Modified Ferrocenes as Primary Driers for Formulations of Alkyd Paints
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Acyl Chlorides
2.2.1. Synthesis of 4-Fluorobenzoyl Chloride
2.2.2. Synthesis of 4-Methoxybenzoyl Chloride
2.2.3. Synthesis of 3,4,5-Trimethoxybenzoyl Chloride
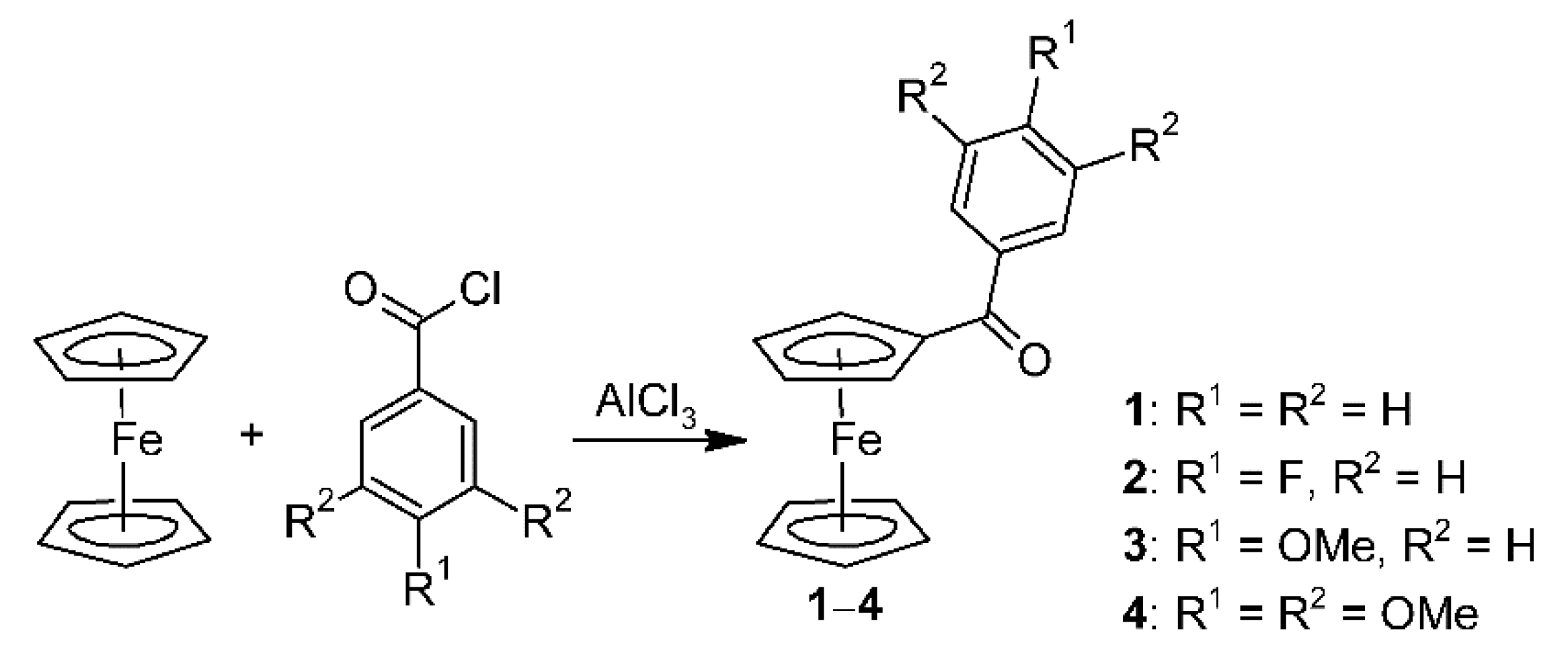
2.3. Synthesis of Modified Ferrocenes
2.3.1. Synthesis of (4-Fluorobenzoyl)ferrocene (2)
2.3.2. Synthesis of (4-Methoxybenzoyl)ferrocene (3)
2.3.3. Synthesis of (3,4,5-Trimethoxybenzoyl)ferrocene (4)
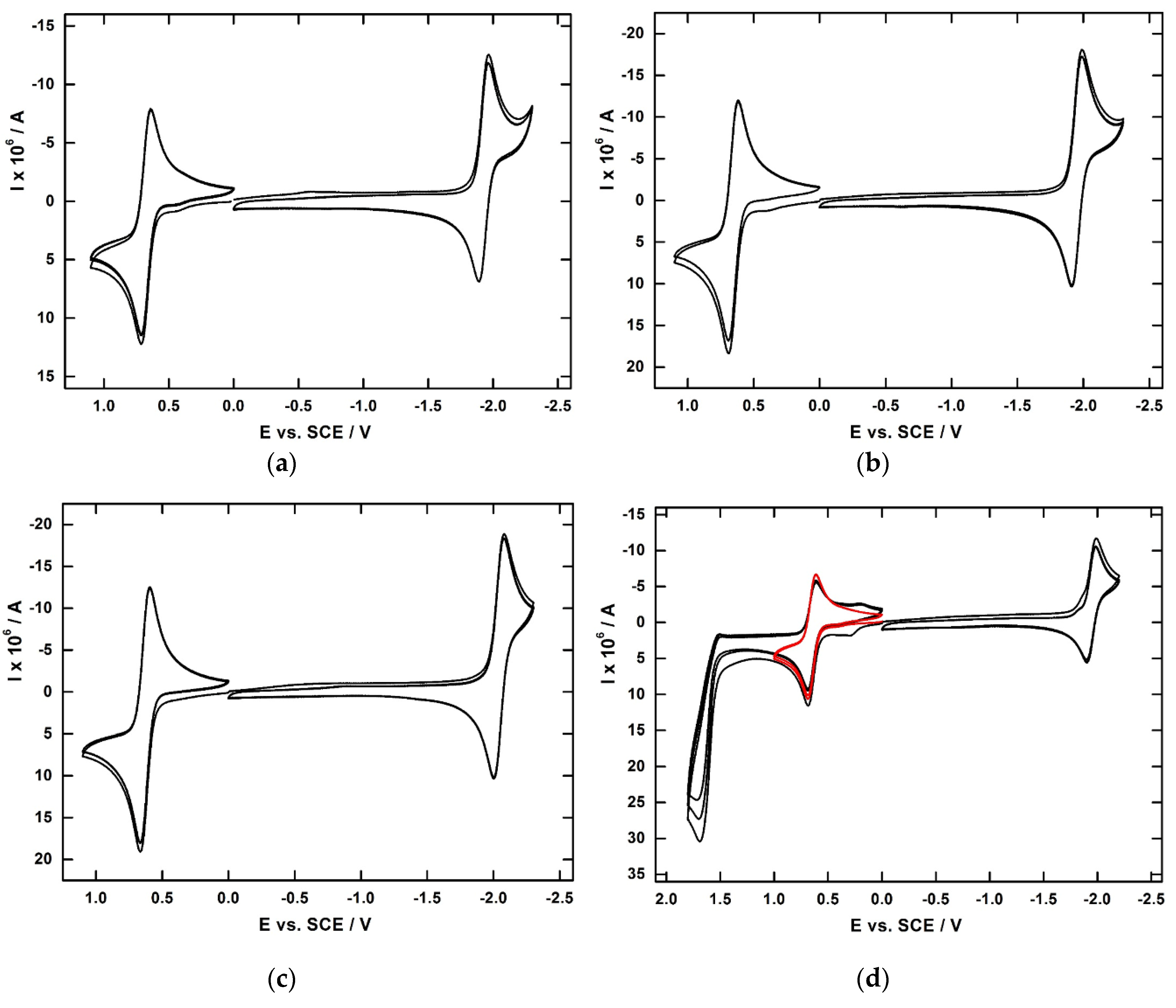
2.4. Electrochemical Studies
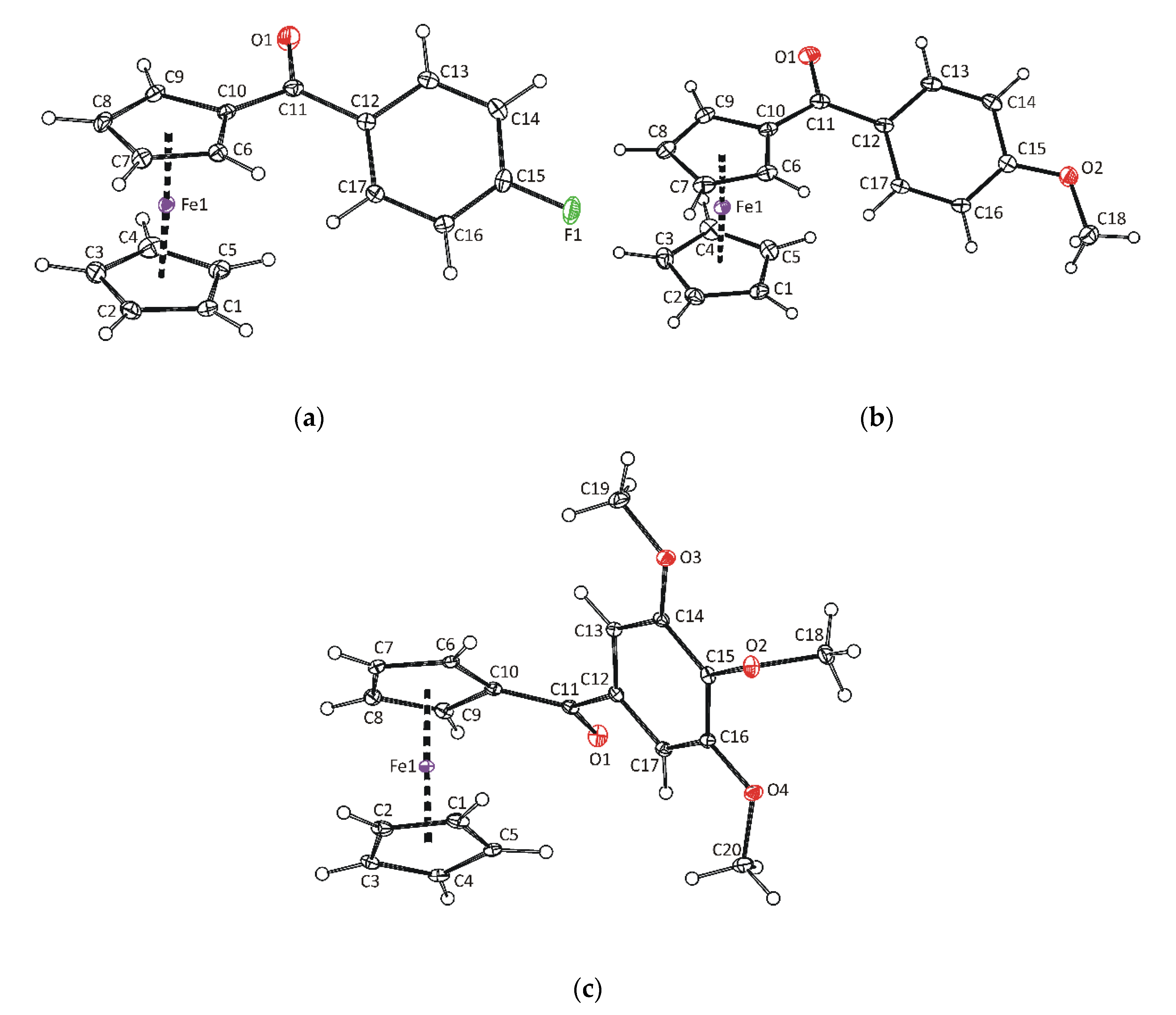
2.5. X-ray Diffraction Analysis
2.6. Preparation of Test Formulations
2.7. Mechanical Tests
2.8. Kinetics of the Autoxidation Process
3. Results and Discussion
3.1. Synthesis and Characterization of Benzoyl Substituted Ferrocenes
3.2. Room Temperature Curing of Alkyd Resin
3.3. Kinetic Studies on the Substituted Ferrocenes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Dai, L.X.; Tu, T.; You, S.L.; Deng, W.P.; Hou, X.L. Asymmetric Catalysis with Chiral Ferrocene Ligands. Acc. Chem. Res. 2003, 36, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Arrayás, R.G.; Adrio, J.; Carretero, J.C. Recent Applications of Chiral Ferrocene Ligands in Asymmetric Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7674–7715. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Zhao, Y.; Wang, L.; Yu, H.; Ren, F.; Saleem, M.; Amer, W.A. Recent research progress in the synthesis and properties of burning rate catalysts based on ferrocene-containing polymers and derivatives. J. Organomet. Chem. 2014, 755, 16–32. [Google Scholar] [CrossRef]
- Li, L.; Shi, J.L.; Yan, J.N.; Zhao, X.G.; Chen, H.G. Mesoporous SBA-15 material functionalized with ferrocene group and its use as heterogeneous catalyst for benzene hydroxylation. Appl. Catal. A Gen. 2004, 263, 213–217. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Ning, P. Ferrocene-Catalyzed Heterogeneous Fenton-like Degradation of Methylene Blue: Influence of Initial Solution pH. Ind. Eng. Chem. Res. 2014, 53, 6334–6340. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Liu, X.; Zhao, Q.; Zhang, H.; Zhang, Y.; Ning, P.; Tian, S. Ferrocene-catalyzed heterogeneous Fenton-like degradation mechanisms and pathways of antibiotics under simulated sunlight: A case study of sulfamethoxazole. J. Hazard. Mater. 2018, 353, 26–34. [Google Scholar] [CrossRef]
- Andrew Lin, K.Y.; Lin, T.Y.; Chena, Y.C.; Lin, Y.F. Ferrocene as an efficient and recyclable heterogeneous catalyst for catalytic ozonation in water. Catal. Commun. 2017, 95, 40–45. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kutal, C. Benzoyl-Substituted Ferrocenes: An Attractive New Class of Anionic Photoinitiators. Macromolecules 2000, 33, 1152–1156. [Google Scholar] [CrossRef]
- Sanderson, C.T.; Palmer, B.J.; Morgan, A.; Murphy, M.; Dluhy, R.A.; Mize, T.; Amster, I.J.; Kutal, C. Classical Metallocenes as Photoinitiators for the Anionic Polymerization of an Alkyl 2-Cyanoacrylate. Macromolecules 2002, 35, 9648–9652. [Google Scholar] [CrossRef]
- Jones, F.N. Alkyd Resins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Honzíček, J. Curing of Air-Drying Paints: A Critical Review. Ind. Eng. Chem. Res. 2019, 58, 12485–12505. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Simpson, N.; Maaijen, K.; Roelofsen, Y.; Hage, R. The Evolution of Catalysis for Alkyd Coatings: Responding to Impending Cobalt Reclassification with Very Active Iron and Manganese Catalysts, Using Polydentate Nitrogen Donor Ligands. Catalysts 2019, 9, 825. [Google Scholar] [CrossRef]
- Matušková, E.; Honzíček, J. Performance of Manganese(III) Acetylacetonate in Solvent-Borne and High-Solid Alkyd Formulations. Materials 2020, 13, 642. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, E.; van Gorkum, R. A study of new manganese complexes as potential driers for alkyd paints. J. Coat. Technol. Res. 2007, 4, 491–503. [Google Scholar] [CrossRef]
- de Boer, J.W.; Wesenhagen, P.V.; Wenker, E.C.M.; Maaijen, K.; Gol, F.; Gibbs, H.; Hage, R. The Quest for Cobalt-Free Alkyd Paint Driers. Eur. J. Inorg. Chem. 2013, 3581–3591. [Google Scholar] [CrossRef]
- Křižan, M.; Vinklárek, J.; Erben, M.; Císařová, I.; Honzíček, J. Autoxidation of alkyd resins catalyzed by iron(II) bispidine complex: Drying performance and in-depth infrared study. Prog. Org. Coat. 2017, 111, 361–370. [Google Scholar] [CrossRef]
- Preininger, O.; Vinklárek, J.; Honzíček, J.; Mikysek, T.; Erben, M. A promising drying activity of environmentally friendly oxovanadium(IV) complexes in air-drying paints. Prog. Org. Coat. 2015, 88, 191–198. [Google Scholar] [CrossRef]
- Preininger, O.; Honzíček, J.; Kalenda, P.; Vinklárek, J. Drying activity of oxovanadium(IV) 2-ethylhexanoate in solvent-borne alkyd paints. J. Coat. Technol. Res. 2016, 13, 479–487. [Google Scholar] [CrossRef]
- Preininger, O.; Charamzová, I.; Vinklárek, J.; Císařová, I.; Honzíček, J. Oxovanadium(IV) complexes bearing substituted pentane-2,4-dionate ligands: Synthesis, structure and drying activity in solvent-borne alkyd paints. Inorg. Chim. Acta 2017, 462, 16–22. [Google Scholar] [CrossRef]
- Charamzová, I.; Machálková, A.; Vinklárek, J.; Císařová, I.; Honzíček, J. Benzyl substituted oxidovanadium(IV) pentane-2,4-dionates: Synthesis, structure and drying properties. Inorg. Chim. Acta 2019, 492, 243–248. [Google Scholar] [CrossRef]
- Charamzová, I.; Vinklárek, J.; Kalenda, P.; Honzíček, J. Application of Oxovanadium Complex Stabilized by N,N,N,N-Chelating Ligand in Air-Drying Paints. Coatings 2018, 8, 204. [Google Scholar] [CrossRef]
- Charamzová, I.; Vinklárek, J.; Kalenda, P.; Císařová, I.; Honzíček, J. Oxidovanadium(V) dithiocarbamates as driers for alkyd binders. J. Coat. Technol. Res. 2020. [Google Scholar] [CrossRef]
- Stava, V.; Erben, M.; Vesely, D.; Kalenda, P. Properties of metallocene complexes during the oxidative crosslinking of air drying coatings. J. Phys. Chem. Solids 2007, 68, 799–802. [Google Scholar] [CrossRef]
- Erben, M.; Veselý, D.; Vinklárek, J.; Honzíček, J. Acyl-substituted ferrocenes as driers for solvent-borne alkyd paints. J. Mol. Catal. A Chem. 2012, 353–354, 13–21. [Google Scholar] [CrossRef]
- Honzíček, J.; Vinklárek, J. Chemical curing of alkyd resin catalyzed by benzoylferrocene: Performance, kinetics, and thickness effects. J. Appl. Polym. Sci. 2018, 135, 46184. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Lu, B.; Wang, Q.; Zhao, M.; Xie, X.; Zhang, Z. Ruthenium-Catalyzed Enantioselective Hydrogenation of Ferrocenyl Ketones: A Synthetic Method for Chiral Ferrocenyl Alcohols. J. Org. Chem. 2015, 80, 9563–9569. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Sammakia, T. The Conversion of tert-Butyl Esters to Acid Chlorides Using Thionyl Chloride. J. Org. Chem. 2017, 82, 3245–3251. [Google Scholar] [CrossRef]
- Zaragoza, F. One-Step Conversion of Methyl Ketones to Acyl Chlorides. J. Org. Chem. 2015, 80, 10370–10374. [Google Scholar] [CrossRef]
- Ghinet, A.; Rigo, B.; Hénichart, J.P.; Le Broc-Ryckewaert, D.; Pommery, J.; Pommery, N.; Thuru, X.; Quesnel, B.; Gautret, P. Synthesis and biological evaluation of phenstatin metabolites. Bioorg. Med. Chem. 2011, 19, 6042–6054. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Khobragade, D.A.; Mahamulkar, S.G.; Pospíšil, L.; Císařová, I.; Rulíšek, L.; Jahn, U. Acceptor-Substituted Ferrocenium Salts as Strong, Single-Electron Oxidants: Synthesis, Electrochemistry, Theoretical Investigations, and Initial Synthetic Application. Chem. Eur. J. 2012, 18, 12267–12277. [Google Scholar] [CrossRef] [PubMed]
- Butler, I.R.; Cullen, W.R.; Rettig, S.J.; Trotter, J. Structure of Benzoylferrocene. Acta Crystallogr. Sect. C Struct. Chem. 1988, 44, 1666–1667. [Google Scholar] [CrossRef]
- Persoz, B. The hardness pendulum. Peint. Pigment. Vernis 1945, 21, 194–201. [Google Scholar]
- Gezici-Koç, Ö.; Thomas, C.A.A.M.; Michel, M.E.B.; Erich, S.J.F.; Huinink, H.P.; Flapper, J.; Duivenvoorde, F.L.; van der Ven, L.G.J.; Adan, O.C.G. In-depth study of drying solvent-borne alkyd coatings in presence of Mn and Fe- based catalysts as cobalt alternatives. Mater. Today Commun. 2016, 7, 22–31. [Google Scholar] [CrossRef]
- van Gorkum, R.; Bouwman, E.; Reedijk, J. Fast Autoxidation of Ethyl Linoleate Catalyzed by [Mn(acac)3] and Bipyridine: A Possible Drying Catalyst for Alkyd Paints. Inorg. Chem. 2004, 43, 2456–2458. [Google Scholar] [CrossRef]
- Charamzová, I.; Vinklárek, J.; Honzíček, J. Effect of primary driers on oxidative drying of high-solid alkyd binder: Investigation of thickness effects by mechanical tests and infrared spectroscopy. Prog. Org. Coat. 2018, 125, 177–185. [Google Scholar] [CrossRef]
| Compound | 2 | 3 | 4 |
|---|---|---|---|
| Formula | C17H13FFeO | C18H16FeO2 | C20H20FeO4 |
| Mr | 308.12 | 320.16 | 380.21 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P (No. 2) | P21/n (No. 14) | P21/c (No. 14) |
| a (Å) | 6.1079(2) | 6.3152(1) | 8.6322(7) |
| b (Å) | 10.1461(3) | 28.2133(6) | 14.7211(10) |
| c (Å) | 10.8772(3) | 8.0592(2) | 13.3636(9) |
| α (°) | 81.068(1) | 90 | 90 |
| β (°) | 76.508(1) | 101.506(1) | 101.811(2) |
| γ (°) | 82.235(1) | 90 | 90 |
| Z | 2 | 4 | 4 |
| V (Å3) | 644.08(3) | 1407.07(5) | 1662.2(2) |
| dc (g∙cm−3) | 1.589 | 1.511 | 1.519 |
| Crystal size | 0.549 × 0.426 × 0.150 | 0.814 × 0.613 × 0.502 | 0.383 × 0.347 × 0.252 |
| Color | Orange | Orange | Orange-red |
| Shape | Prism | Prism | Prism |
| μ (mm−1) | 1.173 | 1.072 | 0.929 |
| F(000) | 316.0 | 664.0 | 792.0 |
| h range | −7, 7 | −7, 8 | −11, 11 |
| k range | −13, 9 | −38, 36 | −19, 19 |
| l range | −14, 14 | −10, 7 | −17, 17 |
| θ range (°) | 1.941, 27.498 | 2.678, 27.494 | 2.410, 27.541 |
| Reflections measured | 8228 | 6585 | |
| Reflections independent (Rint 1) | 2919 (0.0165) | 3213 (0.0143) | 3822 (0.0206) |
| Reflections observed [I > 2σ(I)] | 2684 | 3014 | 3675 |
| No. of parameters | 181 | 191 | 229 |
| GOF 2 | 1.044 | 1.119 | 1.075 |
| R3, wR4 | 0.0239, 0.0572 | 0.0265, 0.0621 | 0.0243, 0.0656 |
| Δρ (e∙Å−3) | 0.369, −0.256 | 0.302, −0.308 | 0.337, −0.465 |
| Compound | EoF (ox1) [V] 1 MeCN/DMF | EoF (red1) [V] 1 MeCN/DMF | ΔE [V] 2 MeCN/DMF |
|---|---|---|---|
| 1 | 0.67/0.70 | −1.93/−1.91 | 2.61/2.61 |
| 2 | 0.65/0.70 | −1.95/−1.90 | 2.61/2.61 |
| 3 | 0.63/0.68 | −2.04/−2.02 | 2.67/2.69 |
| 4 | 0.65/0.69 | −1.95/−1.92 | 2.60/2.61 |
| Parameter | 1 2 | 2 | 3 | 4 |
|---|---|---|---|---|
| Fe–Cg1 3 | 1.6464(10) | 1.6489(7) | 1.6426(8) | 1.6454(6) |
| Fe–Cg2 4 | 1.6549(12) | 1.6517(7) | 1.6508(8) | 1.6563(6) |
| Cg1–Fe–Cg2 3,4 | 177.57(6) | 177.86(4) | 177.68(5) | 177.46(3) |
| α 5 | 14.26(13) | 17.60(9) | 15.18(9) | 10.84(8) |
| β 6 | 26.20(13) | 25.29(8) | 23.60(8) | 44.75(7) |
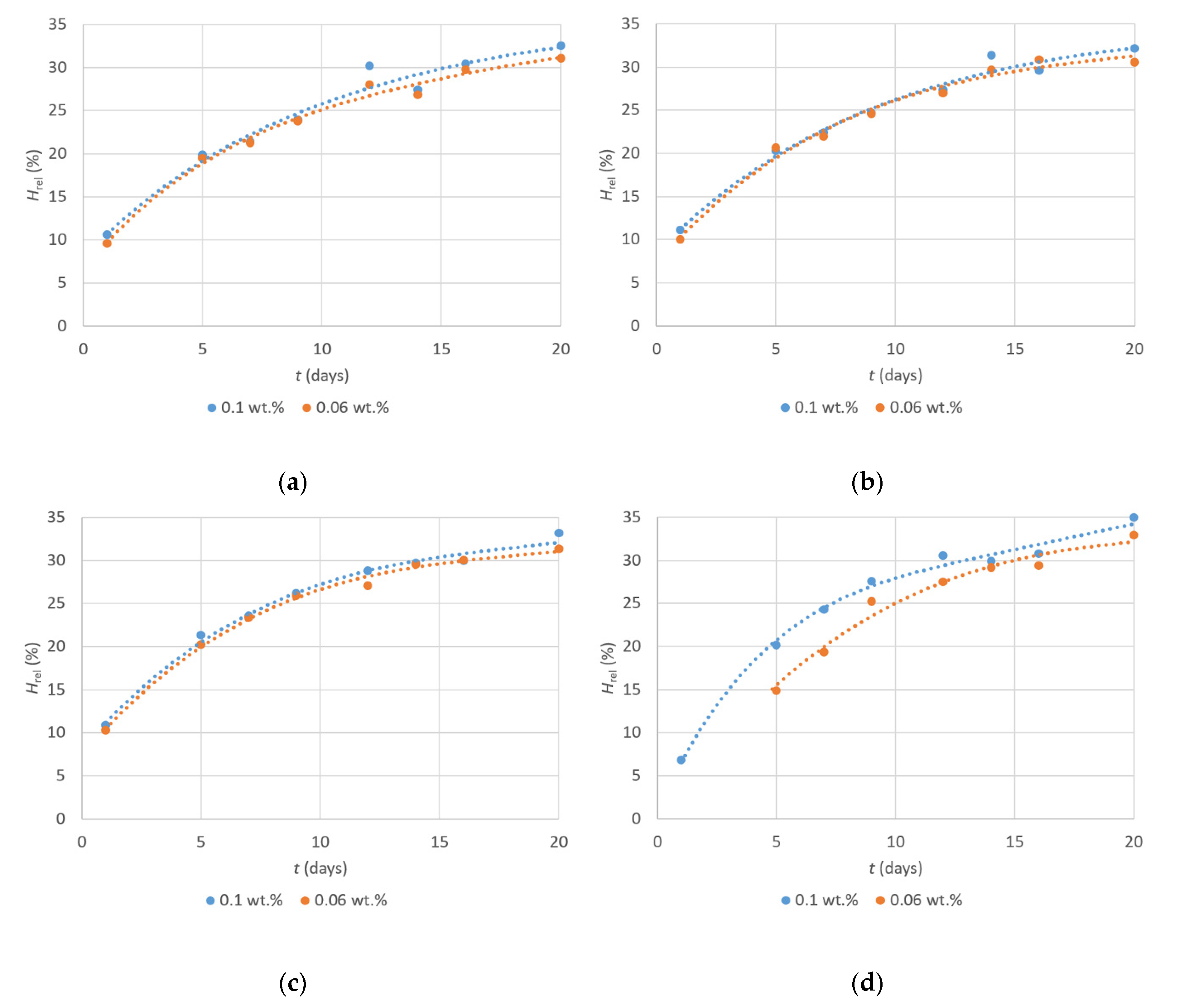
| Drier | Metal Content (wt.%) | τ1 1 (h) | τ2 2 (h) | τ3 3 (h) | Hrel,10d4 (%) | Hrel,100d5 (%) |
|---|---|---|---|---|---|---|
| 1 | 0.1 | 10.1 | 12.1 | 12.1 | 24.9 | 41.9 |
| 0.06 | 19.7 | 21.6 | 21.6 | 24.7 | 41.6 | |
| 0.03 | >24 | >24 | >24 | – 6 | – 6 | |
| 2 | 0.1 | 5.8 | 7.7 | 9.2 | 25.6 | 40.5 |
| 0.06 | 14.1 | 17.2 | >24 | 25.4 | 39.7 | |
| 0.03 | >24 | >24 | >24 | – 6 | – 6 | |
| 3 | 0.1 | 0.9 | 4.5 | 5.1 | 27.0 | 39.7 |
| 0.06 | 4.0 | 5.5 | 7.2 | 26.6 | 39.1 | |
| 0.03 | 9.7 | 14.5 | 16.9 | – 6 | – 6 | |
| 4 | 0.1 | 16.4 | 18.8 | 18.8 | 28.6 | 42.0 |
| 0.06 | 22.5 | >24 | >24 | 26.0 | 40.8 | |
| 0.03 | >24 | >24 | >24 | – 6 | – 6 | |
| Co-2EH | 0.1 | 0.9 | 5.5 | 5.5 | 25.9 | 61.2 |
| 0.06 | 2.7 | 8.1 | 8.1 | 35.3 | 53.5 | |
| 0.03 | 7.9 | 11.3 | >24 | – 6 | – 6 |
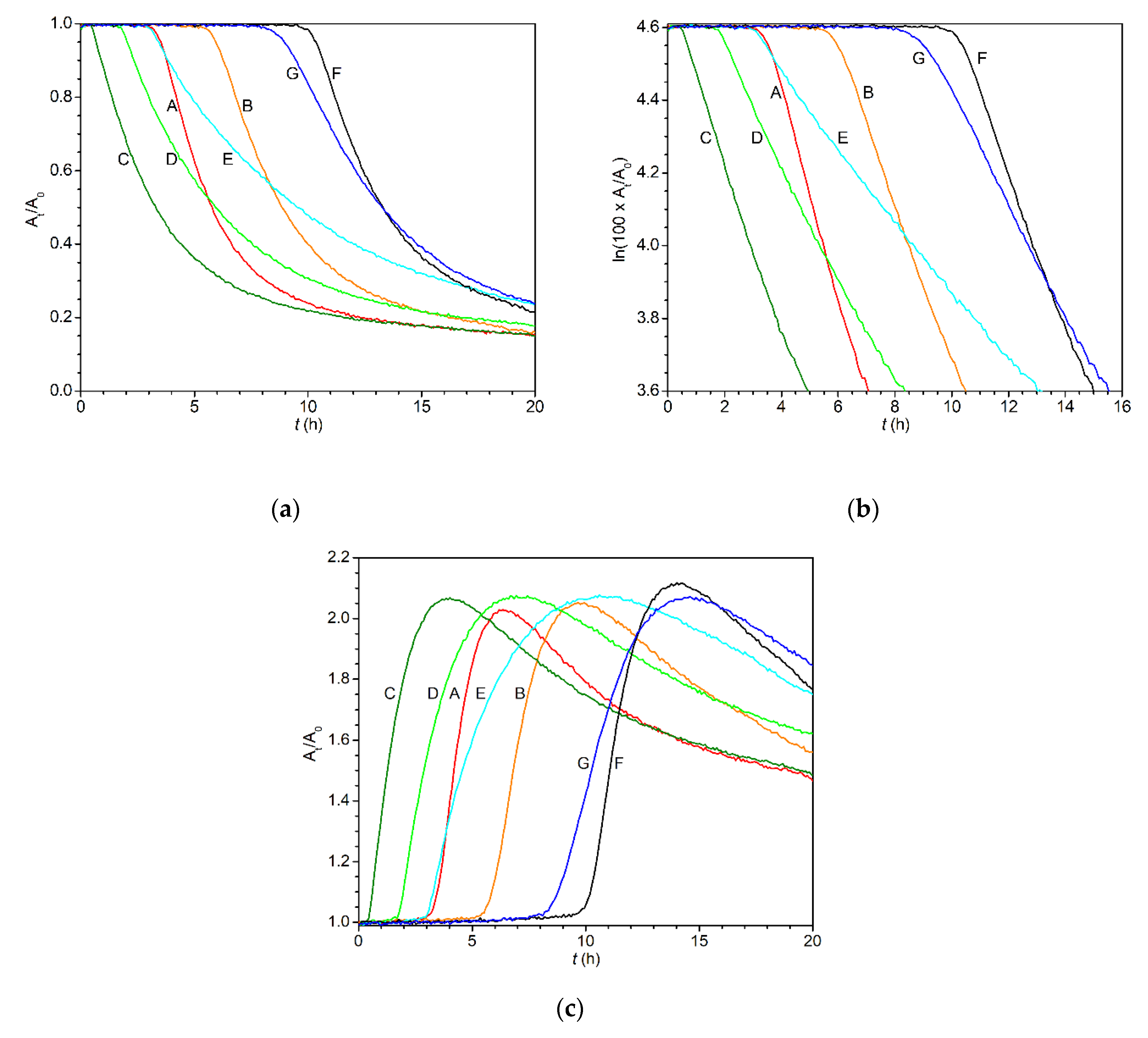
| Drier | Metal Content (wt.%) | kmax1 (h−1) | IT1 (h) | t1/21 (h) | tconj2 (h) |
|---|---|---|---|---|---|
| 1 | 0.1 | 0.30 | 3.4 | 5.8 | 6.5 |
| 0.06 | 0.24 | 5.7 | 8.9 | 9.8 | |
| 3 | 0.1 | 0.25 | 0.5 | 3.3 | 4.0 |
| 0.06 | 0.18 | 1.8 | 5.9 | 7.4 | |
| 0.03 | 0.12 | 3.0 | 9.5 | 11.0 | |
| 4 | 0.1 | 0.23 | 10.3 | 13.3 | 14.1 |
| 0.06 | 0.16 | 8.9 | 13.2 | 14.7 | |
| Co-2EH3 | 0.1 | 2.18 | 0.2 | 0.5 | 0.6 |
| 0.06 | 1.55 | 0.5 | 0.9 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honzíček, J.; Fedorova, T.; Vinklárek, J.; Mikysek, T.; Císařová, I. Modified Ferrocenes as Primary Driers for Formulations of Alkyd Paints. Coatings 2020, 10, 873. https://doi.org/10.3390/coatings10090873
Honzíček J, Fedorova T, Vinklárek J, Mikysek T, Císařová I. Modified Ferrocenes as Primary Driers for Formulations of Alkyd Paints. Coatings. 2020; 10(9):873. https://doi.org/10.3390/coatings10090873
Chicago/Turabian StyleHonzíček, Jan, Tatiana Fedorova, Jaromír Vinklárek, Tomáš Mikysek, and Ivana Císařová. 2020. "Modified Ferrocenes as Primary Driers for Formulations of Alkyd Paints" Coatings 10, no. 9: 873. https://doi.org/10.3390/coatings10090873
APA StyleHonzíček, J., Fedorova, T., Vinklárek, J., Mikysek, T., & Císařová, I. (2020). Modified Ferrocenes as Primary Driers for Formulations of Alkyd Paints. Coatings, 10(9), 873. https://doi.org/10.3390/coatings10090873