Effect of Plating Current Density on the Ball-On-Disc Wear of Sn-Plated Ni Coatings on Cu Foils
Abstract
1. Introduction
2. Materials and Methods
2.1. Plating Bath
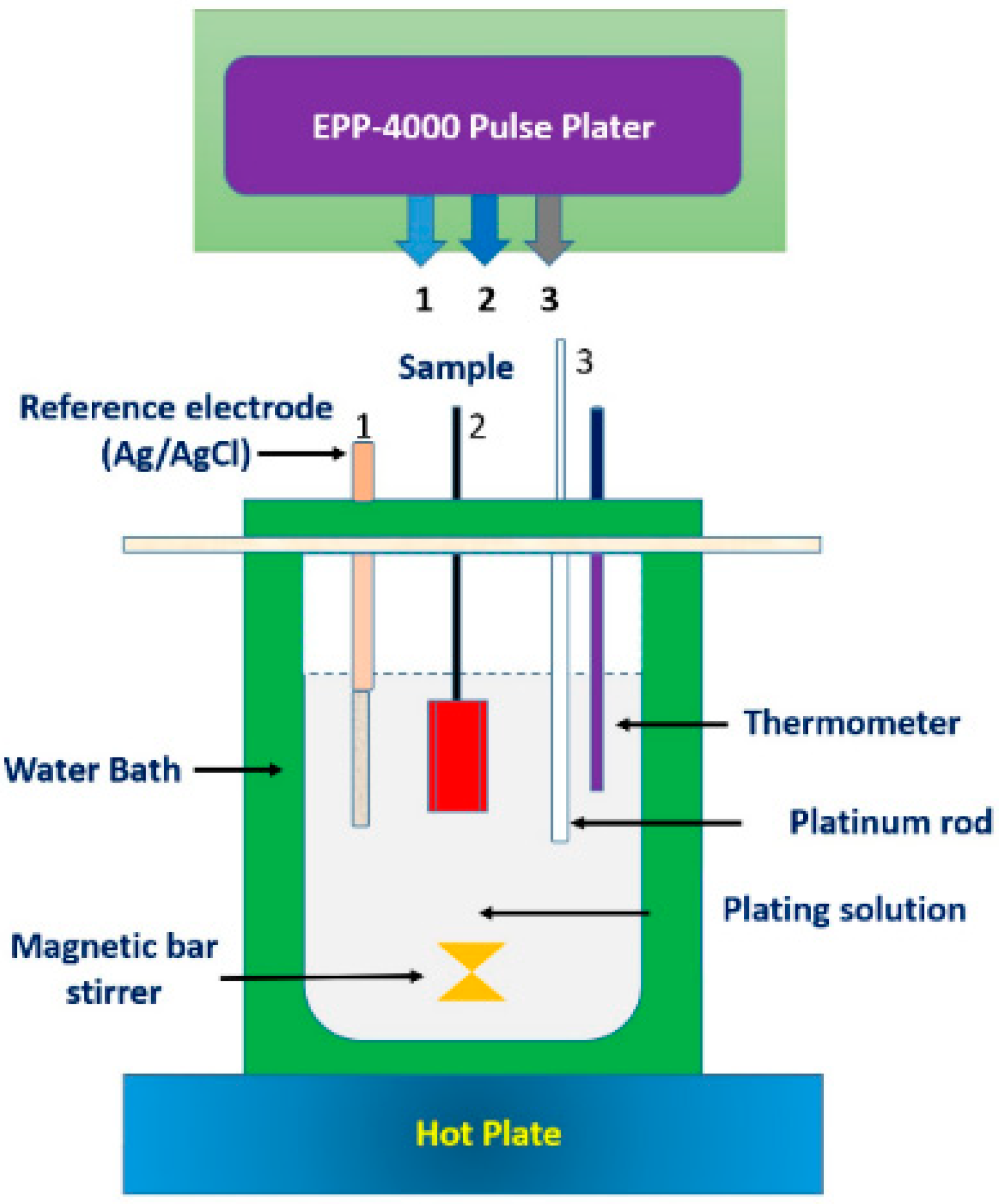
2.2. Plating Cell
2.3. Plating Experiment
2.4. Microstructural Investigations
2.5. Mechanical Tests
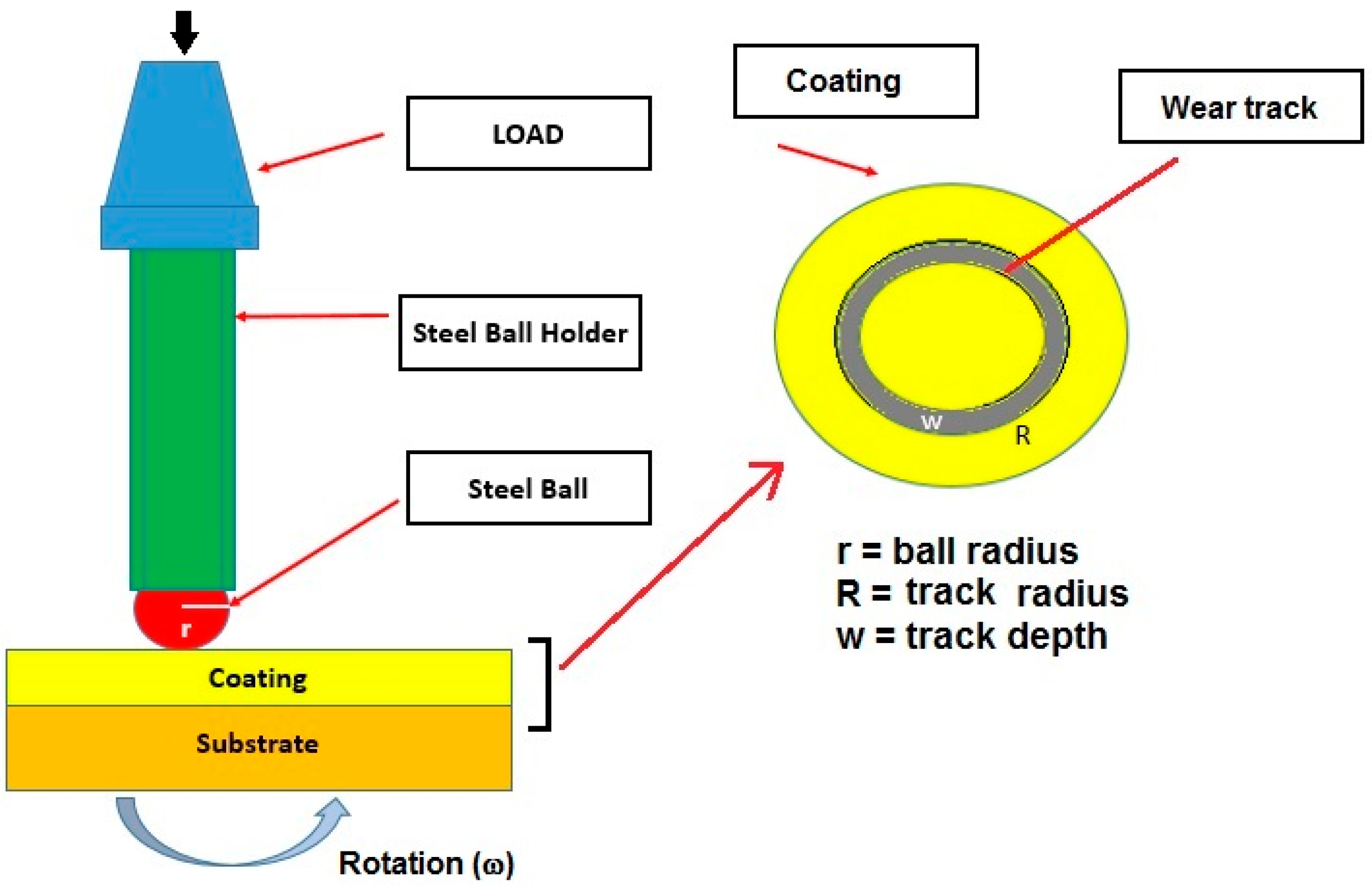
2.6. Tribological Tests
3. Results and Discussions
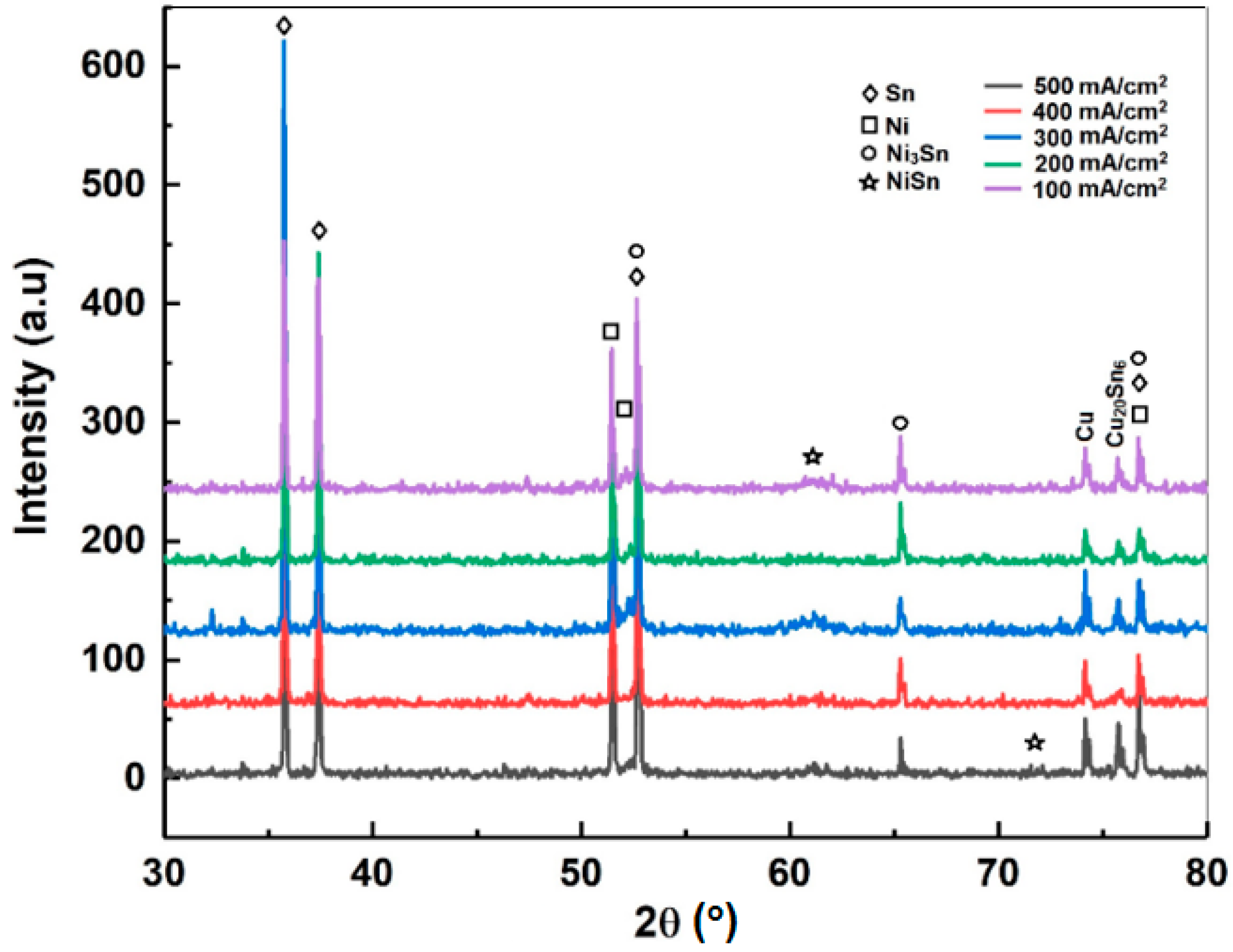
3.1. Phase Evolution
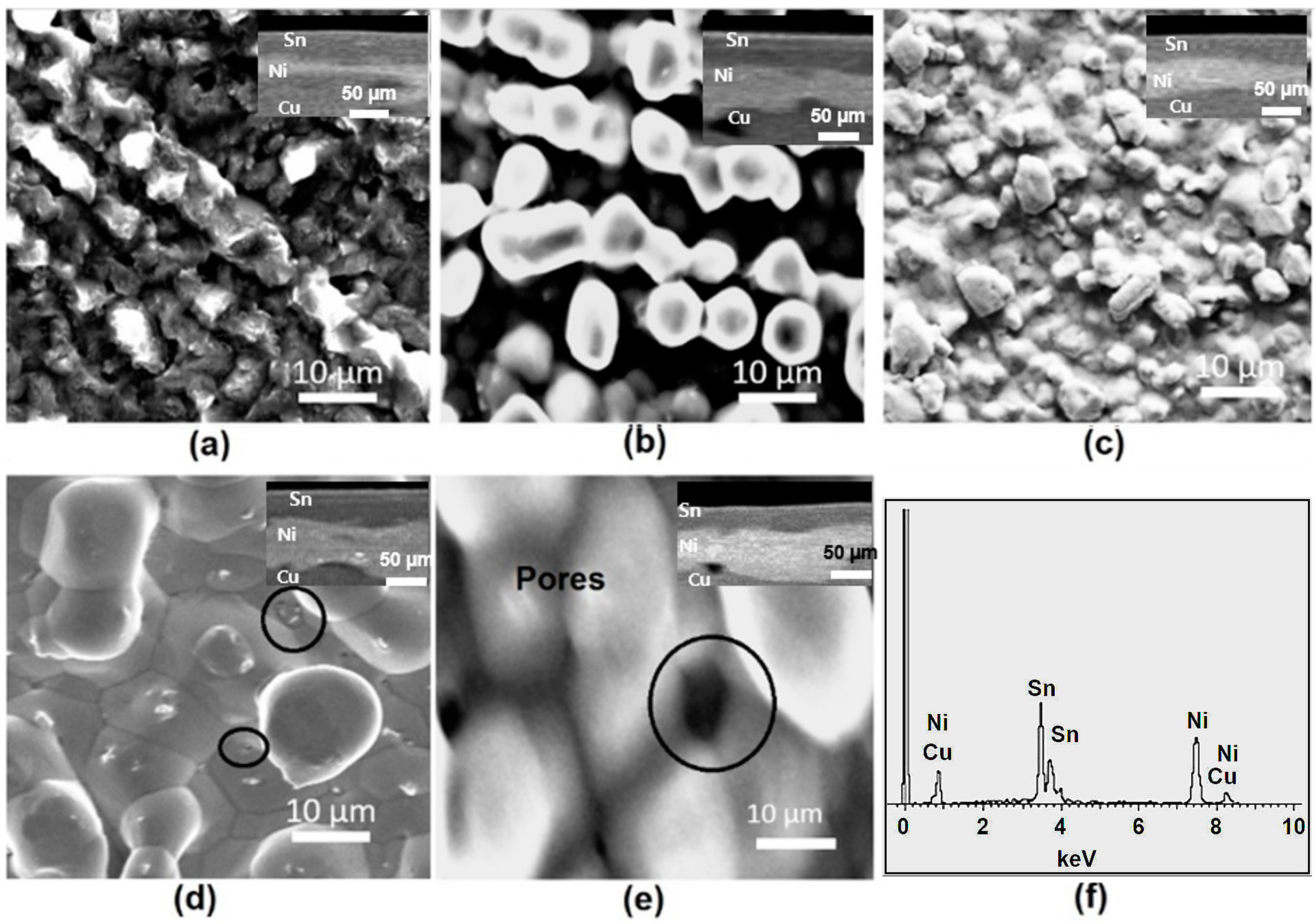
3.2. Morphology
3.3. Deposit Roughness and Thickness
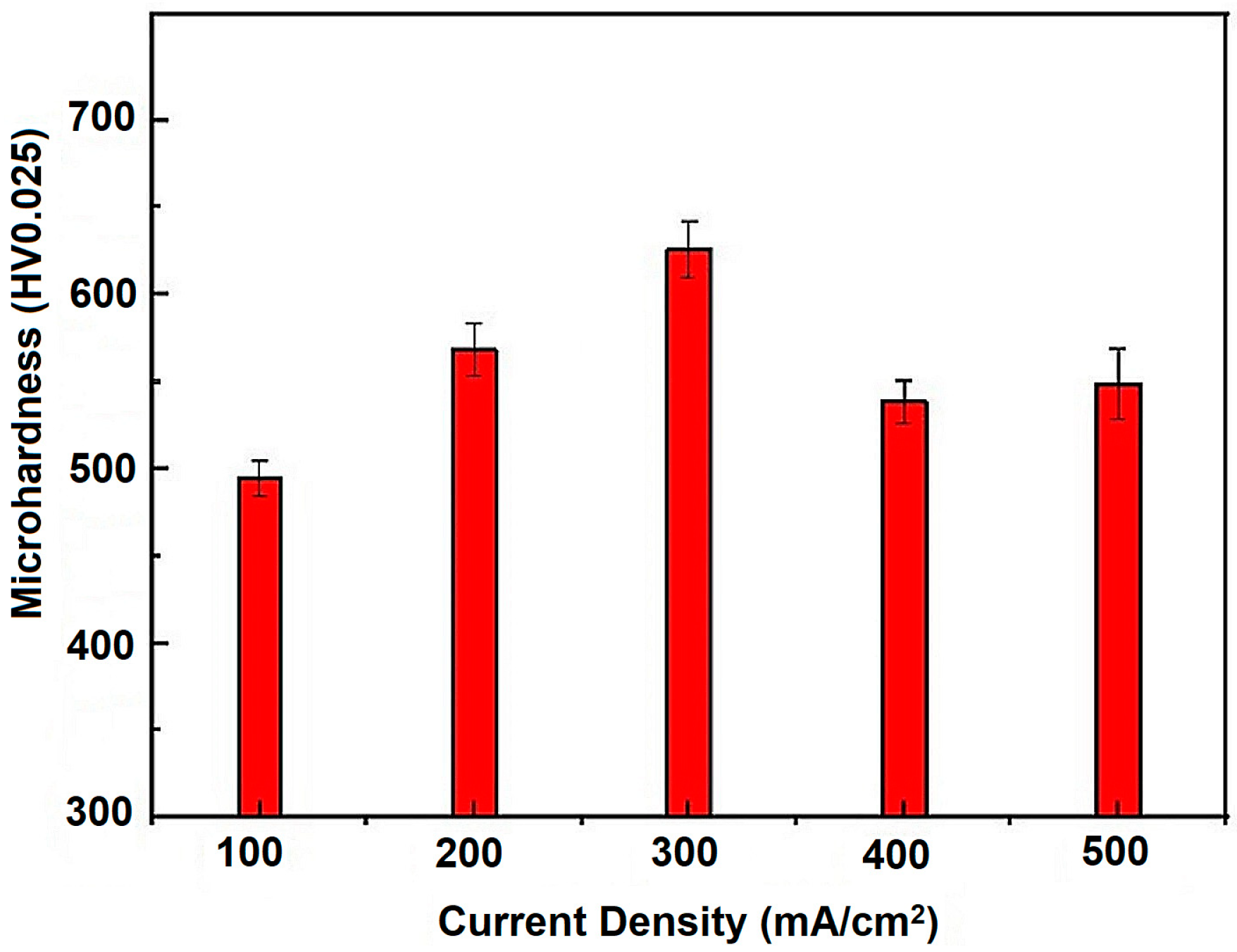
3.4. Deposit Microhardness
3.5. Wear and Friction Studies
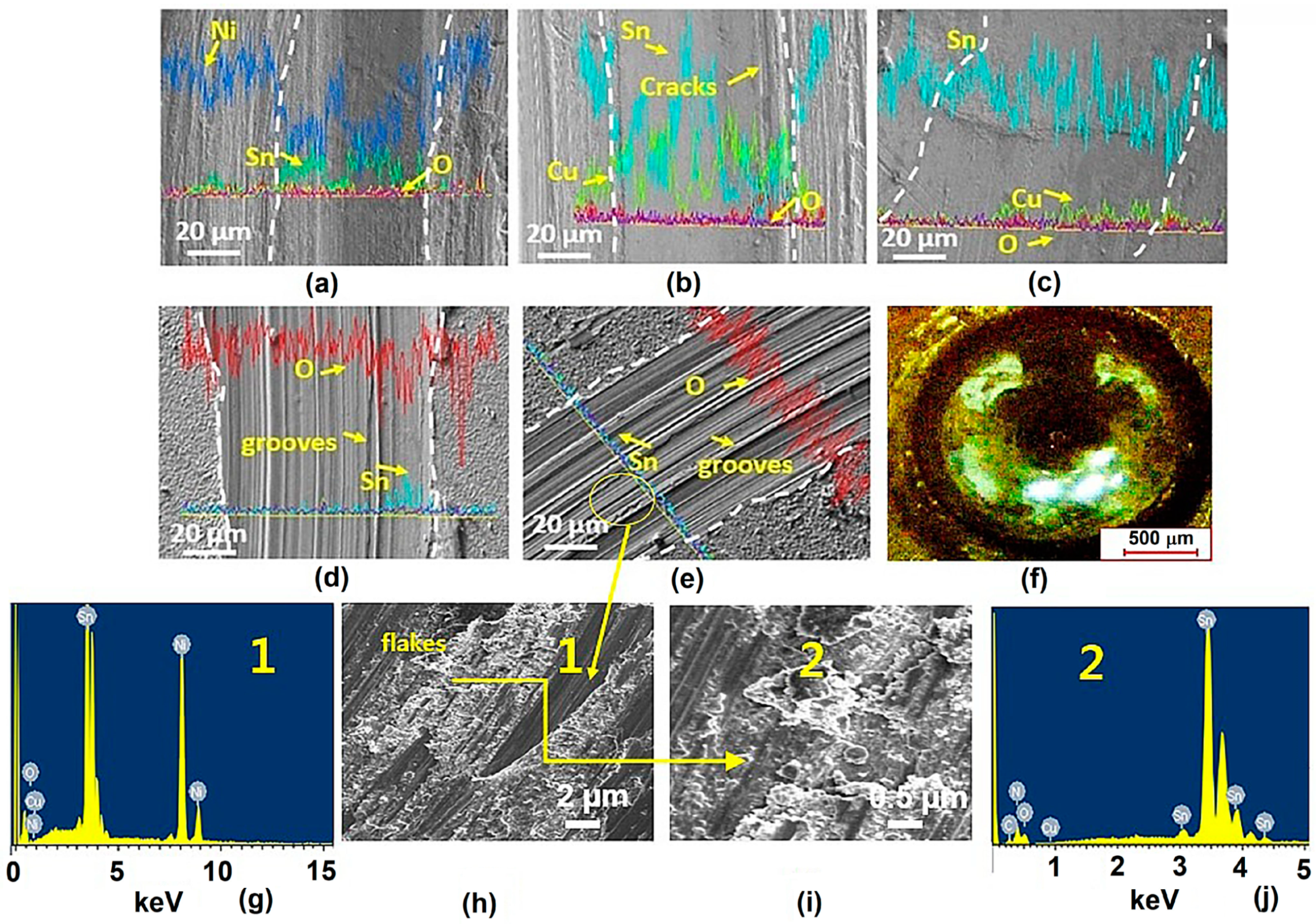
3.5.1. Wear Tracks
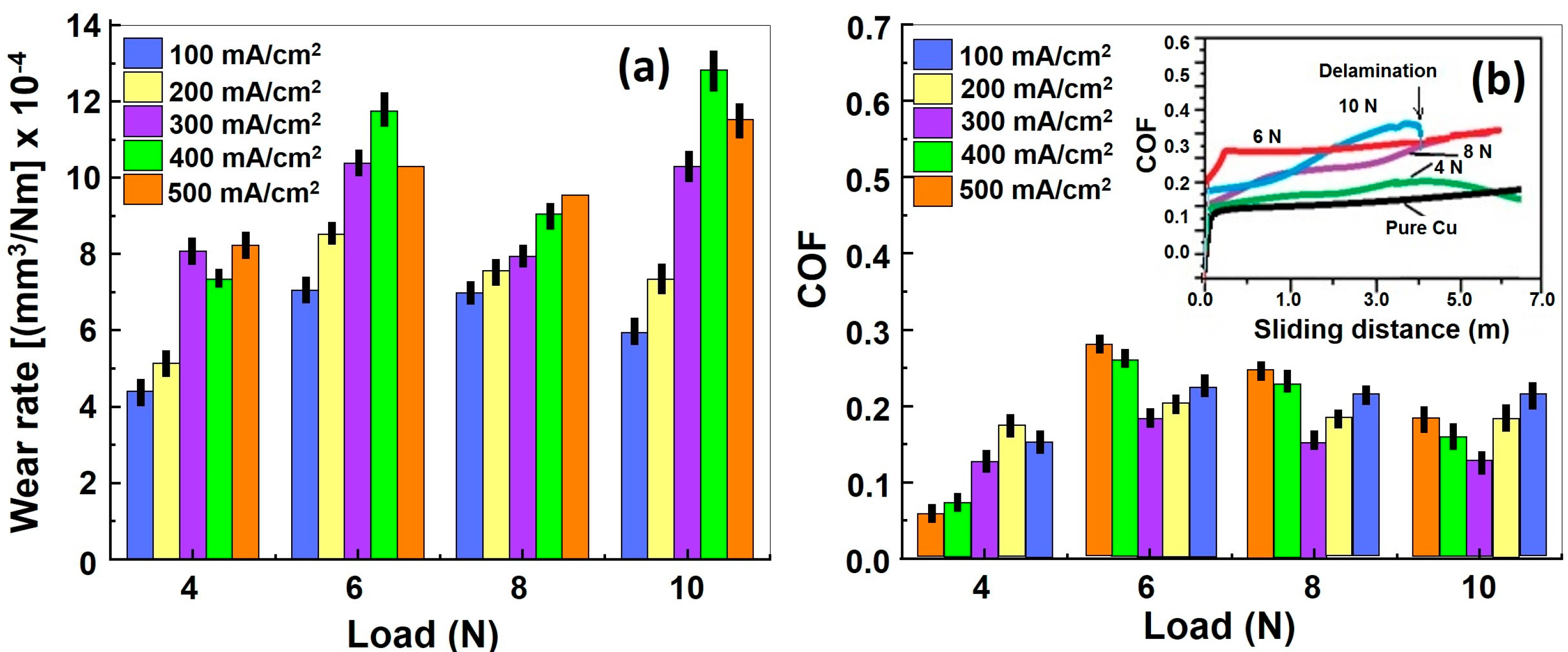
3.5.2. Wear Factor and COF
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osenbach, J.W.; DeLucca, J.M.; Potteiger, B.D.; Amin, A.; Baiocchi, F.A. Sn-whiskers: Truths and myths. In Lead-Free Electronic Solders; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Sharma, A.; Das, S.; Das, K. Effect of different electrolytes on the microstructure, corrosion and whisker growth of pulse plated tin coatings. Microelectron. Eng. 2017, 170, 59–68. [Google Scholar] [CrossRef]
- Hektor, J.; Marijon, J.B.; Ristinmaa, M.; Hall, S.A.; Hallberg, H.; Iyengar, S.; Micha, J.S.; Robach, O.; Grennerat, F.; Castelnau, O. Evidence of 3D strain gradients associated with tin whisker growth. Scr. Mater. 2018, 144, 1–4. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Pulse electrodeposition of lead-free tin-based composites for microelectronic packaging. In Electrodeposition of Composite Materials; Mohamed, A.M.A., Golden, T.D., Eds.; InTech: Rijeka, Croatia, 2016; pp. 253–274. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Pulse electroplating of ultrafine grained tin coating. In Electroplating of Nanostructures; Mahmood, A., Ed.; Intech: Rijeka, Croatia, 2015; pp. 105–129. [Google Scholar] [CrossRef]
- Paunovic, M.; Schlesinger, M. Fundamentals of Electrochemical Deposition; Wiley Interscience: New York, NY, USA, 2006. [Google Scholar]
- Sharma, A.; Cheon, C.S.; Jung, J.P. Recent progress in electroless plating of copper. J. Microelectron. Packag. Soc. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Bates, B.L.; Zhang, Y. Effect of post-deposition heat treatment on electrodeposited NiCoCrAlY coatings. Surf. Eng. 2017, 33, 136–141. [Google Scholar] [CrossRef]
- Jung, D.H.; Sharma, A.; Kim, K.H.; Choo, Y.C.; Jung, J.P. Effect of current density and plating time on Cu electroplating in TSV and low alpha solder bumping. J. Mater. Eng. Perform. 2015, 24, 1107–1115. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Das, K. Fabrication of Sn–Ag/CeO2 electro-composite solder by pulse electrodeposition. Metall. Mater. Trans. A 2013, 44, 5587–5601. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Das, K. Fabrication of Sn nanostructures by template assisted pulse electrodeposition. Surf. Eng. 2016, 32, 378–384. [Google Scholar] [CrossRef]
- Aniekwe, U.V.; Utigard, T.A. High-temperature oxidation of nickel-plated copper vs pure copper. Can. Metall. Q. 1999, 38, 277–281. [Google Scholar] [CrossRef]
- Harput, S. Fields of application of nickel plated copper conductor. In Sarkuysan Electrolytic Copper Document; Sarkuysan Electrolytic Copper Co.: Darıca, Turkey, 2006. [Google Scholar]
- Brusse, J.A.; Ewell, G.J.; Siplon, J.P. Tin Whiskers: Attributes and Mitigation. In Proceedings of the 22nd Capacitor and Resistor Technology Symposium, New Orleans, LA, USA, 25–29 March 2002; pp. 67–80. [Google Scholar]
- Wilson, G.C. The use of tin when alloyed with nickel or lead as a printed circuit finish. Trans. IMF 1972, 50, 109–113. [Google Scholar] [CrossRef]
- Jalota, S.K. Tin-nickel alloy plating. Met. Finish 2001, 99, 320–323. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Das, K. A study on the effect of pulse electrodeposition parameters on the morphology of pure tin coatings. Metall. Mater. Trans. A 2014, 45, 4610–4622. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Das, S.; Das, K. Influence of current density on surface morphology and properties of pulse plated tin films from citrate electrolyte. Appl. Surf. Sci. 2014, 290, 373–380. [Google Scholar] [CrossRef]
- Pewnim, N.; Roy, S. Electrodeposition of tin-rich Cu–Sn alloys from a methanesulfonic acid electrolyte. Electrochim. Acta 2013, 90, 498–506. [Google Scholar] [CrossRef]
- Sharma, A.; Bhattacharya, S.; Sen, R.; Reddy, B.S.B.; Fecht, H.J.; Das, K.; Das, S. Influence of current density on microstructure of pulse electrodeposited tin coatings. Mater. Charact. 2012, 68, 22–32. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminum and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Ungar, T.; Dragomir, I.; Revesz, A.; Borbely, A. The contrast factors of dislocations in cubic crystals: The dislocation model of strain anisotropy in practice. J. Appl. Cryst. 1999, 32, 992–1002. [Google Scholar] [CrossRef]
- Rashidi, A.M.; Amadeh, A. The effect of current density on the grain size of electrodeposited nanocrystalline nickel coatings. Surf. Coat. Technol. 2008, 202, 3772–3776. [Google Scholar] [CrossRef]
- Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear; ASTM G133–05; ASTM International: West Conshohocken, PA, USA, 2016.
- Bajwa, R.S.; Tudela, I.; Verbickas, R.; Palamarciuc, I.; Zhang, Y. Friction and wear performance of Sn-based coatings under hydrodynamic, mixed and boundary lubrication. Trib. Int. 2020, 149, 105695. [Google Scholar] [CrossRef]
- Ilangovan, S.; Sellamuthu, R. Effects of tin on hardness, wear rate and coefficient of friction of Cu–Ni–Sn alloys. J. Eng. Sci. Technol. 2013, 8, 34–43. [Google Scholar]
- Podgursky, V.; Gregor, A.; Adoberg, E.; Kulu, P. Wear of hard coatings, evaluated by means of KaloMax. Proc. Est. Acad. Sci. Eng. 2006, 12, 419–426. [Google Scholar]
- Drozd, K.; Walczak, M.; Szala, M.; Gancarczyk, K. Tribological behavior of AlCrSiN-coated tool steel K340 versus popular tool steel grades. Materials 2020, 13, 4895. [Google Scholar] [CrossRef] [PubMed]
- Szala, M.; Łatka, L.; Walczak, M.; Winnicki, M. Comparative study on the cavitation erosion and sliding wear of cold-sprayed Al/Al2O3 and Cu/Al2O3 coatings, and stainless steel, aluminium alloy, copper and brass. Metals 2020, 10, 856. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, L.; Dwyer-Joyce, R.S. Friction and wear of Cu-15 wt.%Ni-8 wt.%Sn bronze lubricated by grease at room and elevated temperature. Wear 2020, 460–461, 203474. [Google Scholar] [CrossRef]
- Rosoiu, S.P.; Pantazi, A.G.; Petica, A.; Cojocaru, A.; Costovici, S.; Zanella, C.; Visan, T.; Anicai, L.; Enachescu, M. Comparative study of Ni–Sn alloys electrodeposited from choline chloride-based ionic liquids in direct and pulsed current. Coatings 2019, 9, 801. [Google Scholar] [CrossRef]
- Rooksby, H. An X-ray study of tin-nickel electrodeposits. Trans. IMF 1950, 27, 153–169. [Google Scholar] [CrossRef]
- Weil, R.; Cook, H.C. Electron-microscopic observations of the structure of electroplated nickel. J. Electrochem. Soc. 1962, 109, 295–301. [Google Scholar] [CrossRef]
- Schmitz, E.P.S.; Quinaia, S.P.; Garcia, J.R.; de Andrade, C.K.; Lopes, M.C. Influence of commercial organic additives on the nickel electroplating. Int. J. Electrochem. Sci. 2016, 11, 983–997. [Google Scholar]
- Ghosh, S.K.; Grover, A.K.; Dey, G.K.; Totlani, M.K. Nanocrystalline Ni–Cu alloy plating by pulse electrolysis. Surf. Coat. Technol. 2000, 126, 48–63. [Google Scholar] [CrossRef]
- Sharma, A.; Jang, Y.J.; Jung, J.P. Effect of current density on morphology of electroplated tin. Surf. Eng. 2015, 31, 458–464. [Google Scholar] [CrossRef]
- Matsui, I.; Takigawa, Y.; Yokoe, D.; Kato, T.; Uesugi, T.; Higashi, K. Strategy for electrodeposition of highly ductile bulk nanocrystalline metals with a face-centered cubic structure. Mater. Trans. 2014, 55, 1859–1866. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Omidvar, H.; Javanbakht, M.; Mozafari, A. Characterization of pure nickel coatings fabricated under pulse current conditions. Int. J. Mater. Metall. Eng. 2015, 9, 1061–1065. [Google Scholar]
- Bhattacharya, S.; Sharma, A.; Das, S.; Das, K. Synthesis and properties of pulse electrodeposited lead-free tin-based Sn/ZrSiO4 nanocomposite coatings. Metall. Mater. Trans. A 2016, 47, 1292–1312. [Google Scholar] [CrossRef]
- Mangam, V.; Bhattacharya, S.; Das, K.; Das, S. Friction and wear behavior of Cu–CeO2 nanocomposite coatings synthesized by pulsed electrodeposition. Surf. Coat. Technol. 2010, 205, 801–805. [Google Scholar] [CrossRef]
- Lee, K.U.; Jeong, D.K.; Joo, H.G.; Park, Y.W. Simulation for fretting corrosion of tin-coated copper contacts. Mater. Corr. 2011, 62, 352–356. [Google Scholar] [CrossRef]

| Composition of Bath | Quantity (g·L−1) | Composition of Bath | Quantity (g·L−1) | Operating Parameters | Values |
|---|---|---|---|---|---|
| Ni Plating | Sn Plating | ||||
| NiSO4·6H2O | 300 | SnSO4 | 150 | Current Density | Ni: 300 mA/cm2 Sn: 100–500 mA/cm2 |
| NiCl2·6H2O | 45 | H2SO4 | 60 | On-time, Off-time | 0.001 s, 0.011 s |
| H3BO3 | 40 | Triton X-100 | 0.2 | Pulse Frequency | 100 Hz |
| Saccharin Sodium | 0.5 | – | – | Duty Cycle | 9% |
| Sodium Dodecyl Sulfate | 0.1 | – | – | Magnetic Stirring | 300 rpm |
| Substrate | Copper (cold rolled), 2 mm thick, Ra: 1 µm, Microhardness: 130 ± 8 HV | ||||
| Normal Load (N) | 2–10 N |
| Sliding Distance (m) | 7 |
| Linear Velocity (mm/s) | 3.8 |
| Sliding Radius (mm) | 6 |
| Lubricant | Dry |
| Temperature (°C) | 30 °C |
| Environment | Dry air |
| Ball Indenter | WC (Tungsten Carbide), 2.0 mm, Grade G25 |
| Specimen Shape | Sn-plated Ni/Cu (30–55 µm Sn plated on 30 µm Ni over 25 × 25 × 2 mm3 Cu substrate) |
| Ball Indenter Hardness (HV) | 1750 |
| Current Density (mA/cm2) | Composition (wt.%) |
|---|---|
| 100 | Cu: 1.1, Ni: 7.10, Sn: 91.8 |
| 200 | Cu: 0.08, Ni: 9.56, Sn: 90.36 |
| 300 | Cu: 0.05, Ni: 14.68, Sn: 85.27 |
| 400 | Cu: 0.09, Ni: 25.36, Sn: 74.55 |
| 500 | Cu: 1.1, Ni: 34.67, Sn: 64.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Ahn, B. Effect of Plating Current Density on the Ball-On-Disc Wear of Sn-Plated Ni Coatings on Cu Foils. Coatings 2021, 11, 56. https://doi.org/10.3390/coatings11010056
Sharma A, Ahn B. Effect of Plating Current Density on the Ball-On-Disc Wear of Sn-Plated Ni Coatings on Cu Foils. Coatings. 2021; 11(1):56. https://doi.org/10.3390/coatings11010056
Chicago/Turabian StyleSharma, Ashutosh, and Byungmin Ahn. 2021. "Effect of Plating Current Density on the Ball-On-Disc Wear of Sn-Plated Ni Coatings on Cu Foils" Coatings 11, no. 1: 56. https://doi.org/10.3390/coatings11010056
APA StyleSharma, A., & Ahn, B. (2021). Effect of Plating Current Density on the Ball-On-Disc Wear of Sn-Plated Ni Coatings on Cu Foils. Coatings, 11(1), 56. https://doi.org/10.3390/coatings11010056






