Machine Learning Based Prediction of Nanoscale Ice Adhesion on Rough Surfaces
Abstract
1. Introduction
2. Methods
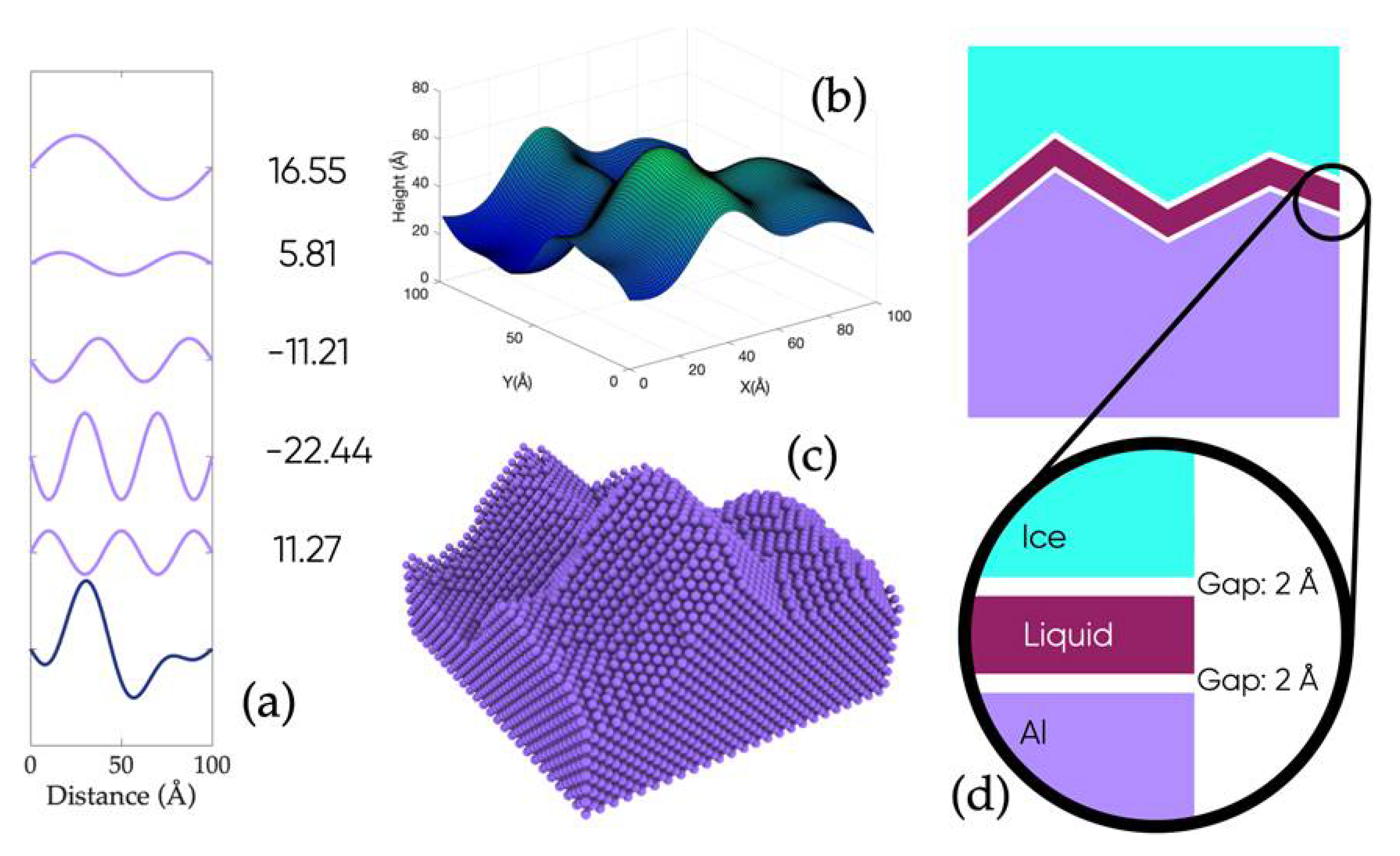
2.1. Large-Scale Atomistic Modeling and MD Simulation of Nanoscale Ice Adhesion
2.2. ML on Nanoscale Ice Adhesion on Rough Surfaces
3. Results and Discussion
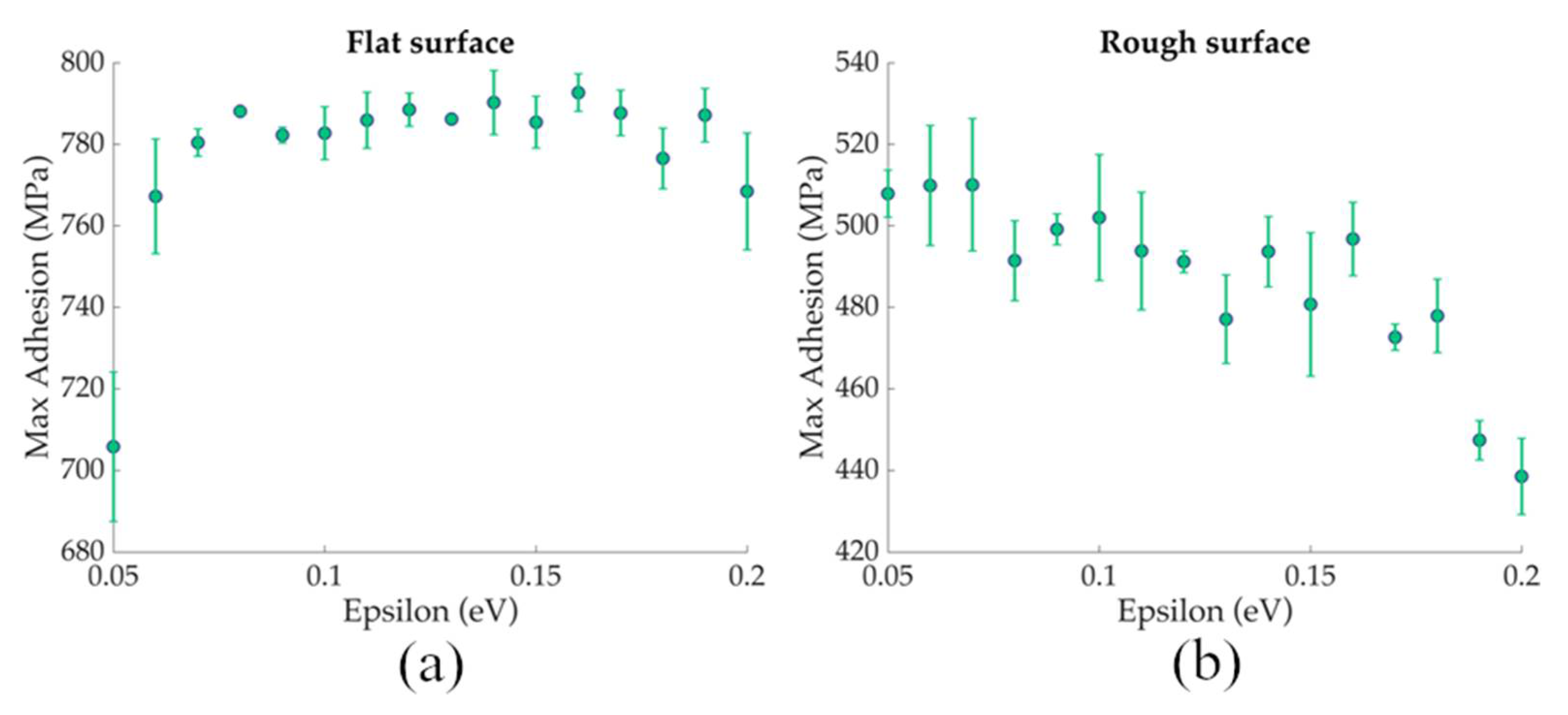
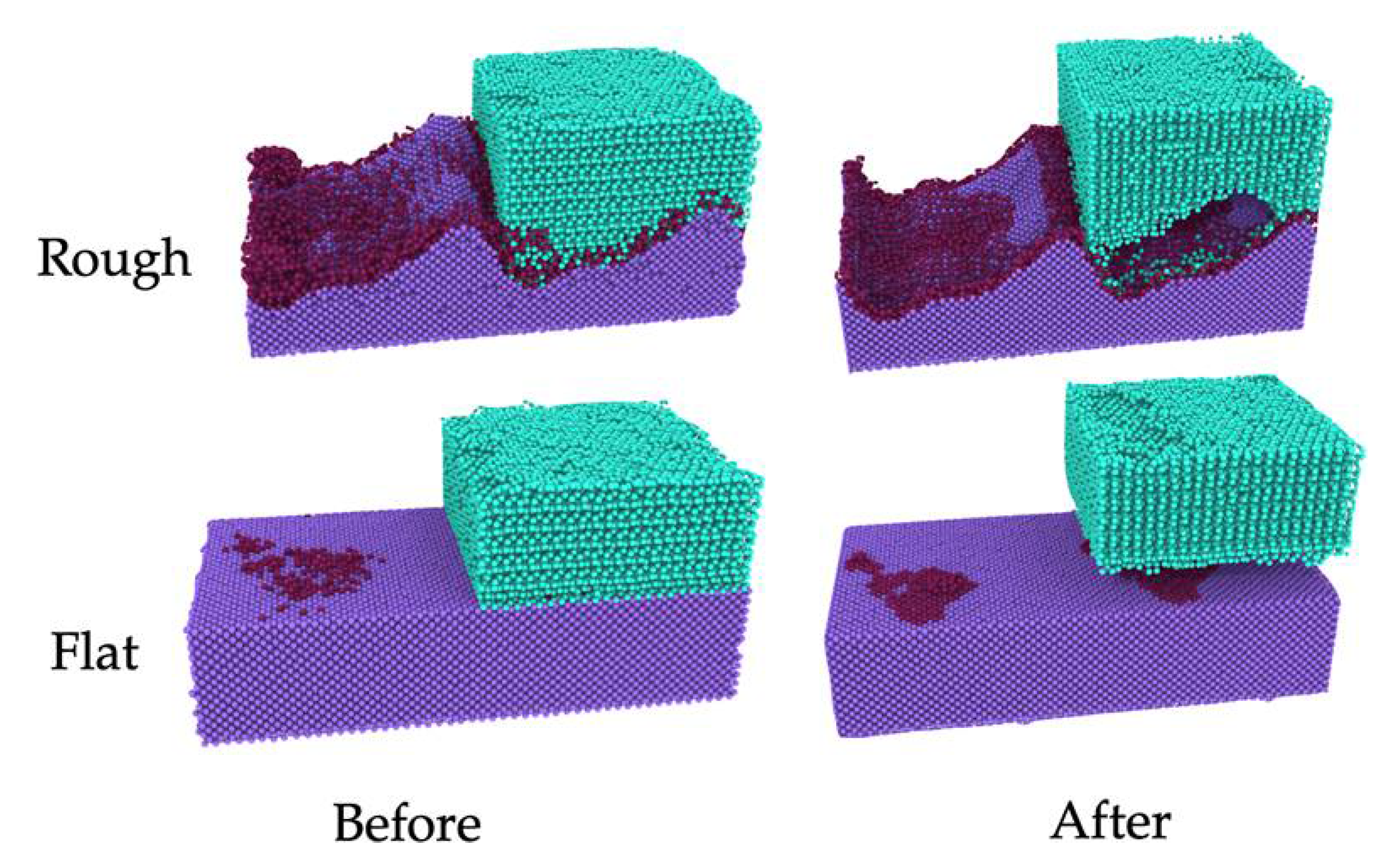
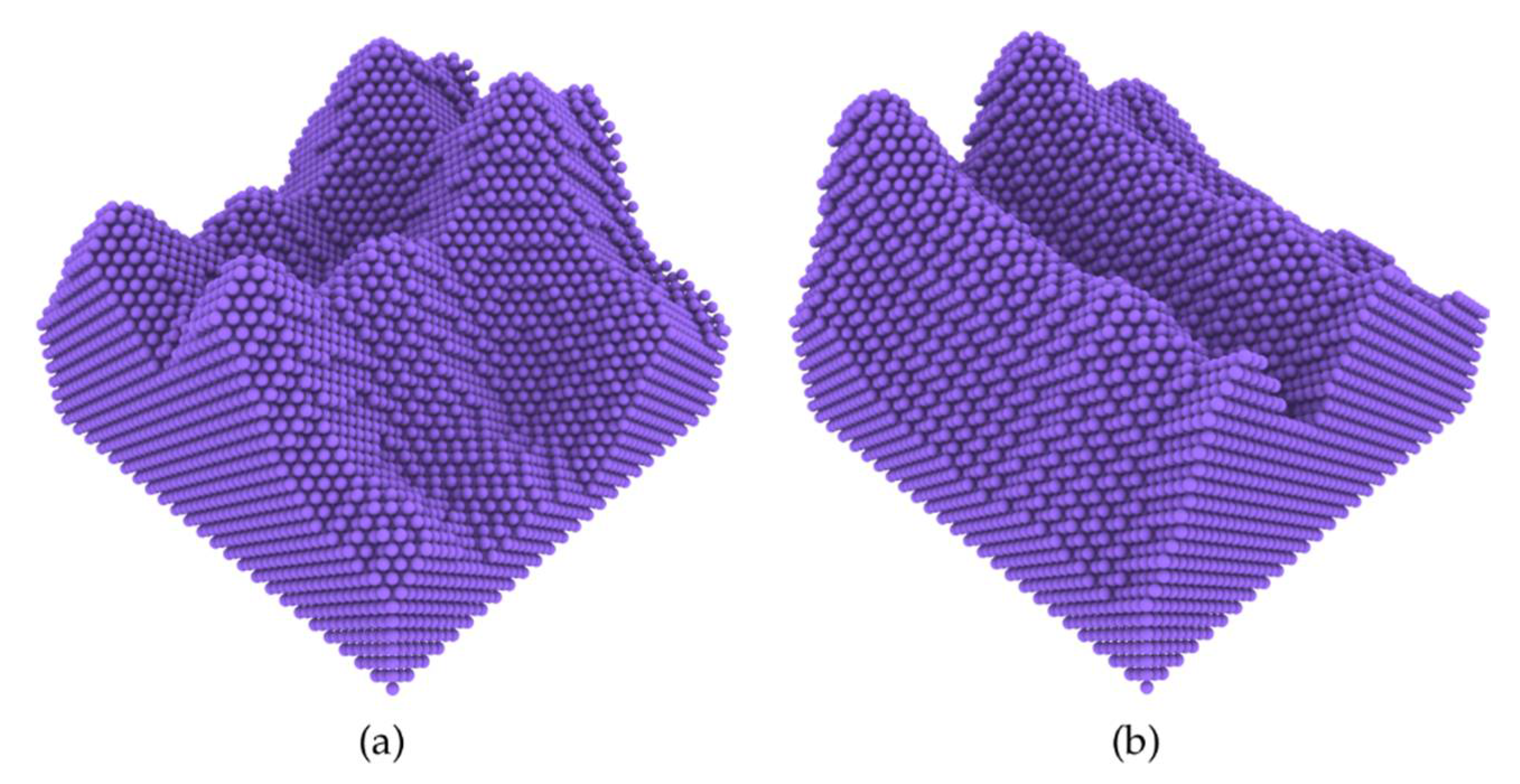
3.1. Nanoscale Ice Adhesion on Rough Surfaces
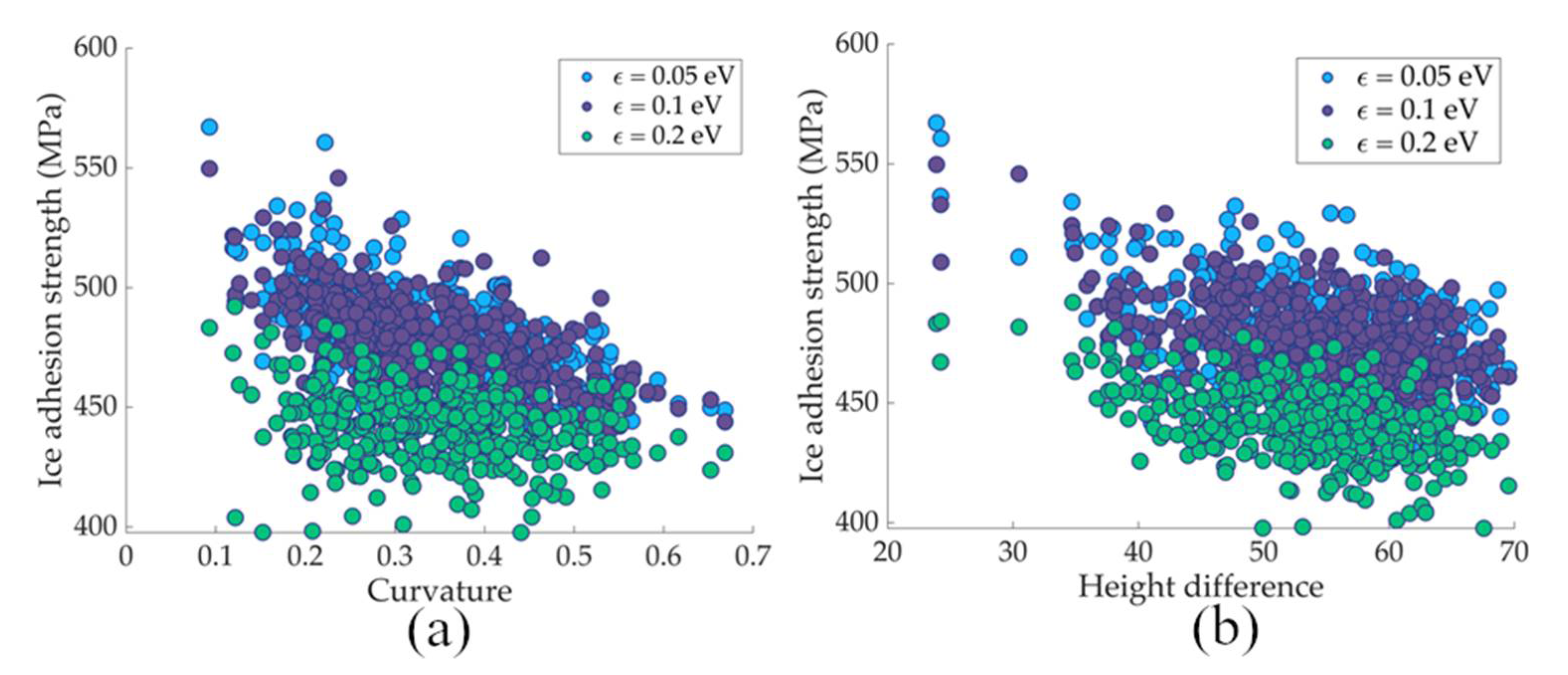
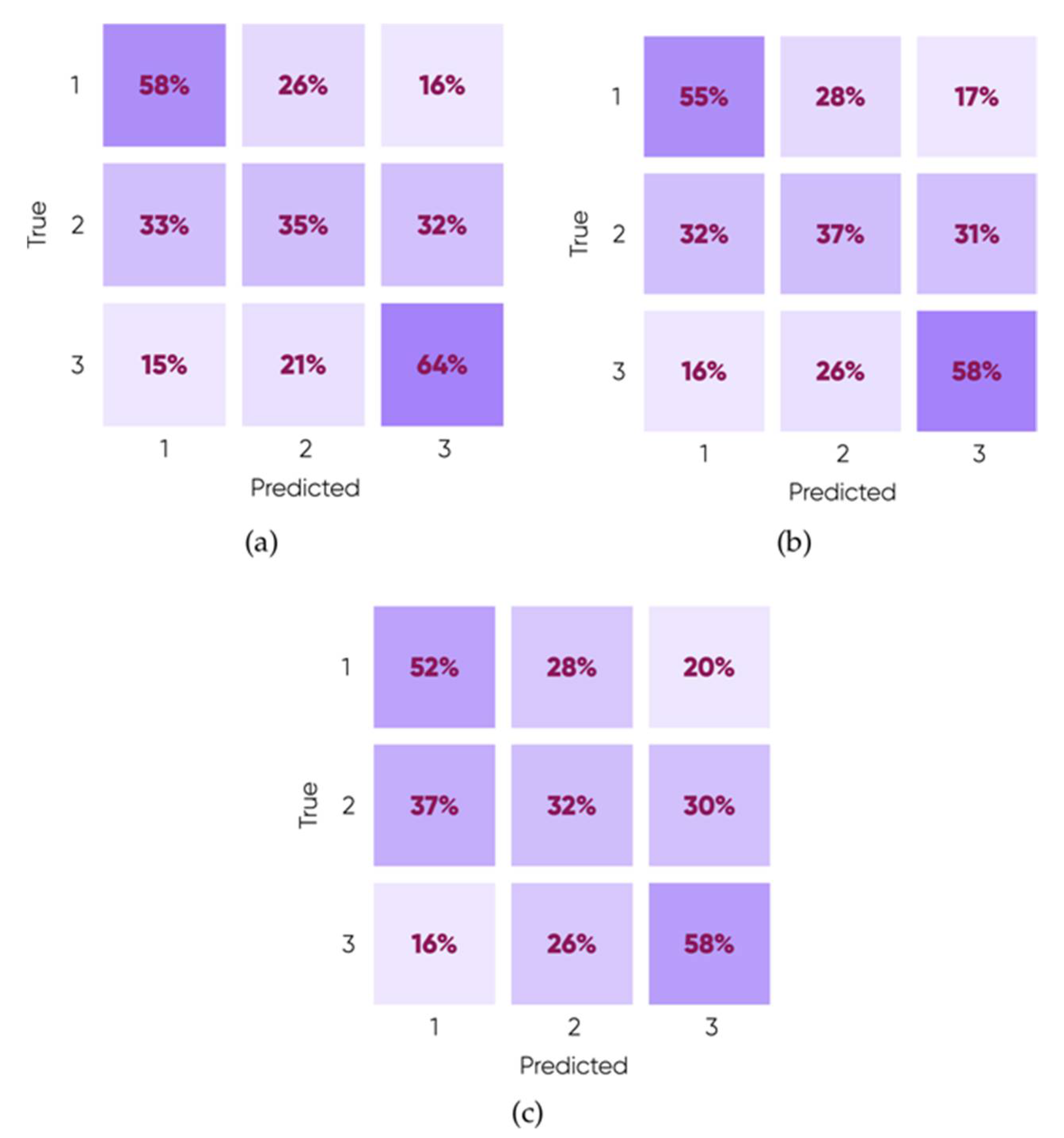
3.2. ML Prediction of Nanoscale Ice Adhesion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, L.; Huang, Z.; Priezjev, N.V.; Chen, S.; Luo, K.; Hu, H. A significant reduction of ice adhesion on nanostructured surfaces that consist of an array of single-walled carbon nanotubes: A molecular dynamics simulation study. Appl. Surf. Sci. 2018, 437, 202–208. [Google Scholar] [CrossRef]
- Grizen, M.; Maitra, T.; Bradley, J.P.; Tiwari, M.K. Nanotextured Aluminum-Based Surfaces with Icephobic Properties. Heat Transf. Eng. 2020, 41, 1663–1672. [Google Scholar] [CrossRef]
- Green, S. A Study of U.S. Inflight Icing Accidents and Incidents, 1978 to 2002. In Proceedings of the 44th AIAA Aerospace Sciences Meeting and Exhibit; American Institute of Aeronautics and Astronautics (AIAA), Reno, NV, USA, 9–12 January 2006. [Google Scholar]
- Hochart, C.; Fortin, G.; Perron, J.; Ilinca, A. Wind turbine performance under icing conditions. Wind. Energy 2008, 11, 319–333. [Google Scholar] [CrossRef]
- Jiménez, A.A.; Márquez, F.P.G.; Moraleda, V.B.; Muñoz, C.Q.G. Linear and nonlinear features and machine learning for wind turbine blade ice detection and diagnosis. Renew. Energy 2019, 132, 1034–1048. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Su, F. Damage accumulation model of ice detach behavior in ultrasonic de-icing technology. Renew. Energy 2020, 153, 1396–1405. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between Water Wettability and Ice Adhesion. ACS Appl. Mater. Interfaces 2010, 2, 3100–3110. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Jones, P.R.; Patankar, N.A. Thermodynamics of sustaining liquid water within rough icephobic surfaces to achieve ultra-low ice adhesion. Sci. Rep. 2019, 9, 258. [Google Scholar] [CrossRef]
- Laforte, J.; Allaire, M.; Laflamme, J. State-of-the-art on power line de-icing. Atmos. Res. 1998, 46, 143–158. [Google Scholar] [CrossRef]
- Sojoudi, H.; Wang, M.; Boscher, N.; McKinley, G.H.; Gleason, K.K. Durable and scalable icephobic surfaces: Similarities and distinctions from superhydrophobic surfaces. Soft Matter 2016, 12, 1938–1963. [Google Scholar] [CrossRef]
- Zhuo, Y.; Xiao, S.; Amirfazli, A.; He, J.; Zhang, Z. Polysiloxane as icephobic materials—The past, present and the future. Chem. Eng. J. 2020, 127088. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, J.; Wang, Q.; Chen, Q.; Ding, J. Verification of Icephobic/Anti-icing Properties of a Superhydrophobic Surface. ACS Appl. Mater. Interfaces 2013, 5, 3370–3381. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, G.; Tian, Q. Effects of nano-fluorocarbon coating on icing. Appl. Surf. Sci. 2012, 258, 7219–7224. [Google Scholar] [CrossRef]
- Liao, R.; Zuo, Z.; Guo, C.; Yuan, Y.; Zhuang, A. Fabrication of superhydrophobic surface on aluminum by continuous chemical etching and its anti-icing property. Appl. Surf. Sci. 2014, 317, 701–709. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of Ice-free Nanostructured Surfaces Based on Repulsion of Impacting Water Droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef]
- Li, T.; Ibáñez-Ibáñez, P.F.; Håkonsen, V.; Wu, J.; Xu, K.; Zhuo, Y.; Luo, S.; He, J.; Zhang, Z. Self-Deicing Electrolyte Hydrogel Surfaces with Pa-level Ice Adhesion and Durable Antifreezing/Antifrost Per-formance. ACS Appl. Mater. Interfaces 2020, 12, 35572–35578. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J.; Wang, Y.; Chen, Q.; Ding, J.; Wang, Q. Ice-phobic Coatings Based on Silicon-Oil-Infused Polydimethylsiloxane. ACS Appl. Mater. Interfaces 2013, 5, 4053–4062. [Google Scholar] [CrossRef]
- Sojoudi, H.; McKinley, G.H.; Gleason, K.K. Linker-free grafting of fluorinated polymeric cross-linked network bilayers for durable reduction of ice adhesion. Mater. Horizons 2014, 2, 91–99. [Google Scholar] [CrossRef]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings—Screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef]
- He, Z.; Xiao, S.; Gao, H.; He, J.; Zhang, Z. Multiscale crack initiator promoted super-low ice adhesion surfaces. Soft Matter 2017, 13, 6562–6568. [Google Scholar] [CrossRef]
- Shen, Q.; Qiu, J.; Liu, G.; Lv, K.; Zhang, Y. Rough surface simulation and electrical contact transient performance. In Proceedings of the 2016 Prognostics and System Health Management Conference (PHM-Chengdu), Chengdu, China, 19–21 October 2016; Institute of Electrical and Electronics Engineers (IEEE): Piscataway Township, NJ, USA, 2016; pp. 1–6. [Google Scholar]
- Xiao, S.; He, J.; Zhang, Z. Nanoscale deicing by molecular dynamics simulation. Nanoscale 2016, 8, 14625–14632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Skallerud, B.H.; Wang, F.; Zhang, Z.; He, J. Enabling sequential rupture for lowering atomistic ice adhesion. Nanoscale 2019, 11, 16262–16269. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; He, J.; Zhang, Z. Modeling nanoscale ice adhesion. Acta Mech. Solida Sin. 2017, 30, 224–226. [Google Scholar] [CrossRef]
- Singh, J.K.; Müller-Plathe, F. On the characterization of crystallization and ice adhesion on smooth and rough surfaces using molecular dynamics. Appl. Phys. Lett. 2014, 104, 21603. [Google Scholar] [CrossRef]
- Metya, A.K.; Singh, J.K. Ice adhesion mechanism on lubricant-impregnated surfaces using molecular dynamics simula-tions. Mol. Simul. 2019, 45, 394–402. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Kumar, S.V.; Bobji, M.; Chakradhar, R.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Saito, H.; Takai, K.; Yamauchi, G. Water- and ice-repellent coatings. Surf. Coat Int. 1997, 80, 168–171. [Google Scholar] [CrossRef]
- Rahman, M.A.; Jacobi, A.M. Condensation, frost formation, and frost melt-water retention characteristics on micro-grooved brass surfaces under natural convection. Heat Transf. Eng. 2013, 34, 1147–1155. [Google Scholar] [CrossRef]
- Ghalmi, Z.; Menini, R.; Farzaneh, M. Theoritical studies and quantification of ice adhesion mechanisms. In Proceedings of the 13th International Workshop on Atmospheric Icing of Structures, IWAIS, Andermatt, Switzerland, 8–11 September 2009. [Google Scholar]
- Chen, D.; Gelenter, M.D.; Hong, M.; Cohen, R.E.; McKinley, G.H. Icephobic Surfaces Induced by Interfacial Nonfrozen Water. ACS Appl. Mater. Interfaces 2017, 9, 4202–4214. [Google Scholar] [CrossRef]
- Eberle, P.; Tiwari, M.K.; Maitra, T.; Poulikakos, D. Rational nanostructuring of surfaces for extraordinary icephobicity. Nanoscale 2014, 6, 4874–4881. [Google Scholar] [CrossRef]
- He, Z.; Vågenes, E.T.; Delabahan, C.; He, J.; Zhang, Z. Room Temperature Characteristics of Polymer-Based Low Ice Adhesion Surfaces. Sci. Rep. 2017, 7, srep42181. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-Icing Superhydrophobic Coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Kulinich, S.A.; Farzaneh, M. On ice-releasing properties of rough hydrophobic coatings. Cold Reg. Sci. Technol. 2011, 65, 60–64. [Google Scholar] [CrossRef]
- Coasne, B.; Galarneau, A.; Pellenq, R.J.M.; Di Renzo, F. Adsorption, intrusion and freezing in porous silica: The view from the nanoscale. Chem. Soc. Rev. 2013, 42, 4141–4171. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.B.; De La Llave, E.; Welke, K.; Scherlis, D.A.; Molinero, V. Freezing, melting and structure of ice in a hydrophilic nanopore. Phys. Chem. Chem. Phys. 2010, 12, 4124–4134. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Stott, S.L.; Toner, M. Molecular dynamics at the interface between ice and poly (vinyl alcohol) and ice recrys-tallization inhibition. Langmuir 2017, 34, 5116–5123. [Google Scholar] [CrossRef]
- Alba-Simionesco, C.; Coasne, B.; Dosseh, G.; Dudziak, G.; Gubbins, K.E.; Radhakrishnan, R.; Sliwinska-Bartkowiak, M. Effects of confinement on freezing and melting. J. Phys. Condens. Matter 2006, 18, R15–R68. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Hiemenz, P.C. Principles of Colloid and Surface Chemistry; M. Dekker: New York, NY, USA, 1986; Volume 188. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; John Wiley and Sons: New York, NY, USA, 2010. [Google Scholar]
- Webber, J.B.W. Studies of nano-structured liquids in confined geometries and at surfaces. Prog. Nucl. Magn. Reson. Spectrosc. 2010, 56, 78–93. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, S.; Zhuo, Y.; Ding, W.; He, J.; Zhang, Z. Liquid layer generators for excellent icephobicity at extremely low temperatures. Mater. Horizons 2019, 6, 2063–2072. [Google Scholar] [CrossRef]
- Zhuo, Y.; Xiao, S.; Håkonsen, V.; Li, T.; Wang, F.; He, J.; Zhang, Z. Ultrafast self-healing and highly transparent coating with mechanically durable icephobicity. Appl. Mater. Today 2020, 19, 100542. [Google Scholar] [CrossRef]
- Bell, I.G. Models for the specific adhesion of cells to cells. Science 1978, 200, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Zednik, C. Solving the Black Box Problem: A Normative Framework for Explainable Artificial Intelligence. Philos. Technol. 2019, 1–24. [Google Scholar] [CrossRef]
- Samuel, A.L. Some Studies in Machine Learning Using the Game of Checkers. II—Recent Progress. IBM J. Res. Dev. 1967, 11, 601–617. [Google Scholar] [CrossRef]
- Rønneberg, S.; Laforte, C.; Volat, C.; He, J.; Zhang, Z. The effect of ice type on ice adhesion. AIP Adv. 2019, 9, 055304. [Google Scholar] [CrossRef]
- Solveyra, E.G.; De La Llave, E.; Scherlis, D.A.; Molinero, V. Melting and Crystallization of Ice in Partially Filled Nanopores. J. Phys. Chem. B 2011, 115, 14196–14204. [Google Scholar] [CrossRef] [PubMed]
- Molinero, V.; Moore, E.B. Water Modeled as an Intermediate Element between Carbon and Silicon†. J. Phys. Chem. B 2009, 113, 4008–4016. [Google Scholar] [CrossRef]
- Stillinger, F.H.; Weber, T.A. Computer simulation of local order in condensed phases of silicon. Phys. Rev. B 1985, 31, 5262–5271. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys. Rev. B 1984, 29, 6443–6453. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Harrington, P. Machine Learning in Action; Manning Publications Co.: New York, NY, USA, 2012. [Google Scholar]
- Matlab, V. 7.10. 0 (R2010a); The MathWorks Inc.: Natick, MA, USA, 2010.
- Friedman, J.; Hastie, T.; Tibshirani, R. The Elements of Statistical Learning; Springer Series in Statistics: New York, NY, USA, 2001; Volume 1. [Google Scholar]
- Fitzner, M.; Sosso, G.C.; Cox, S.J.; Michaelides, A. The many faces of heterogeneous ice nucleation: Interplay between surface morphology and hydrophobi-city. J. Am. Chem. Soc. 2015, 137, 13658–13669. [Google Scholar] [CrossRef] [PubMed]
- Urata, C.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Self-lubricating organogels (SLUGs) with exceptional syneresis-induced anti-sticking properties against vis-cous emulsions and ices. J. Mater. Chem. A 2015, 3, 12626–12630. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Chai, T.; Draxler, R. Root mean square error (RMSE) or mean absolute error (MAE)?—Arguments against avoiding RMSE in the literature. Geosci. Model. Dev. 2014, 7, 1247–1250. [Google Scholar] [CrossRef]
- Itaoka, K. Regression and interpretation low R-Squares. In Proceedings of the Presentation at Social Research Network 3rd Meeting, Tokyo, Japan, 12–13 April 2012. [Google Scholar]
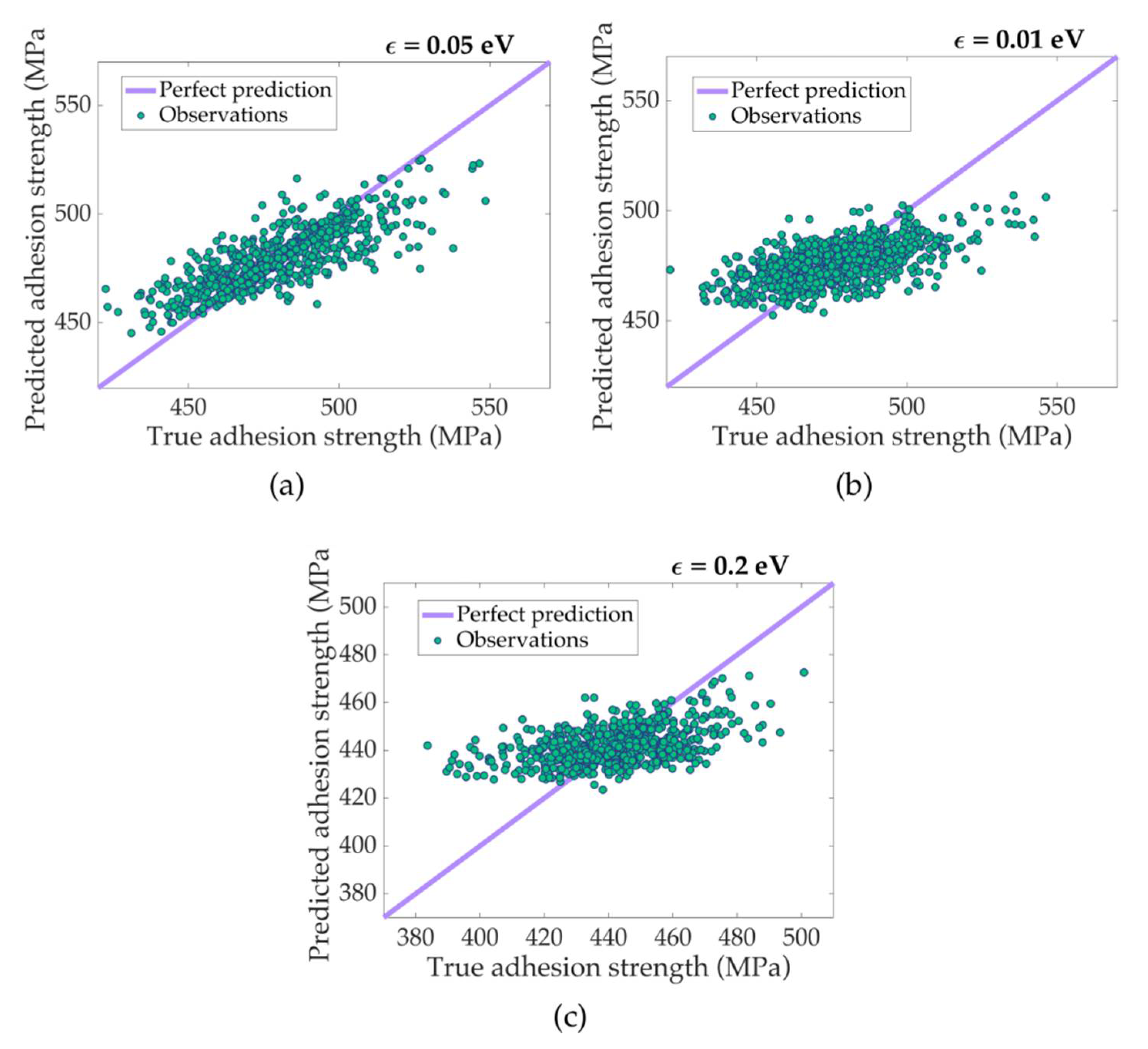
| Tensile Pulling-Regression | |||
|---|---|---|---|
| = 0.05 eV | = 0.1 eV | = 0.2 eV | |
| Observations | 752 | 752 | 634 |
| Linear | RMSE: 17.08 | RMSE: 16.29 | RMSE: 16.43 |
| R2: 0.33 | R2: 0.29 | R2: 0.20 | |
| Quadratic | RMSE: 17.06 | RMSE: 16.29 | RMSE: 16.73 |
| R2: 0.33 | R2: 0.29 | R2: 0.17 | |
| Cubic | RMSE: 17.35 | RMSE: 17.00 | RMSE: 17.16 |
| R2: 0.31 | R2: 0.23 | R2: 0.12 | |
| Fine Gaussian | RMSE: 20.81 | RMSE: 19.32 | RMSE: 18.28 |
| R2: 0.01 | R2: 0 | R2: 0 | |
| Medium Gaussian | RMSE: 16.86 | RMSE: 16.72 | RMSE: 16.64 |
| R2: 0.35 | R2: 0.25 | R2: 0.18 | |
| Coarse Gaussian | RMSE: 17.01 | RMSE: 16.22 | RMSE: 16.37 |
| R2: 0.34 | R2: 0.30 | R2: 0.20 | |
| Tensile Pulling-Classifications | |||
|---|---|---|---|
| = 0.05 eV | = 0.1 eV | = 0.2 eV | |
| Observations | 752 | 752 | 634 |
| Linear | 52.2% | 48.0% | 44.3% |
| Quadratic | 49.7% | 46.1% | 43.7% |
| Cubic | 45.0% | 41.9% | 40.1% |
| Fine Gaussian | 45.2% | 39.0% | 34.9% |
| Medium Gaussian | 50.1% | 49.7% | 45.0% |
| Coarse Gaussian | 51.1% | 49.1% | 43.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringdahl, S.; Xiao, S.; He, J.; Zhang, Z. Machine Learning Based Prediction of Nanoscale Ice Adhesion on Rough Surfaces. Coatings 2021, 11, 33. https://doi.org/10.3390/coatings11010033
Ringdahl S, Xiao S, He J, Zhang Z. Machine Learning Based Prediction of Nanoscale Ice Adhesion on Rough Surfaces. Coatings. 2021; 11(1):33. https://doi.org/10.3390/coatings11010033
Chicago/Turabian StyleRingdahl, Simen, Senbo Xiao, Jianying He, and Zhiliang Zhang. 2021. "Machine Learning Based Prediction of Nanoscale Ice Adhesion on Rough Surfaces" Coatings 11, no. 1: 33. https://doi.org/10.3390/coatings11010033
APA StyleRingdahl, S., Xiao, S., He, J., & Zhang, Z. (2021). Machine Learning Based Prediction of Nanoscale Ice Adhesion on Rough Surfaces. Coatings, 11(1), 33. https://doi.org/10.3390/coatings11010033







