E-Beam Deposition of Scandia-Stabilized Zirconia (ScSZ) Thin Films Co-Doped with Al
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Walter, E.J.; Lewis, S.P.; Rappe, A.M. First principles study of carbon monoxide adsorption on zirconia-supported copper. Surf. Sci. 2001, 495, 44–50. [Google Scholar] [CrossRef][Green Version]
- Sriubas, M.; Kainbayev, N.; Virbukas, D.; Bočkute, K.; Rutkuniene, Ž.; Laukaitis, G. Structure and conductivity studies of scandia and alumina doped zirconia thin films. Coatings 2019, 9, 317. [Google Scholar] [CrossRef]
- Zavodinsky, V.G. The mechanism of ionic conductivity in stabilized cubic zirconia. Phys. Solid State 2004, 46, 453–457. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T. Oxygen-ion conducting electrolyte materials for solid oxide fuel cells. Ionics 2000, 6, 1–21. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, W.S.; Choi, S.H.; Kim, J.; Lee, H.W.; Lee, J.H. Characterization of ZrO2 co-doped with Sc2O3 and CeO2 electrolyte for the application of intermediate temperature SOFCs. Solid State Ion. 2005, 176, 33–39. [Google Scholar] [CrossRef]
- Liu, Y.; Lao, L.E. Structural and electrical properties of ZnO-doped 8 mol % yttria-stabilized zirconia. Solid State Ion. 2006, 177, 159–163. [Google Scholar] [CrossRef]
- Mahato, N.; Gupta, A.; Balani, K. Doped zirconia and ceria-based electrolytes for solid oxide fuel cells: A review. Nanomater. Energy 2012, 1, 27–45. [Google Scholar] [CrossRef]
- Sarat, S.; Sammes, N.; Smirnova, A. Bismuth oxide doped scandia-stabilized zirconia electrolyte for the intermediate temperature solid oxide fuel cells. J. Power Sources 2006, 160, 892–896. [Google Scholar] [CrossRef]
- Ishii, T. Structural phase transition and ionic conductivity in 0.88ZrO2(0.12 − x)Sc2O3xAl2O3. Solid State Ion. 1995, 78, 333–338. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, M.; Bi, Z.; Dong, Y.; Zhang, H.; Zhang, J.; Feng, Z.; Li, C. Structure and impedance of ZrO2 doped with Sc2O3 and CeO2. Mater. Lett. 2005, 59, 2579–2582. [Google Scholar] [CrossRef]
- Yamamoto, O.; Arati, Y.; Takeda, Y.; Imanishi, N.; Mizutani, Y.; Kawai, M.; Nakamura, Y. Electrical conductivity of stabilized zirconia with ytterbia and scandia. Solid State Ion. 1995, 79, 137–142. [Google Scholar] [CrossRef]
- Omar, S.; Belda, A.; Escardino, A.; Bonanos, N. Ionic conductivity ageing investigation of 1Ce10ScSZ in different partial pressures of oxygen. Solid State Ion. 2011, 184, 2–5. [Google Scholar] [CrossRef]
- Hirano, M.; Oda, T.; Ukai, K.; Mizutani, Y. Effect of Bi2O3 additives in Sc stabilized zirconia electrolyte on a stability of crystal phase and electrolyte properties. Solid State Ion. 2003, 158, 215–223. [Google Scholar] [CrossRef]
- Belous, A.G.; V’Yunov, O.I.; Gunes, V.; Bohnke, O. Ionic and electronic conductivities of yttria- and scandia-stabilized zirconia. Inorg. Mater. 2014, 50, 1235–1241. [Google Scholar] [CrossRef]
- Guo, C.X.; Wang, J.X.; He, C.R.; Wang, W.G. Effect of alumina on the properties of ceria and scandia co-doped zirconia for electrolyte-supported SOFC. Ceram. Int. 2013, 39, 9575–9582. [Google Scholar] [CrossRef]
- Agarkov, D.A.; Borik, M.A.; Bredikhin, S.I.; Burmistrov, I.N.; Eliseeva, G.M.; Kolotygin, V.A.; Kulebyakin, A.V.; Kuritsyna, I.E.; Lomonova, E.E.; Milovich, F.O.; et al. Structure and transport properties of zirconia crystals co-doped by scandia, ceria and yttria. J. Mater. 2019, 5, 273–279. [Google Scholar] [CrossRef]
- Zhang, F.; Reveron, H.; Spies, B.C.; van Meerbeek, B.; Chevalier, J. Trade-off between fracture resistance and translucency of zirconia and lithium-disilicate glass ceramics for monolithic restorations. Acta Biomater. 2019, 91, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Dasari, H.P.; Ahn, J.S.; Ahn, K.; Park, S.Y.; Hong, J.; Kim, H.; Yoon, K.J.; Son, J.W.; Lee, H.W.; Lee, J.H. Synthesis sintering and conductivity behavior of ceria-doped Scandia-stabilized zirconia. Solid State Ion. 2014, 263, 103–109. [Google Scholar] [CrossRef]
- Hirata, T.; Asari, E.; Kitajima, M. Infrared and raman spectroscopic studies of ZrO2 polymorphs doped with Y2O3 or CeO2. J. Solid State Chem. 1994, 110, 201–207. [Google Scholar] [CrossRef]
- Li, L.; Wang, W. Synthesis and characterization of monoclinic ZrO2 nanorods by a novel and simple precursor thermal decomposition approach. Solid State Commun. 2003, 127, 639–643. [Google Scholar] [CrossRef]
- Huy, L.D.; Laffez, P.; Daniel, P.; Jouanneaux, A.; Khoi, N.T.; Siméone, D. Structure and phase component of ZrO2 thin films studied by Raman spectroscopy and X-ray diffraction. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2003, 104, 163–168. [Google Scholar] [CrossRef]
- Porozova, S.E.; Smetkin, A.A.; Solnyshkov, I.V. Surface composition and structure of highly porous materials based zirconia stabilized with yttria. Russ. J. Non-Ferrous Met. 2017, 58, 664–669. [Google Scholar] [CrossRef]
- Clarke, D.R.; Adar, F. Measurement of the crystallographically transformed zone produced by fracture in ceramics containing tetragonal zirconia. J. Am. Ceram. Soc. 1982, 65, 284–288. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of crystal structure of nanosized ZrO2 on photocatalytic degradation of methyl orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Gazzoli, D.; Mattei, G.; Valigi, M. Raman and X-ray investigations of the incorporation of Ca2+ and Cd2+ in the ZrO2 structure. J. Raman Spectrosc. 2007, 38, 824–831. [Google Scholar] [CrossRef]
- Nomura, K.; Mizutani, Y.; Kawai, M.; Nakamura, Y.; Yamamoto, O. Aging and raman scattering study of scandia and yttria doped zirconia. Solid State Ion. 2000, 132, 235–239. [Google Scholar] [CrossRef]
- Kumar, A.; Jaiswal, A.; Sanbui, M.; Omar, S. Scandia stabilized zirconia-ceria solid electrolyte (xSc1CeSZ, 5 < x < 11) for IT-SOFCs: Structure and conductivity studies. Scr. Mater. 2016, 121, 10–13. [Google Scholar] [CrossRef]
- Xue, Q.; Huang, X.; Zhang, H.; Xu, H.; Zhang, J.; Wang, L. Synthesis and characterization of high ionic conductivity ScSZ core/shell nanocomposites. J. Rare Earths 2017, 35, 567–573. [Google Scholar] [CrossRef]
- Li, M.; Feng, Z.; Ying, P.; Xin, Q.; Li, C. Phase transformation in the surface region of zirconia and doped zirconia detected by UV Raman spectroscopy. Phys. Chem. Chem. Phys. 2003, 5, 5326–5332. [Google Scholar] [CrossRef]
- Lyamina, G.; Ilela, A.; Khasanov, O.; Petyukevich, M.; Vaitulevich, E. Synthesis of Al2O3–ZrO2 powders from differently concentrated suspensions with a spray drying technique. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2016; Volume 1772, p. 020011. [Google Scholar] [CrossRef]
- Morant, C.; Sanz, J.M.; Galán, L.; Soriano, L.; Rueda, F. An XPS study of the interaction of oxygen with zirconium. Surf. Sci. 1989, 218, 331–345. [Google Scholar] [CrossRef]
- Milanov, A.P.; Xu, K.; Cwik, S.; Parala, H.; de los Arcos, T.; Becker, H.W.; Rogalla, D.; Cross, R.; Paul, S.; Devi, A. Sc2O3, Er2O3, and Y2O3 thin films by MOCVD from volatile guanidinate class of rare-earth precursors. Dalt. Trans. 2012, 41, 13936–13947. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J. Phys. Chem. C. 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Raja, J.; Nguyen, C.P.T.; Lee, C.; Balaji, N.; Chatterjee, S.; Jang, K.; Kim, H.; Yi, J. Improved data retention of InSnZnO nonvolatile memory by H2O2 treated Al2O3 tunneling layer: A cost-effective method. IEEE Electron Device Lett. 2016, 37, 1272–1275. [Google Scholar] [CrossRef]
- Corsi, J.S.; Fu, J.; Wang, Z.; Lee, T.; Ng, A.K.; Detsi, E. Hierarchical bulk nanoporous aluminum for on-site generation of hydrogen by hydrolysis in pure water and combustion of solid fuels. ACS Sustain. Chem. Eng. 2019, 7, 11194–11204. [Google Scholar] [CrossRef]
- Srdić, V.V.; Winterer, M. Aluminum-doped zirconia nanopowders: Chemical vapor synthesis and structural analysis by rietveld refinement of X-ray diffraction data. Chem. Mater. 2003, 15, 2668–2674. [Google Scholar] [CrossRef]
- Dahl, G.T.; Döring, S.; Krekeler, T.; Janssen, R.; Ritter, M.; Weller, H.; Vossmeyer, T. Alumina-doped zirconia submicro-particles: Synthesis, thermal stability, and microstructural characterization. Materials 2019, 12, 2856. [Google Scholar] [CrossRef]
- Yoo, Y.W.; Jeon, W.; Lee, W.; An, C.H.; Kim, S.K.; Hwang, C.S. Structure and electrical properties of Al-doped HfO2 and ZrO2 films grown via atomic layer deposition on Mo electrodes. ACS Appl. Mater. Interfaces 2014, 6, 22474–22482. [Google Scholar] [CrossRef]
- Spiga, S.; Rao, R.; Lamagna, L.; Wiemer, C.; Congedo, G.; Lamperti, A.; Molle, A.; Fanciulli, M.; Palma, F.; Irrera, F. Structural and electrical properties of atomic layer deposited Al-doped ZrO2 films and of the interface with TaN electrode. J. Appl. Phys. 2012, 112, 014107. [Google Scholar] [CrossRef]
- Gorji, N.E. Oxygen incorporation into CdS/CdTe thin film solar cells. Opt. Quantum Electron 2015, 47, 2445–2453. [Google Scholar] [CrossRef]
- Siu, G.G.; Stokes, M.J.; Liu, Y. Variation of fundamental and higher-order raman spectra of (formula presented) nanograins with annealing temperature. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 3173–3179. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Madden, P.A.; Norberg, S.T.; Hull, S. Structural disorder in doped zirconias, part II: Vacancy ordering effects and the conductivity maximum. Chem. Mater. 2011, 23, 1365–1373. [Google Scholar] [CrossRef]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline CeO2 thin films. Solid State Ion. 2002, 149, 99–105. [Google Scholar] [CrossRef]


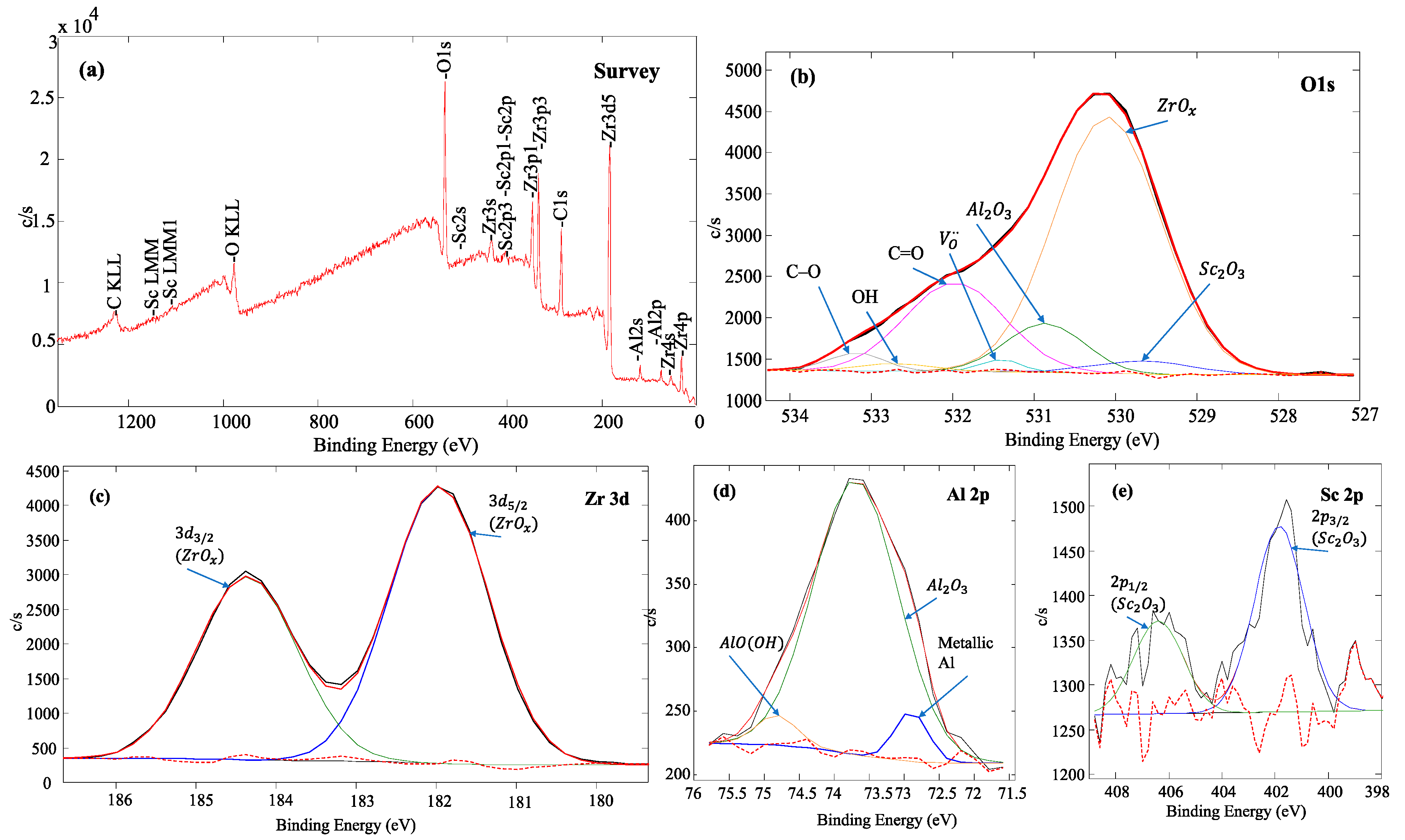
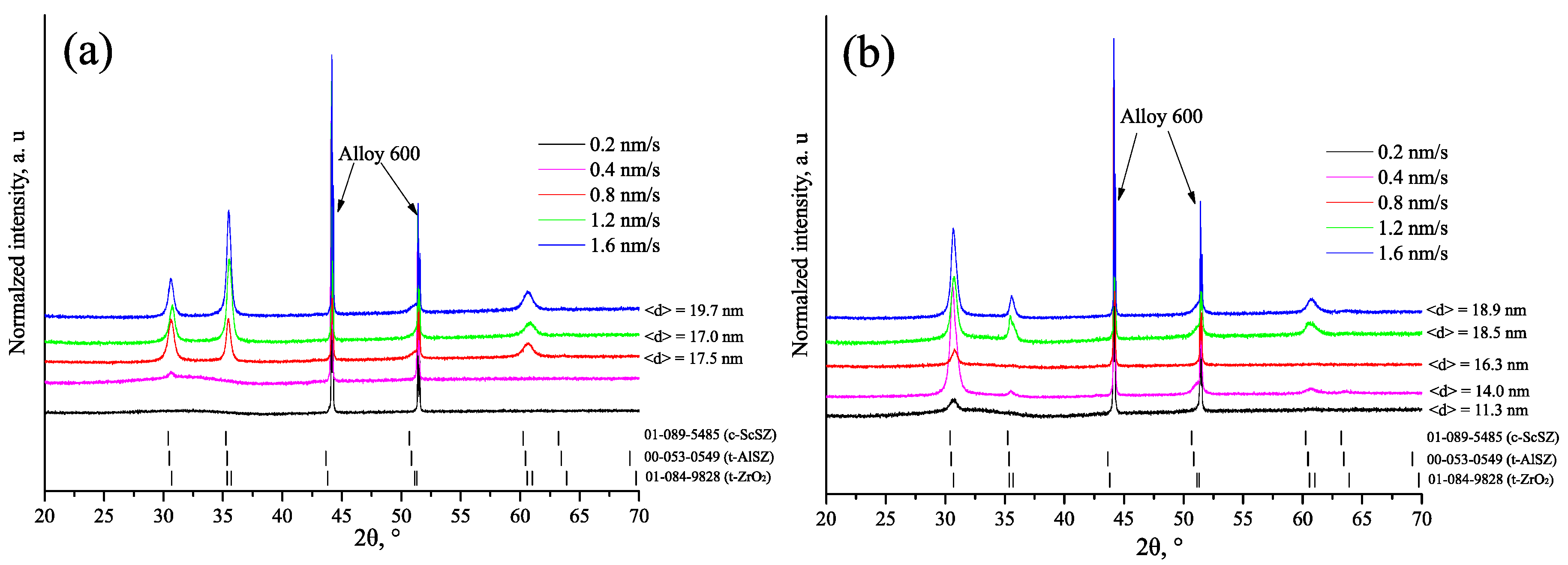
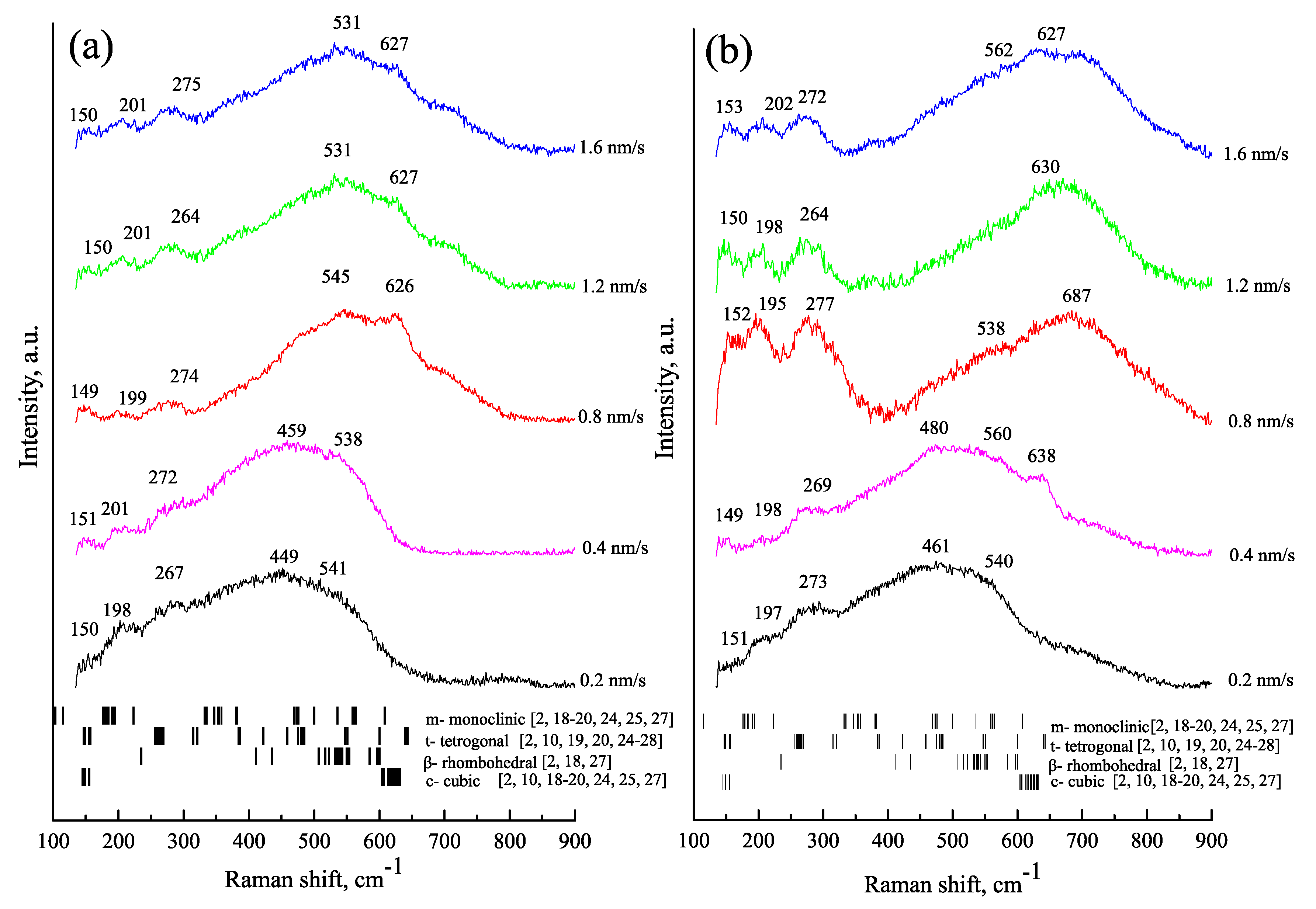
| vd, nm/s | Td = 300 °C | Td = 600 °C | ||||||
|---|---|---|---|---|---|---|---|---|
| cO, at.% | cAl, at.% | cSc, at.% | cZr, at.% | cO, at.% | cAl, at.% | cSc, at.% | cZr, at.% | |
| 0.2 | 51.4 | 20.2 | 3.1 | 25.3 | 54.2 | 12.2 | 3.6 | 30.0 |
| 0.4 | 49.5 | 12.4 | 3.6 | 34.5 | 49.8 | 12.5 | 3.4 | 34.3 |
| 0.8 | 53.4 | 3.8 | 7.4 | 35.4 | 50.6 | 15.8 | 3.6 | 30.0 |
| 1.2 | 51.5 | 6.4 | 8.7 | 33.5 | 53.6 | 8.0 | 6.7 | 31.8 |
| 1.6 | 54.1 | 3.0 | 6.8 | 36.0 | 51.2 | 7.1 | 7.1 | 34.5 |
| Powder | 52.0 | 12.6 | 3.9 | 32.5 | - | |||
| Deposition Rate (nm/s) | Substrate Temperature (°C) | |||||
|---|---|---|---|---|---|---|
| 300 | 600 | 300 | 600 | 300 | 600 | |
| Monoclinic | Tetragonal + Rhombohedral | Cubic | ||||
| 0.2 | 35% | 28% | 65% | 72% | 0% | 0% |
| 0.4 | 19% | 12% | 81% | 88% | 0% | 0% |
| 0.8 | 6% | 54% | 41% | 46% | 53% | 0% |
| 1.2 | 19% | 20% | 26% | 26% | 55% | 53% |
| 1.6 | 19% | 18% | 26% | 23% | 55% | 59% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kainbayev, N.; Sriubas, M.; Bockute, K.; Virbukas, D.; Laukaitis, G. E-Beam Deposition of Scandia-Stabilized Zirconia (ScSZ) Thin Films Co-Doped with Al. Coatings 2020, 10, 870. https://doi.org/10.3390/coatings10090870
Kainbayev N, Sriubas M, Bockute K, Virbukas D, Laukaitis G. E-Beam Deposition of Scandia-Stabilized Zirconia (ScSZ) Thin Films Co-Doped with Al. Coatings. 2020; 10(9):870. https://doi.org/10.3390/coatings10090870
Chicago/Turabian StyleKainbayev, Nursultan, Mantas Sriubas, Kristina Bockute, Darius Virbukas, and Giedrius Laukaitis. 2020. "E-Beam Deposition of Scandia-Stabilized Zirconia (ScSZ) Thin Films Co-Doped with Al" Coatings 10, no. 9: 870. https://doi.org/10.3390/coatings10090870
APA StyleKainbayev, N., Sriubas, M., Bockute, K., Virbukas, D., & Laukaitis, G. (2020). E-Beam Deposition of Scandia-Stabilized Zirconia (ScSZ) Thin Films Co-Doped with Al. Coatings, 10(9), 870. https://doi.org/10.3390/coatings10090870





