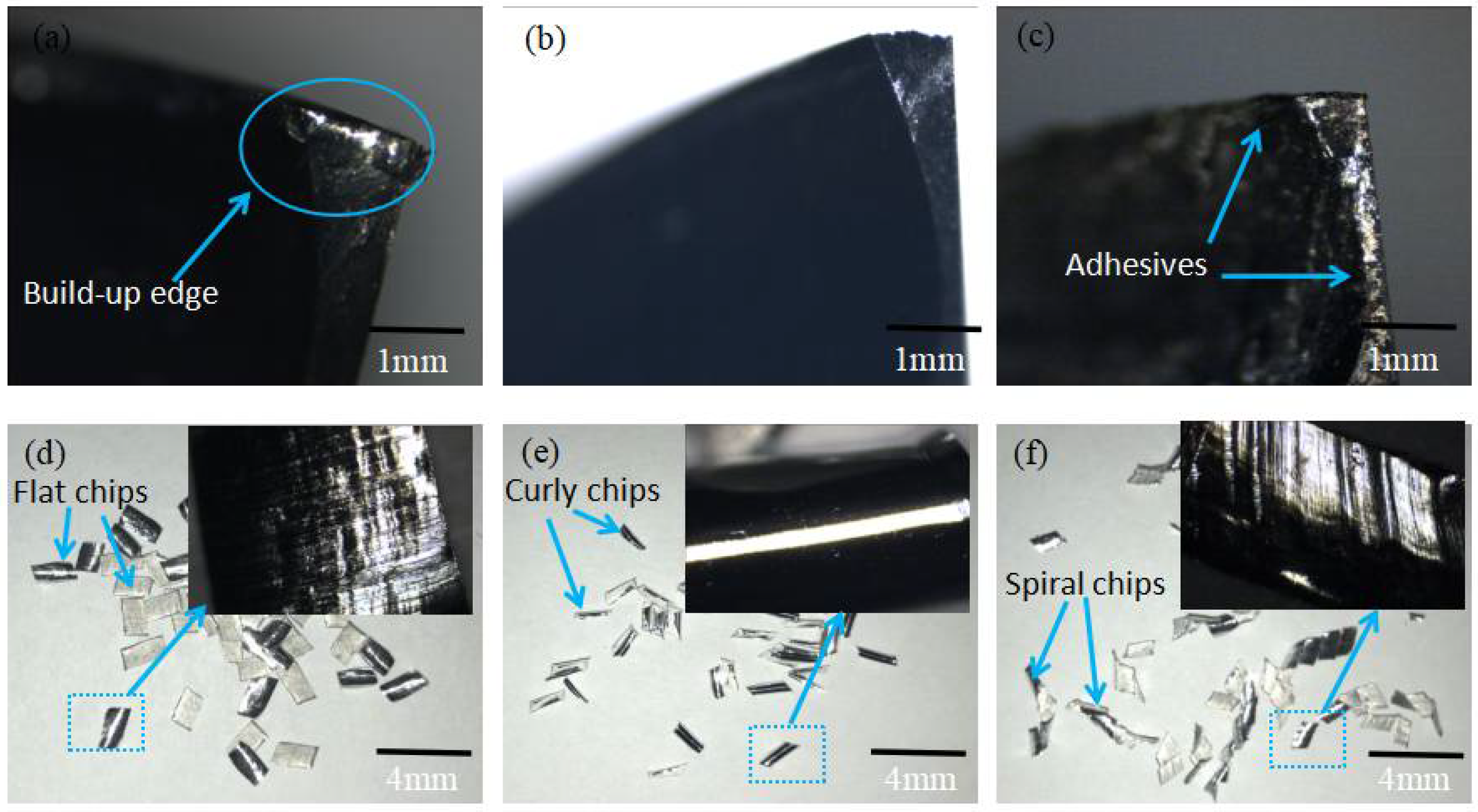
3.4. Cutting Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, C.J.; Kim, J.Y.; Lee, S.K.; Ko, D.C.; Kim, B.M. Design of mechanical clinching tools for joining of aluminium alloy sheets. Mater. Des. 2010, 31, 1854–1861. [Google Scholar] [CrossRef]
- Ahmad, R.; Wahab, N.A.; Hasan, S.; Harun, Z.; Rahman, M.M.; Shahizan, N.R. Effect of Erbium Addition on the Microstructure and Mechanical Properties of Aluminium Alloy. Key Eng. Mater. 2019, 796, 62–66. [Google Scholar] [CrossRef]
- Deshpande, V.S.; Fleck, N.A. High strain rate compressive behaviour of aluminium alloy foams. Int. J. Impact Eng. 2000, 24, 277–298. [Google Scholar] [CrossRef]
- Lingappa, M.S.; Srinath, M.S.; Amarendra, H.J. Microstructural and mechanical investigation of aluminium alloy (Al 1050) melted by microwave hybrid heating. Mater. Res. Express 2017, 4, 076504. [Google Scholar] [CrossRef]
- Shaikh, R.S.; Firfiray, B.A.; Pawaskar, S.H.; Khan, A.R.; Hafiz, Y. A Review Paper on the Surface Optimization of Aluminium Alloy in Milling. Int. J. Sci. Res. Dev. 2016, 4, 1523–1530. [Google Scholar]
- Adnan, A. The effect of cutting process on surface microstructure and hardness of pure and Al 6061 aluminium alloy. Eng. Sci. Technol. Int. J. 2015, 18, 303–308. [Google Scholar]
- Robertson, J. Hard amorphous (diamond-like) carbons. Prog. Solid State Chem. 1991, 21, 199–333. [Google Scholar] [CrossRef]
- Tallant, D.R.; Parmeter, J.E.; Siegal, M.P.; Simpson, R.L. The thermal stability of diamond-like carbon. Diam. Relat. Mater. 1995, 4, 191–199. [Google Scholar] [CrossRef]
- Pei, Y.T.; Galvan, D.; de Hosson, J.T.M. Tribological behavior and thermal stability of TiC/a-C:H nanocomposite coatings. Diam. Relat. Mater. 1995, 4, 191–199. [Google Scholar] [CrossRef][Green Version]
- Coldwell, H.L.; Dewes, R.C.; Aspinwall, D.K.; Renevier, N.M.; Teer, D.G. The use of soft/lubricating coatings when dry drilling BS L168 aluminium alloy. Surf. Coat. Technol. 2004, 177, 716–726. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Shockley, J.M.; Vo, P.; Chromik, R.R. Tribological Behavior of a Cold-Sprayed Cu–MoS2 Composite Coating During Dry Sliding Wear. Tribol. Lett. 2016, 62, 9. [Google Scholar] [CrossRef]
- Sugihara, T.; Enomoto, T. Improving anti-adhesion in aluminum alloy cutting by micro stripe texture. Precis. Eng. 2012, 36, 229–237. [Google Scholar] [CrossRef]
- Nizar, M.; Arimatsu, N.; Kawamitsu, H.; Takai, K.; Fukumoto, M. Study on optimal surface property of WC-Co cutting tool for aluminium alloy cutting. Mater. Sci. Eng. 2016, 114, 1–11. [Google Scholar] [CrossRef]
- Çakır, A.; Yağmur, S.; Kavak, N.; Küçüktürk, G.; Şeker, U. The effect of minimum quantity lubrication under different parameters in the turning of AA7075 and AA2024 aluminium alloys. Int. J. Adv. Manuf. Technol. 2016, 84, 2515–2521. [Google Scholar] [CrossRef]
- Dhar, N.R.; Kamruzzaman, M.; Ahmed, M. Effect of minimum quantity lubrication (MQL) on tool wear and surface roughness in turning AISI-4340 steel. J. Mater. Process. Technol. 2006, 172, 299–304. [Google Scholar] [CrossRef]
- Konca, E.; Cheng, Y.-T.; Weiner, A.M.; Dasch, J.M.; Alpas, A.T. Effect of test atmosphere on the tribological behaviour of the non-hydrogenated diamond-like carbon coatings against 319 aluminum alloy and tungsten carbide. Surf. Coat. Technol. 2005, 200, 1783–1791. [Google Scholar] [CrossRef]
- Shi, J.; Gong, Z.; Wang, Y.-F.; Gao, K.; Zhang, J. Friction and wear of hydrogenated and hydrogen-free diamond-like carbon films: Relative humidity dependent character. Appl. Surf. Sci. 2017, 422, 147–154. [Google Scholar] [CrossRef]
- Mabuchi, Y.; Higuchi, T.; Inagaki, Y.; Kousaka, H.; Umehara, N. Wear analysis of hydrogen-free diamond-like carbon coatings under a lubricated condition. Wear 2013, 298–299, 48–56. [Google Scholar] [CrossRef]
- Tyagi, A.; Walia, R.S.; Murtaza, Q.; Pandey, S.M.; Tyagi, P.K.; Bajaj, B. A critical review of diamond like carbon coating for wear resistance applications. Int. J. Refract. Met. Hard Mater. 2019, 78, 107–122. [Google Scholar] [CrossRef]
- Wang, L.; Cui, L.; Lu, Z.; Hui, Z. Understanding the unusual friction behavior of hydrogen-free diamond-like carbon films in oxygen atmosphere by first-principles calculations. Carbon 2016, 100, 556–563. [Google Scholar] [CrossRef]
- Modabberasl, A.; Kameli, P.; Ranjbar, M.; Salamati, H.; Ashiri, R. Fabrication of DLC thin films with improved diamond-like carbon character by the application of external magnetic field. Carbon 2015, 94, 485–493. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ji, L.; Wu, Y.-X.; Li, H.; Hui, S.; Liu, X.; Ye, Y.; Chen, J.; Zhou, H.; Liu, L. The role of trace Ti concentration on the evolution of microstructure and properties of duplex doped Ti(Ag)/DLC films. Vacuum 2015, 115, 23–30. [Google Scholar] [CrossRef]
- Huang, L.; Yuan, J.; Li, C.; Hong, D.-B. Microstructure, tribological and cutting performance of Ti-DLC/α-C:H multilayer film on cemented carbide. Surf. Coat. Technol. 2018, 353, 163–170. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, B.; Zhou, Y.; Zhang, J. Improving the internal stress and wear resistance of DLC film by low content Ti doping. Solid State Sci. 2013, 20, 17–22. [Google Scholar] [CrossRef]
- Tamor, M.A.; Vassell, W.C. Raman “fingerprinting” of amorphous carbon films. J. Appl. Phys. 1994, 76, 3823–3830. [Google Scholar] [CrossRef]
- Casiraghi, C.; Ferrari, A.C.; Robertson, J. Raman spectroscopy of hydrogenated amorphous carbons. Phys. Rev. B 2005, 72, 85401. [Google Scholar] [CrossRef]
- Hanyu, H.; Kamiya, S.; Murakami, Y.; Kondoh, Y. The improvement of cutting performance in semi-dry condition by the combination of DLC coating and CVD smooth surface diamond coating. Surf. Coat. Technol. 2005, 200, 1137–1141. [Google Scholar] [CrossRef]
- Fukui, H.; Okida, J.; Omori, N.; Moriguchi, H.; Tsuda, K. Cutting performance of DLC coated tools in dry machining aluminum alloys. Surf. Coat. Technol. 2004, 187, 70–76. [Google Scholar] [CrossRef]
- Vandevelde, T.C.S.; Vandierendonck, K.; Stappen, M.V.; Mong, W.D.; Perremans, P. Cutting applications of DLC, hard carbon and diamond films. Surf. Coat. Technol. 1999, 113, 80–85. [Google Scholar] [CrossRef]
- Hartley, K.A.; Duffy, J.; Hawley, R.H. Measurement of the temperature profile during shear band formation in steels deforming at high strain rates. J. Mech. Phys. Solids 1987, 35, 283–301. [Google Scholar] [CrossRef]
- Mason, J.J.; Rosakis, A.J.; Ravichandran, G. On the strain and strain rate dependence of the fraction of plastic work converted to heat: An experimental study using high speed infrared detectors and the Kolsky bar. Mech. Mater. 1994, 17, 135–145. [Google Scholar] [CrossRef]
- Potdar, Y.K.; Zehnder, A.T. Temperature and deformation measurements in transient metal cutting. Exp. Mech. 2004, 44, 1–9. [Google Scholar] [CrossRef]
- Longbottom, J.M.; Lanham, J.D. Cutting temperature measurement while machining-a review. Aircr. Eng. Aerosp. Technol. Int. J. 2005, 77, 122–130. [Google Scholar] [CrossRef]
- Silva, M.B.D.; Wallbank, J. Cutting temperature: Prediction and measurement methods—A review. J. Mater. Process. Technol. 1999, 88, 195–202. [Google Scholar] [CrossRef]
- Vernaza-Peña, K.M.; Mason, J.J.; Li, M. Experimental study of the temperature field generated during orthogonal machining of an aluminum alloy. Exp. Mech. 2002, 42, 221–229. [Google Scholar] [CrossRef]
- Sun, S.; Geng, Y.; Tian, L.; Chen, S.; Yan, Y.; Hu, S. Density functional theory study of imidazole, benzimidazole and 2-mercaptobenzimidazole adsorption onto clean Cu(1 1 1) surface. Corros. Sci. 2012, 63, 140–147. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Li, X.; Liu, X.; Zhang, G.; Lu, Z. Experimental and model studies about the lubrication of physisorbed isobutane molecules on hydrogenated diamond-like carbon films. Surf. Coat. Technol. 2019, 357, 759–767. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2010, 27, 1787–1799. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Sen, F.G.; Qi, Y.; Alpas, A.T. Material transfer mechanisms between aluminum and fluorinated carbon interfaces. Acta Mater. 2011, 59, 2601–2614. [Google Scholar] [CrossRef]
- Qi, Y.; Konca, E.; Alpas, A. Atmospheric effects on the adhesion and friction between non-hydrogenated diamond-like carbon (DLC) coating and aluminum–A first principles investigation. Surf. Sci. 2006, 600, 2955–2965. [Google Scholar] [CrossRef]
- Qi, Y.; Hector, L.G. Adhesion and adhesive transfer at aluminum/diamond interfaces:A first-principles study. Phys. Rev. B 2004, 69, 1681–1685. [Google Scholar] [CrossRef]
- Siegel, J.D.J.; Hector, L.G.; Adams, J.B. Ab initio study of Al-ceramic interfacial adhesion. Phys. Rev. B 2003, 67, 552–555. [Google Scholar] [CrossRef]
- Nosé, S.; Ubar, H. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 2002, 100, 191–198. [Google Scholar] [CrossRef]
- Jin, N.; Yang, Y.; Li, J.; Luo, X.; Huang, B.; Sun, Q.; Guo, P. First-principles calculation on β-SiC(111)/α-WC(0001) interface. J. Appl. Phys. 2014, 115, 5811–5836. [Google Scholar] [CrossRef]
- Segall, M.D.; Pickard, C.J.; Shah, R.; Pickard, M.C.P. Population analysis in plane wave electronic structure calculations. Mol. Phys. 1996, 89, 571–577. [Google Scholar] [CrossRef]
- Segall, M.D.; Shah, R.; Pickard, C.J. Population analysis of plane-wave electronic structure calculations of bulk materials. Phys. Rev. B 1996, 54, 16317–16320. [Google Scholar] [CrossRef]
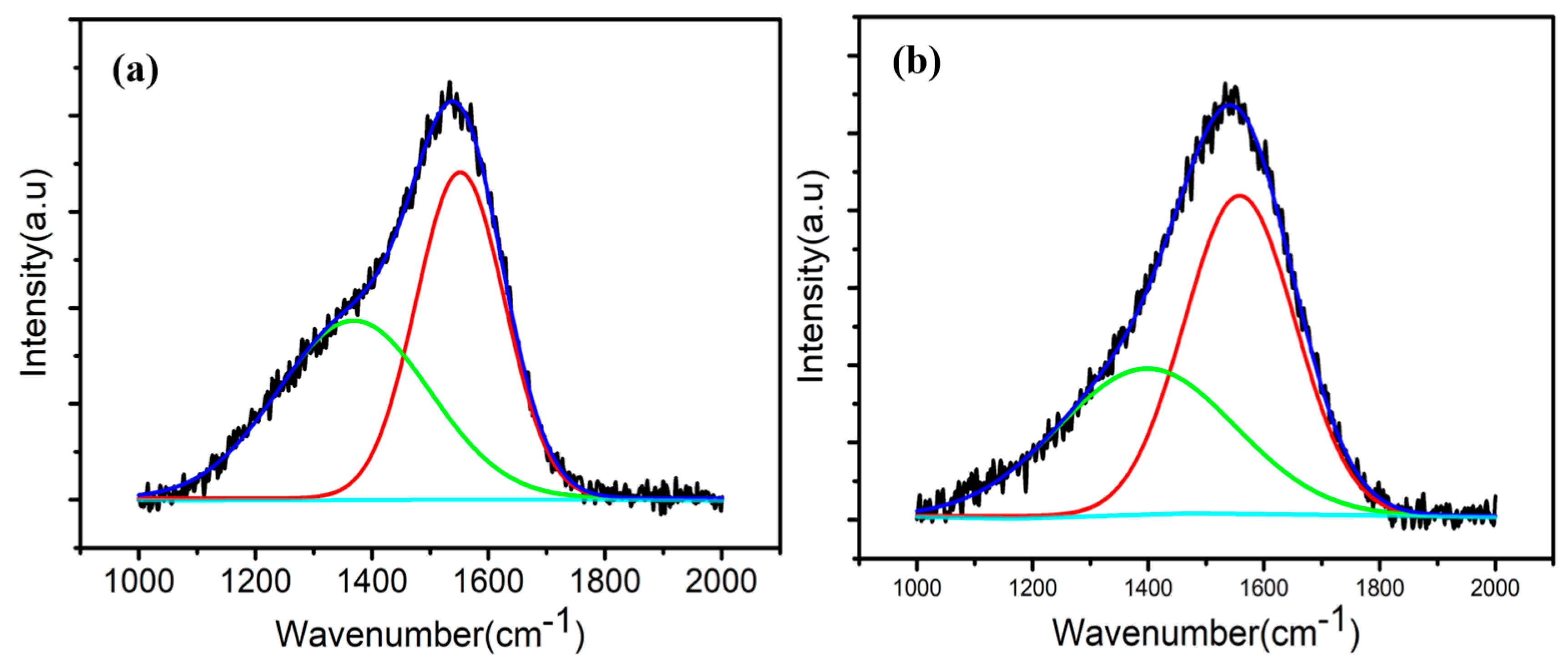


| Coatings | D-Peak Position (cm−1) | D-Peak FWHM (cm−1) | G-Peak Position (cm−1) | G-Peak FWHM (cm−1) | ID/IG |
|---|---|---|---|---|---|
| a-C:H | 1390 | 361 | 1558 | 225 | 0.74 |
| a-C | 1368 | 306 | 1551 | 178 | 0.93 |
| Interface | Interfacial Distance (Å) | Work of Adhesion (Wad) | |
|---|---|---|---|
| Unrelaxed | Fully Relaxed | ||
| a-C:H/Al | 1.81 | 1.52 | 5.27 J/m2 |
| a-C/Al | 1.81 | 1.74 | 4.21 J/m2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, B.; Zhang, E.-g.; Zhou, Q.; Lin, R.-c.; Du, H.-m. Research on the Performance of Diamond-Like Carbon Coatings on Cutting Aluminum Alloy: Cutting Experiments and First-Principles Calculations. Coatings 2021, 11, 63. https://doi.org/10.3390/coatings11010063
Huang B, Zhang E-g, Zhou Q, Lin R-c, Du H-m. Research on the Performance of Diamond-Like Carbon Coatings on Cutting Aluminum Alloy: Cutting Experiments and First-Principles Calculations. Coatings. 2021; 11(1):63. https://doi.org/10.3390/coatings11010063
Chicago/Turabian StyleHuang, Biao, Er-geng Zhang, Qiong Zhou, Rong-chuan Lin, and Hao-ming Du. 2021. "Research on the Performance of Diamond-Like Carbon Coatings on Cutting Aluminum Alloy: Cutting Experiments and First-Principles Calculations" Coatings 11, no. 1: 63. https://doi.org/10.3390/coatings11010063
APA StyleHuang, B., Zhang, E.-g., Zhou, Q., Lin, R.-c., & Du, H.-m. (2021). Research on the Performance of Diamond-Like Carbon Coatings on Cutting Aluminum Alloy: Cutting Experiments and First-Principles Calculations. Coatings, 11(1), 63. https://doi.org/10.3390/coatings11010063




