Study of the Adhesion of Silicate-Based Coating Formulations on a Wood Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Coatings
2.3. Rheological Measurements
2.4. Application of Coatings on Wood Substrate
2.5. Pretreatment of the Wood Samples
2.6. Characterization of the Samples
2.6.1. Pull-Off Adhesion Test of the Samples
2.6.2. Cross-Cut Analysis of the Samples
2.6.3. Scratch Resistance of the Samples
2.6.4. Resistance to Impact of the Samples
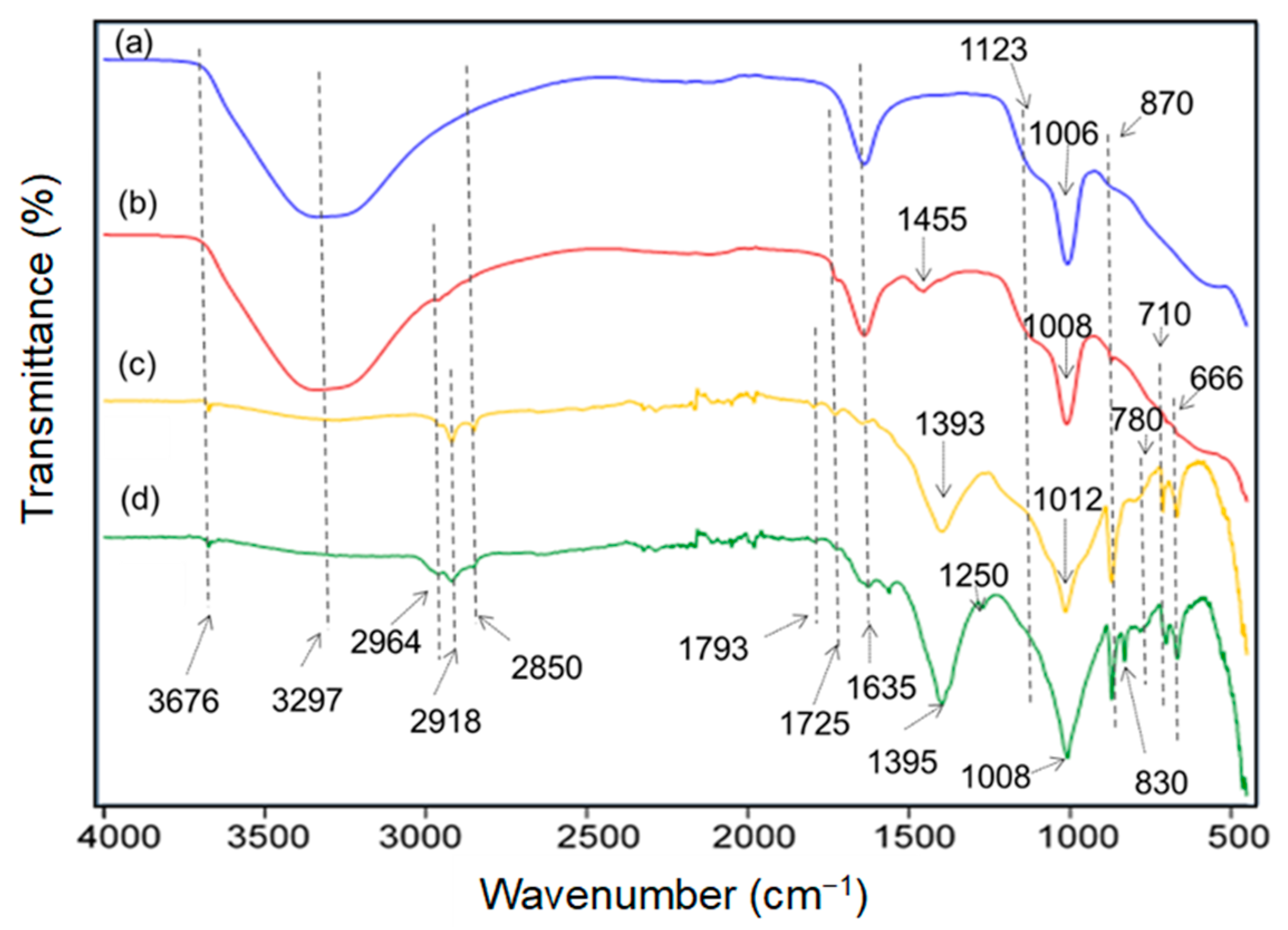
2.6.5. ATR-FTIR of the Samples
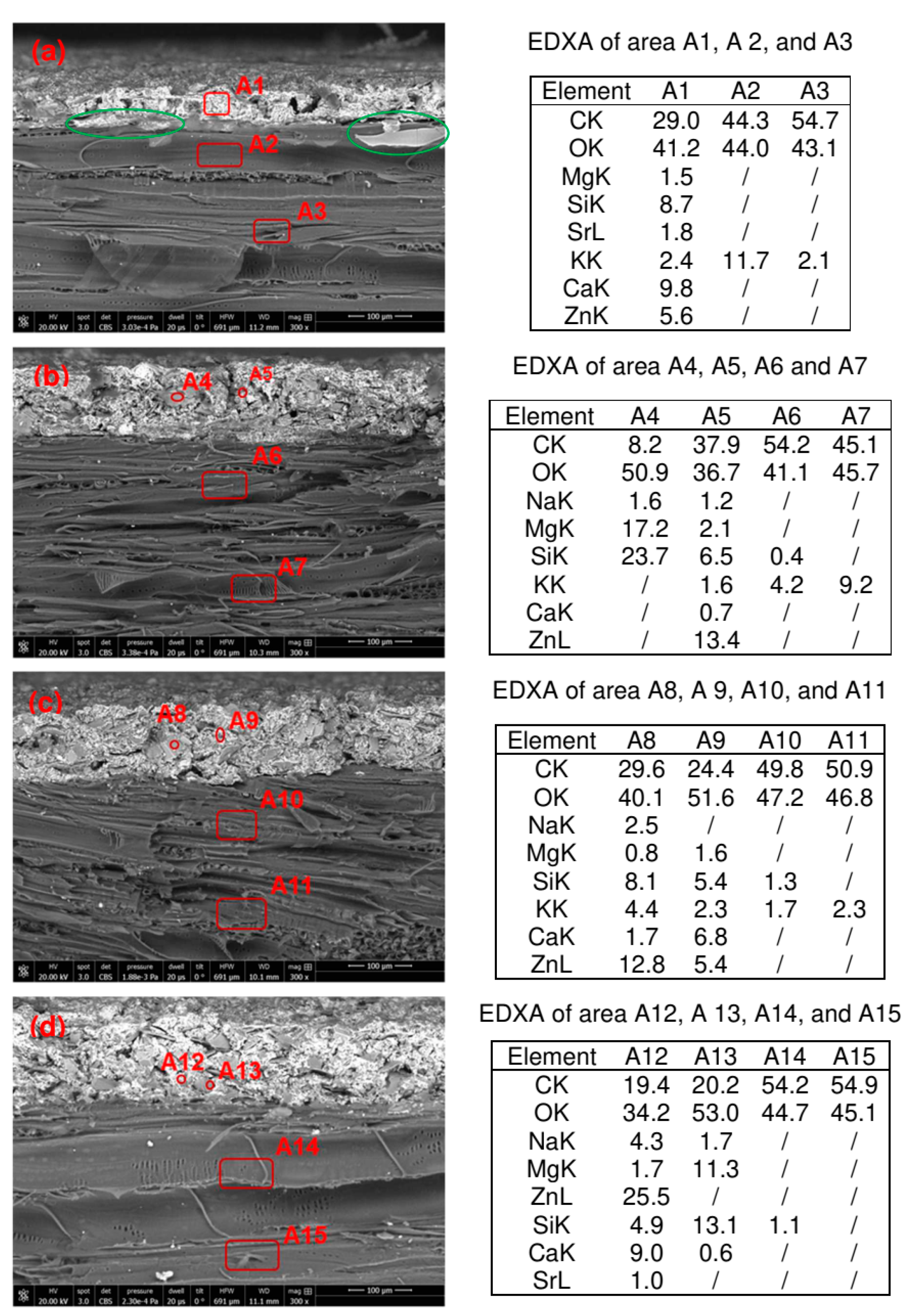
2.6.6. SEM-EDAX of the Samples
2.6.7. Confocal Laser Scanning Microscopy Analyses of the Samples
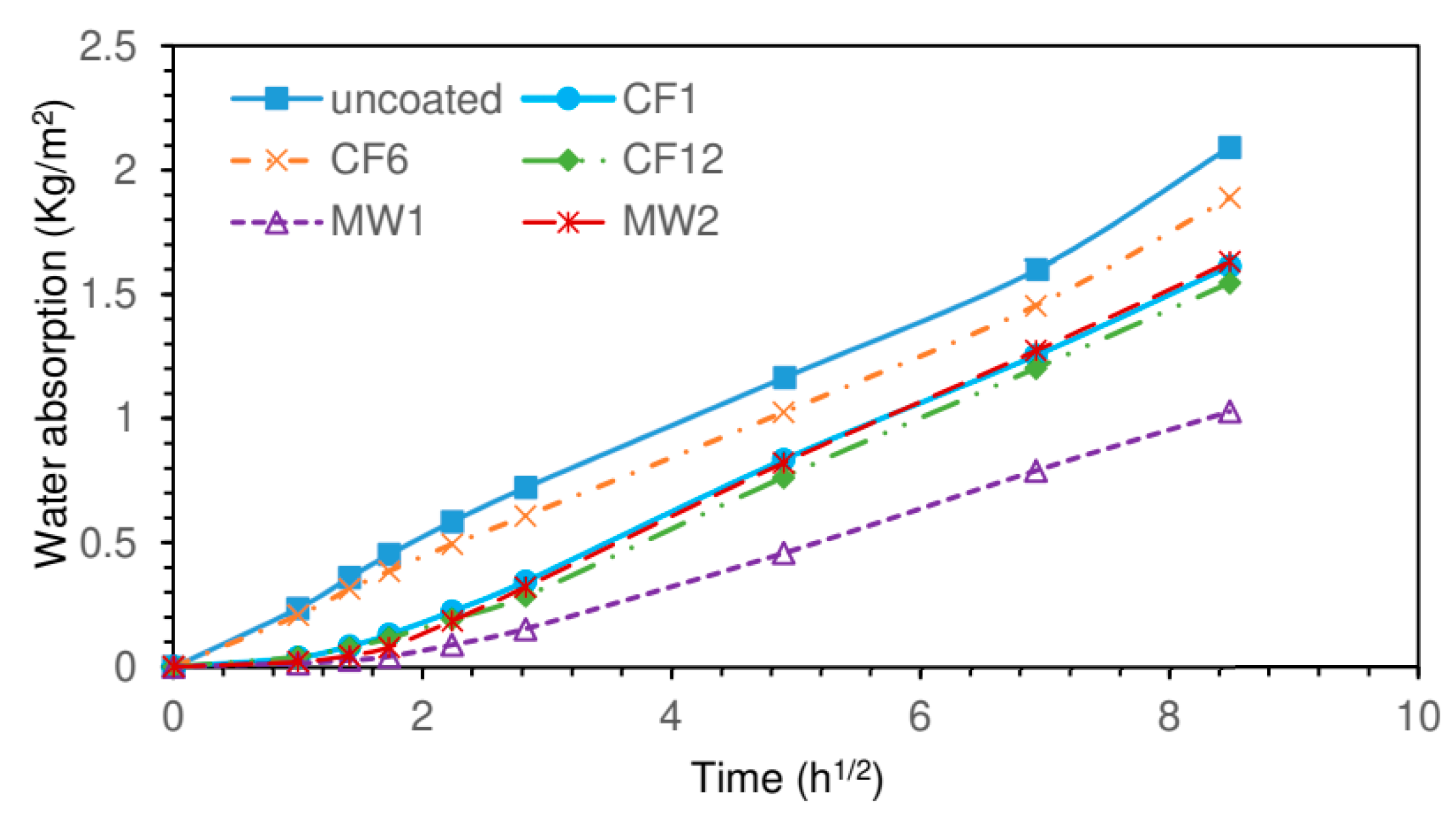
2.6.8. Water Absorption Measurements
3. Results and Discussion
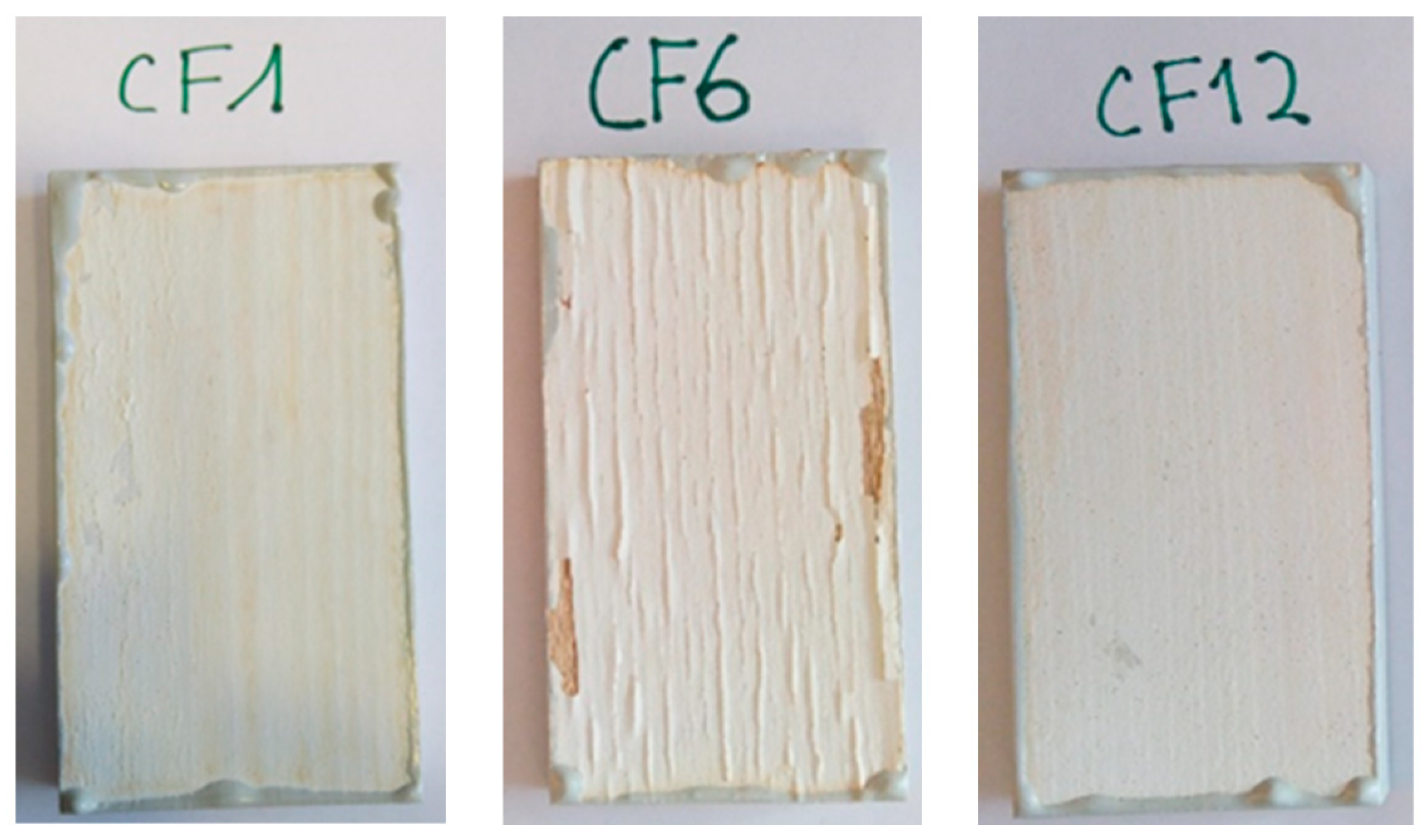
3.1. Coating Performances
3.2. Adhesion Mechanisms
3.3. ATR-FTIR Analyses
3.4. Influence of Wood Mineralization
3.5. Water Resistance of the Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popescu, C.-M.; Pfriem, A. Treatments and modification to improve the reaction to fire of wood and wood based products—An overview. Fire Mater. 2020, 44, 100–111. [Google Scholar] [CrossRef]
- Borges, C.C.; Denzin, T.G.H.; Moreira, C.T.; Duarte, P.J.; Junqueira, T.A. Nanoparticles-based wood preservatives: The next generation of wood protection? CERNE 2018, 24, 397–407. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, M.; Jia, Z.; Jiao, Y.; Cai, L.; Liang, D.; Li, J. Cu thin films on wood surface for robust superhydrophobicity by magnetron sputtering treatment with perfluorocarboxylic acid. Eur. J. Wood Prod. 2019, 77, 115–123. [Google Scholar] [CrossRef]
- Köhler, R.; Sauerbier, P.; Ohms, G.; Viöl, W.; Militz, H. Wood protection through plasma powder deposition—An alternative coating process. Forests 2019, 10, 898. [Google Scholar] [CrossRef]
- Parashar, G.; Bajpayee, M.; Kamani, P.K. Water-borne, non-toxic, high-performance inorganic silicate coatings. Surf. Coat. Int. B 2003, 86, 209–216. [Google Scholar] [CrossRef]
- Pal, S.; Contaldi, V.; Licciulli, A.; Marzo, F. Self-cleaning mineral paint for application in architectural heritage. Coatings 2016, 6, 48. [Google Scholar] [CrossRef]
- Loganina, V.I. Polymer silicate paints for interior decorating. Contemp. Eng. Sci. 2015, 8, 171–177. [Google Scholar] [CrossRef]
- Fraci, A.T. Waterborne Silicates in Coatings and Construction Chemicals in Inorganic Zinc Coatings, History, Chemistry, Properties, Applications and Alternatives, 2nd ed.; Francis, R.A., Ed.; The Australasian Corrosion Association Inc.: Victoria, Australia, 2013; pp. 227–258. [Google Scholar]
- Kumar, S.P.; Takamori, S.; Arakia, H.; Kuroda, S. Flame retardancy of clay–sodium silicate composite coatings on wood for construction purposes. RSC Adv. 2015, 5, 34109–34116. [Google Scholar] [CrossRef]
- Kazmina, O.; Lebedeva, E.; Mitina, N.; Kuzmenko, A. Fire-proof silicate coatings with magnesium-containing fire retardant. J. Coat. Technol. Res. 2018, 15, 543–554. [Google Scholar] [CrossRef]
- Ramasamy, S.; Hussin, K.; Al Bakri, M.M.A.; Ghazali, C.M.R.; Sandhu, A.V.; Binhussain, M.; Shahedan, N.F. Adhesiveness of kaolin based coating material on lumber wood. Key Eng. Mater. 2016, 673, 47–54. [Google Scholar] [CrossRef]
- Ramasamy, S.; Al Bakri Abdullah, M.M.; Kamarudin, H.; Yue, H.; Jin, W. Improvement of kaolin based geopolymer coated wood substrates for use in NaOH molarity. Mater. Sci. Forum 2019, 967, 241–249. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Haque, S.; Sanjayan, J. Behavior of fly ash geopolymer as fire resistant coating for timber. J. Sustain. Cem. Based Mater. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Standard ISO 4624. Paints and Varnishes-Pull Off Test for Adhesion. 2016. Available online: https://www.iso.org/standard/62351.html (accessed on 5 January 2021).
- Standard EN ISO 2409. Paints and Varnishes-Cross Cut Test. 1997. Available online: https://www.iso.org/standard/76041.html (accessed on 5 January 2021).
- Standard EN ISO 1518-1: 2000 Paint and Varnishes-Scratch Test. Available online: https://standards.iteh.ai/catalog/standards/cen/a2ff84bf-9c58-4b9e-a651-4c3899ae56fa/en-iso-1518-2000 (accessed on 5 January 2021).
- Standard ISO 4211-4. Furniture-Tests for Surfaces-Assessment of Resistance to Impact. 1995. Available online: https://standards.iteh.ai/catalog/standards/sist/44c7b7b9-ec61-485e-8909-6e317e0e9f72/sist-iso-4211-4-1995 (accessed on 5 January 2021).
- Chen, G.C. Treatment of wood with polysilicic acid derived from sodium silicate for fungal decay protection. Wood Fiber Sci. 2009, 410, 220–228. [Google Scholar]
- Furuno, T.; Shimada, K.; Uehara, T.; Jodai, S. Combinations of wood and silicate II. Wood-mineral composites using water glass and reactants of barium chloride, boric acid, and borax, and their properties. Mokuzai Gakkaishi 1992, 38, 448–457. [Google Scholar]
- Thougaard, L.; Hayden, J.P. Wood Preservation Method Using Sodium Silicate and Sodium Bicarbonate, WO 2014/101979 A2. Available online: https://patents.google.com/patent/WO2014101979A2/en (accessed on 5 January 2021).
- EN 927-5:2006—Paints and Varnishes—Coating Materials and Coating Systems for Exterior Wood—Part 5: Assessment of the Liquid Water Permeability. Available online: https://www.en-standard.eu/csn-en-927-5-paints-and-varnishes-coating-materials-and-coating-systems-for-exterior-wood-part-5-assessment-of-the-liquid-water-permeability/ (accessed on 5 January 2021).
- Meijer, M.D. A review of interfacial aspects in wood coatings: Wetting, surface energy, substrate penetration and adhesion. COST E18 Final Seminar; European Cooperation in Science and Technology: Brussels, Belgium, 2005. [Google Scholar]
- Sonmez, A.; Budakci, M.; Bayram, M. Effect of wood moisture content on adhesion of varnish coatings. Sci. Res. Essays 2009, 4, 1432–1437. [Google Scholar]
- Kesik, H.I.; Akyildiz, M.H. Effect of the heat treatment on the adhesion strength of water based wood varnishes. Wood Res. 2015, 60, 987–994. [Google Scholar]
- Hazir, E.; Koc, K.H. Evaluation of wood surface coating performance using water based, solvent based and powder coating. Maderas. Cienc. Tecnol. 2019, 21, 467–480. [Google Scholar] [CrossRef]
- Oblak, L.; Kričej, B.; Lipušček, I. The comparison of the coating systems according to the basis criteria. Wood Res. 2006, 51, 77–86. [Google Scholar]
- Çakıcıer, N.; Korkut, S.; Korkut, D.S. Varnish layer hardness, scratch resistance, and glossiness of various wood species as affected by heat treatment. BioResources 2011, 6, 1648–1658. [Google Scholar]
- Ahola, P. Adhesion between paints and wooden substrates: Effects of pre-treatments and weathering of wood. Mater. Struct. 1995, 28, 350–356. [Google Scholar] [CrossRef]
- De Meijer, M.; Van de Velde, B.; Militz, H. Rheological approach to the capillary penetration of coating into wood. J. Coat. Technol. 2001, 73, 39. [Google Scholar] [CrossRef]
- Furuno, T.; Uehara, T.; Jodai, S. Combinations of wood and silicate I. Impregnation by water glass and applications of aluminum sulfate and calcium chloride as reactants. Mokuzai Gakkaishi 1991, 37, 462–472. [Google Scholar]
- Pereyra, A.M.; Giudice, C.A. Flame-retardant impregnants for woods based on alkaline silicates. Fire Saf. J. 2009, 44, 497–503. [Google Scholar] [CrossRef]
- Arora, S.; Laha, A.; Majumdar, A.; Butola, B.S. Prediction of rheology of shear thickening fluids using phenomenological and artificial neural network models. Korea Aust. Rheol. J. 2017, 29, 185–193. [Google Scholar] [CrossRef]
- Ge, J.; Tan, Z.; Li, W.; Zhang, H. The rheological properties of shear thickening fluid reinforced with SiC nanowires. Results Phys. 2017, 7, 3369–3372. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.H.; Wang, P.; Zhu, W.L.; Chen, X.Y. The viscosity properties of zinc-rich coatings from sodium silicate solution modified with aluminium chloride. Pigment Resin Technol. 2009, 38, 153–158. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, W.; Yang, Q. The viscosity properties of sodium silicate solutions. J. Solut. Chem. 2008, 37, 73–83. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S. Characterizing and modeling the rheological performances of potassium silicate solutions. J. Solution Chem. 2016, 45, 1890–1901. [Google Scholar] [CrossRef]
- Fischer, E.K. Rheological properties of commercial paints. J. Colloid Sci. 1950, 5, 271–281. [Google Scholar] [CrossRef]
- Lambert, J.B.; Lu, G.; Singer, S.R.; Kolb, V.M. Silicate complexes of sugars in aqueous solution. J. Am. Chem. Soc. 2004, 126, 9611–9625. [Google Scholar] [CrossRef]
- Bobrowski, A.; Stypuła, B.; Hutera, B.; Kmita, A.; Drożyński, D.; Starowicz, M. FTIR spectroscopy of water glass—The binder moulding modified by ZnO nanoparticles. Metalurgija 2012, 51, 477–480. [Google Scholar]
- Khan, A.S.; Khalid, H.; Sarfraz, Z.; Khan, M.; Iqbal, J.; Muhammad, N.; Fareed, M.A.; Rehman, I.U. Vibrational spectroscopy of selective dental restorative materials. Appl. Spectrosc. Rev. 2016. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R00Si(OR’)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Cai, G.-B.; Chen, S.-F.; Liu, L.; Jiang, J.; Yao, H.-B.; Xu, A.-W.; Yu, S.-H. 1,3-Diamino-2-hydroxypropane-N, N, N′, N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation, transformation and crystal growth. Cryst. Eng. Comm. 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Ferrage, E.; Martin, F.; Petit, S.; Pejo-Soucaille, S.; Micoud, P.; Fourty, G.; Ferret, J.; Salvi, S.; De Parseval, P.; Fortune, J.P. Evaluation of talc morphology using FTIR and H/D substitution. Clay Miner. 2003, 38, 141–150. [Google Scholar] [CrossRef]
- Giraudo, N.; Bergdolt, S.; Wohlgemuth, J.; Welle, A.; Schuhmann, R.; Königer, F.; Thissen, P. Calcium Silicate Phases Explained by High-Temperature-Resistant Phosphate Probe Molecules. Langmuir 2016, 32, 13577–13584. [Google Scholar] [CrossRef]
- Maddalena, R.; Hall, C.; Hamilton, A. Effect of silica particle size on the formation of calcium silicate hydrate [C-S-H] using thermal analysis. Thermochim. Acta 2019, 672, 142–149. [Google Scholar] [CrossRef]
- Li, K.-M.; Jiang, J.-G.; Tian, S.-C.; Chen, X.-J.; Yan, F. Influence of silica types on synthesis and performance of amine−silica hybrid materials used for CO2 capture. J. Phys. Chem. C 2014, 118, 2454–2462. [Google Scholar] [CrossRef]
- Latella, B.A.; Ignat, M.; Barbe, C.J.; Cassidy, D.J.; Bartlett, J.R. Adhesion behaviour of organically-modified silicate coatings on stainless steel. J. Sol Gel Sci. Technol. 2003, 26, 765–770. [Google Scholar] [CrossRef]



| Samples | Water Glass (g) | Acrylic Resin (g) | CaCO3 (g) | ZnO (g) | Talc (g) | Gly (g) | Xyl (g) | Sucr (g) | Dex (g) | TiO2 (g) | Water (g) | Silicon (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF1 a | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 4 | 0.2 |
| CF2 a | 35 | 4 | 10 | 5 | 5 | 4 | 0 | 0 | 0 | 0 | 4 | 0.2 |
| CF3 a | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 4 | 0 | 0 | 4 | 0.2 |
| CF4 a | 35 | 4 | 10 | 5 | 5 | 0 | 4 | 0 | 0 | 0 | 4 | 0.2 |
| CF5 a | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 0 | 4 | 0 | 4 | 0.2 |
| CF6 a | 35 | 5 | 30 | 10 | 5 | 0 | 0 | 0 | 0 | 0 | 15 | 0.2 |
| CF7 a | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 0 | 0 | 5 | 20 | 0.2 |
| CF8 b | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 4 | 0.2 |
| CF9 c | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 4 | 0.2 |
| CF10 d | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 4 | 0.2 |
| CF11 e | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 4 | 0.2 |
| CF12 f | 35 | 4 | 10 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 4 | 0.2 |
| Coating Formulation | Pull-Off Adhesion Strength (MPa) # | Assessment of the Cross-Cut Test (2 mm) | Scratch Resistance (N) | Assessment of the Impact Test * | |
|---|---|---|---|---|---|
| 100 mm | 400 mm | ||||
| CF1 | 2.68 (0.33) | 1 | 6 | 4 (4.5) | 3 (6.3) |
| CF2 | 1.44 (0.40) | 3 | 5 | 3.5 (5.2) | 2.5 (6.7) |
| CF3 | 1.72 (0.08) | 2 | 6 | 4 (4.5) | 3 (6.3) |
| CF4 | 1.21 (0.11) | 3 | 4 | 3 (5.8) | 2.5 (6.8) |
| CF5 | 2.25 (0.08) | 1 | 5 | 4 (4.2) | 3 (6.6) |
| CF6 | 1.07 (0.13) | 5 | 2 | 4 (5.8) | 2.5 (7.0) |
| CF7 | 2.22 (0.06) | 1 | 3 | 4 (3.5) | 2 (6.3) |
| CF8 | 0.84 (0.15) | 4 | 2 | 2.5 (4.5) | 2 (6.1) |
| CF9 | 0.95 (0.08) | 4 | 3 | 2.5 (4.8) | 2 (7.0) |
| CF10 | 2.65 (0.46) | 2 | 5 | 4 (4.0) | 2.5 (7.1) |
| CF11 | 2.71 (0.37) | 2 | 4 | 4 (4.8) | 3 (6.3) |
| CF12 | 2.65 (0.22) | 1 | 5 | 4 (4.6) | 3 (6.5) |
| Bands | Assignments |
|---|---|
| 3676 cm−1 | Stretching of isolated and mutually hydrogen bonded- Si–OH and Mg–OH |
| 3297 cm−1 | Stretching O–H in water and silanol SiO–H hydrogen bonded- with water |
| 2935, 2918, 2850 cm−1 | Asymmetric and symmetric stretching of C–H in methylene and methyl groups |
| 1793, 1725 cm−1 | C=O carbonyls (CO32− and carbonyl in acrylic resins |
| 1635 cm−1 | Bending vibration of water (H–O–H) |
| 1455, 1393 cm−1 | Asymmetric stretching of CO32− |
| 1120 cm−1 | Asymmetric Stretching of Si–O–Si in highly cross-liked zone, e.g., (Si–O)4 closed cage |
| 1012, 1008, 1006 cm−1 | Asymmetric stretching of Si–O in short siloxane-chain |
| 872 cm−1 | Out of plane bending (CO32−), symmetric stretching Si–O |
| 780 cm−1 | –CH3 rocking and Si–C wagging stretching in Si–CH3 |
| 710 cm−1 | In plane bending (CO32−) |
| 666 cm−1 | Stretching of Si–O–Si in tetrahedral unit, stretching of Si–O–Mg |
| Pre-Mineralized Wood | Pull-off Adhesion Strength (MPa) # | Assessment of the Cross-Cut Test | Scratch Resistance (N) | Assessment of the Impact Test (400 mm) * |
|---|---|---|---|---|
| MW1 | 3.8 (0.26) | 1 | 6 | 3.5 (6.4) |
| MW2 | 2.6 (0.18) | 1 | 5.5 | 3.5 (6.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheumani Yona, A.M.; Žigon, J.; Dahle, S.; Petrič, M. Study of the Adhesion of Silicate-Based Coating Formulations on a Wood Substrate. Coatings 2021, 11, 61. https://doi.org/10.3390/coatings11010061
Cheumani Yona AM, Žigon J, Dahle S, Petrič M. Study of the Adhesion of Silicate-Based Coating Formulations on a Wood Substrate. Coatings. 2021; 11(1):61. https://doi.org/10.3390/coatings11010061
Chicago/Turabian StyleCheumani Yona, Arnaud Maxime, Jure Žigon, Sebastian Dahle, and Marko Petrič. 2021. "Study of the Adhesion of Silicate-Based Coating Formulations on a Wood Substrate" Coatings 11, no. 1: 61. https://doi.org/10.3390/coatings11010061
APA StyleCheumani Yona, A. M., Žigon, J., Dahle, S., & Petrič, M. (2021). Study of the Adhesion of Silicate-Based Coating Formulations on a Wood Substrate. Coatings, 11(1), 61. https://doi.org/10.3390/coatings11010061







