Corrosion Behavior and Biological Activity of Micro Arc Oxidation Coatings with Berberine on a Pure Magnesium Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Drugs
2.2. Sample Preparation
2.3. Coating Characterization
2.4. Electrochemical Test
2.5. Immersion Tests
2.6. Slow-Release Drug Measurement
3. Results and Discussion
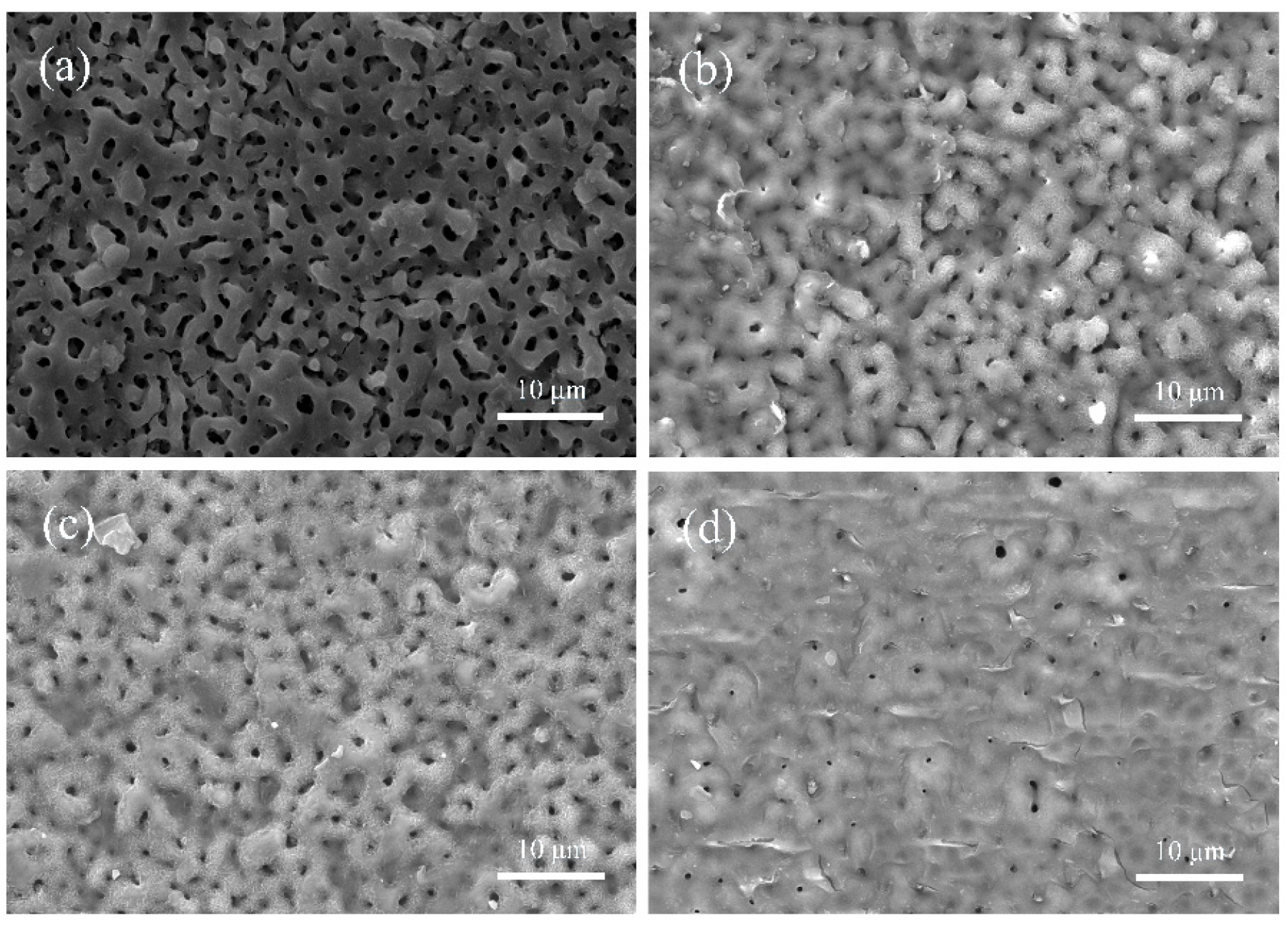
3.1. SEM Analysis
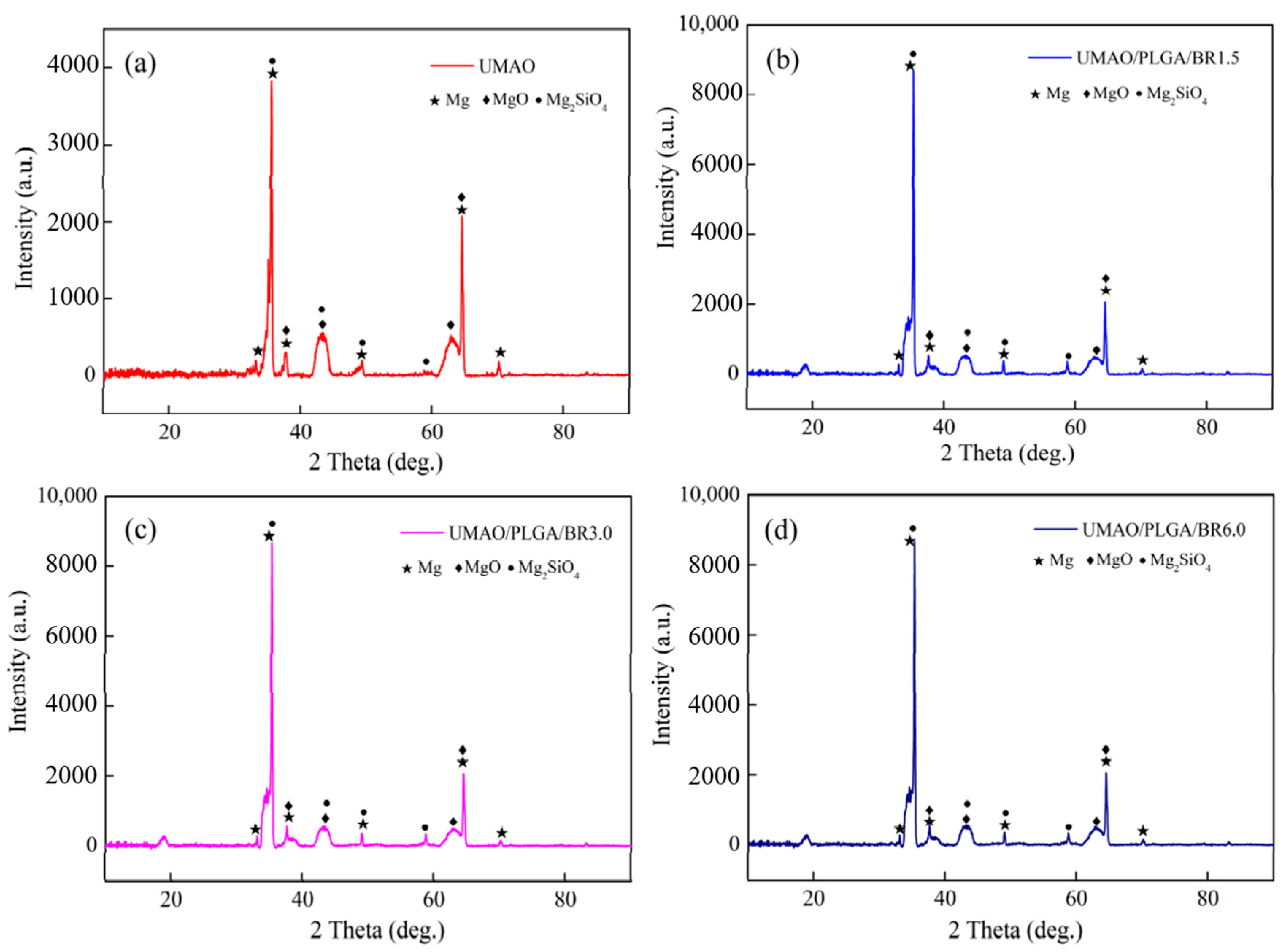
3.2. Phase Analysis of the Coatings
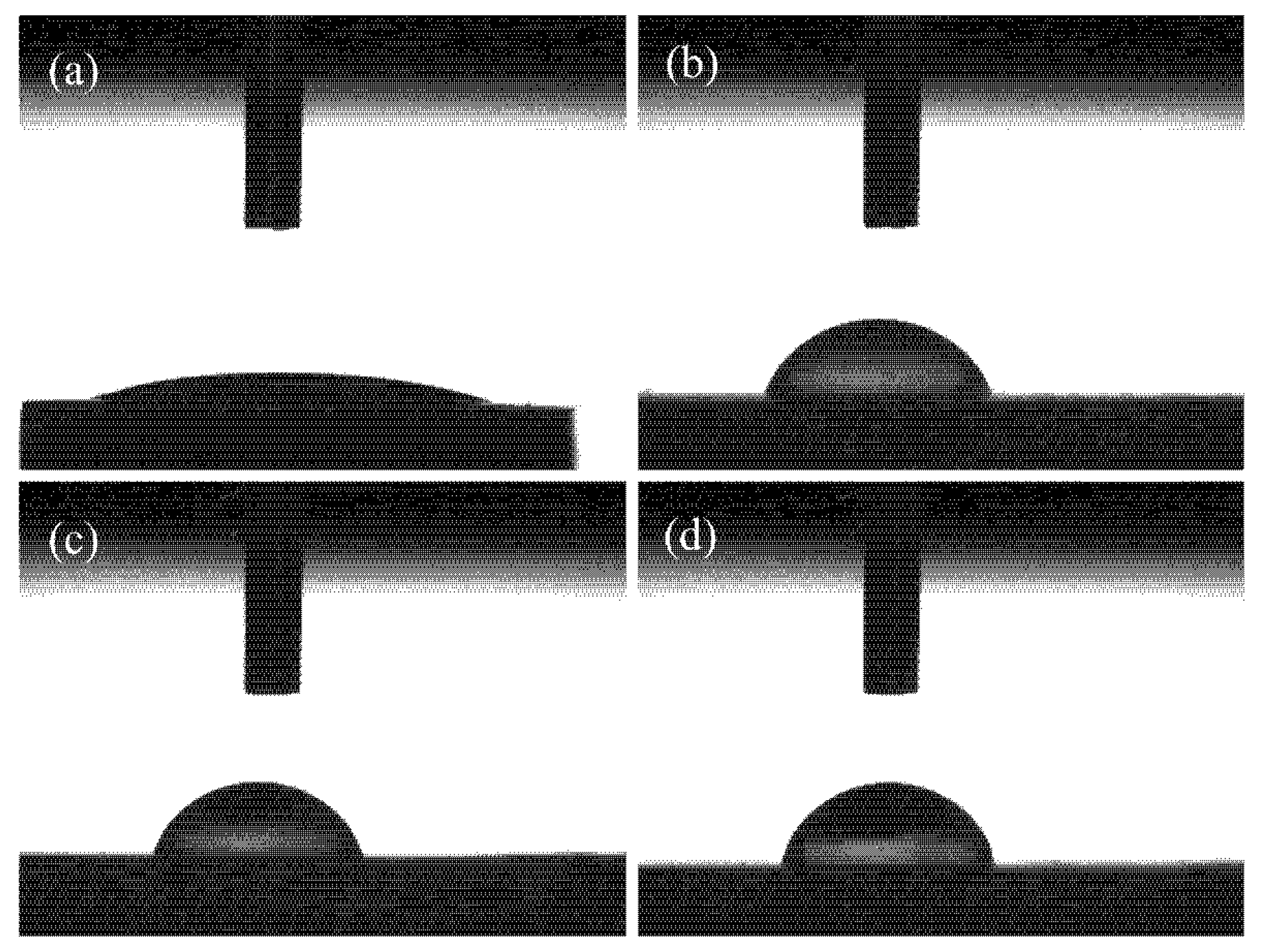
3.3. Contact Angle Analysis
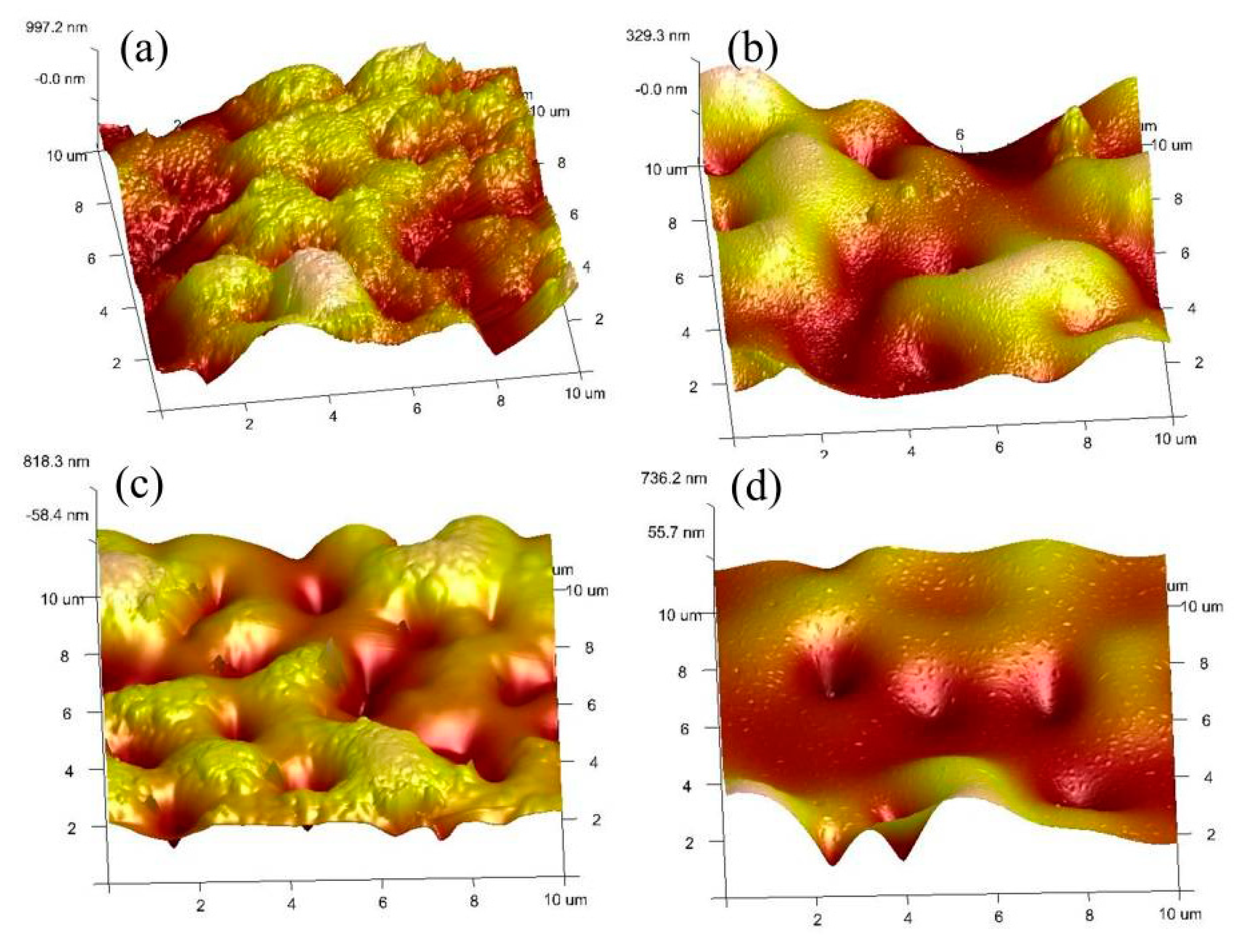
3.4. Coating Roughness
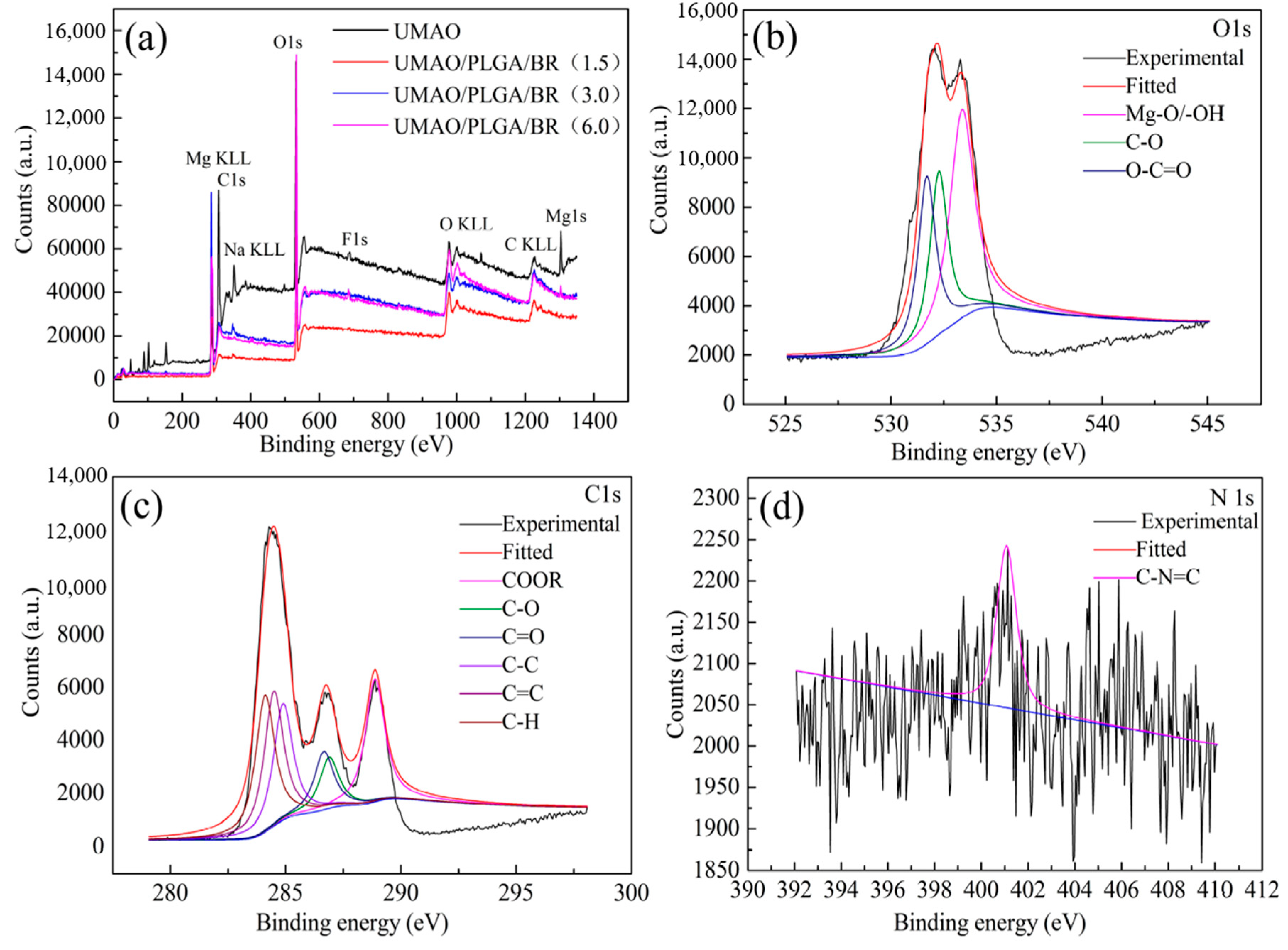
3.5. XPS Analysis
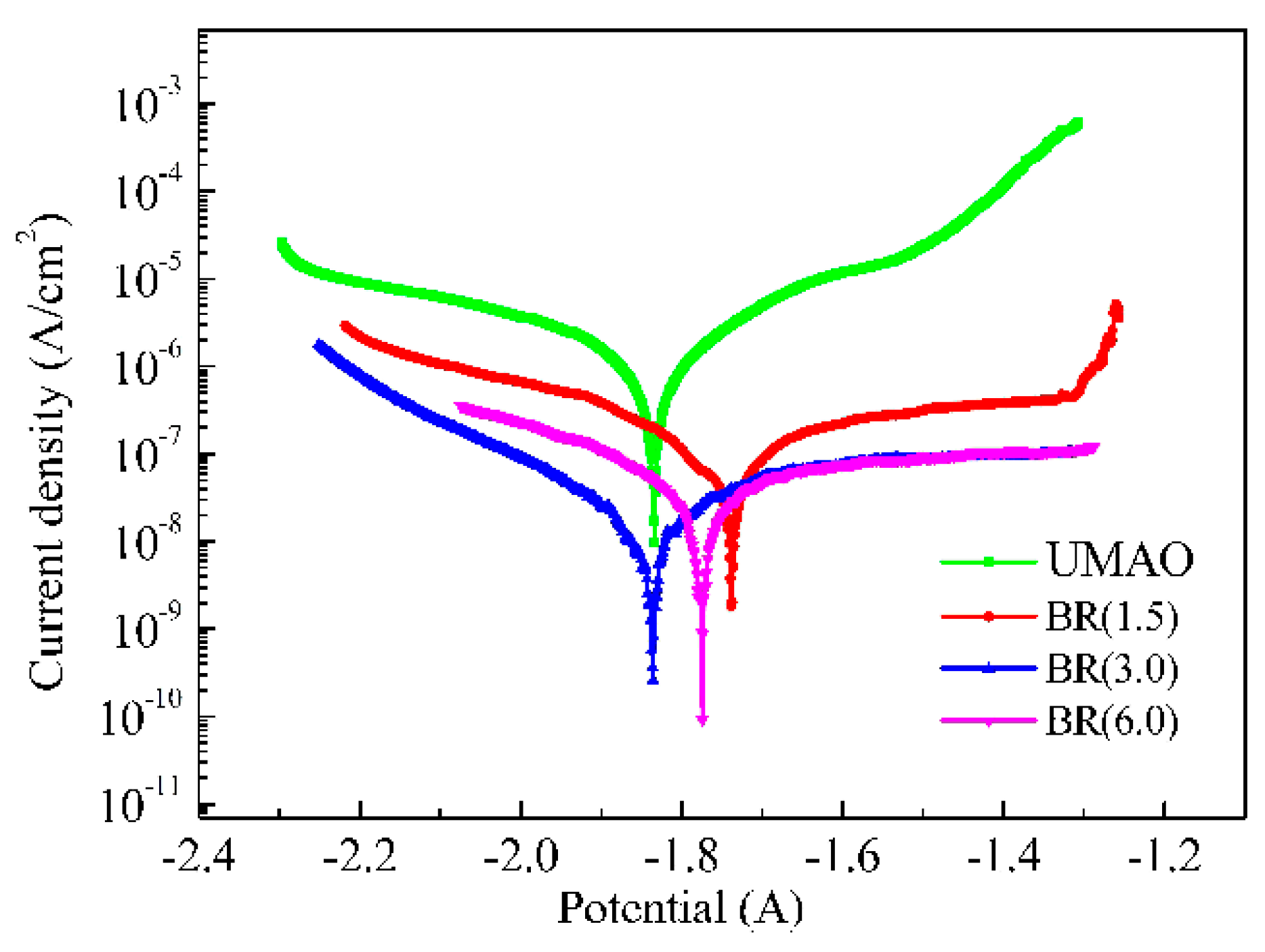
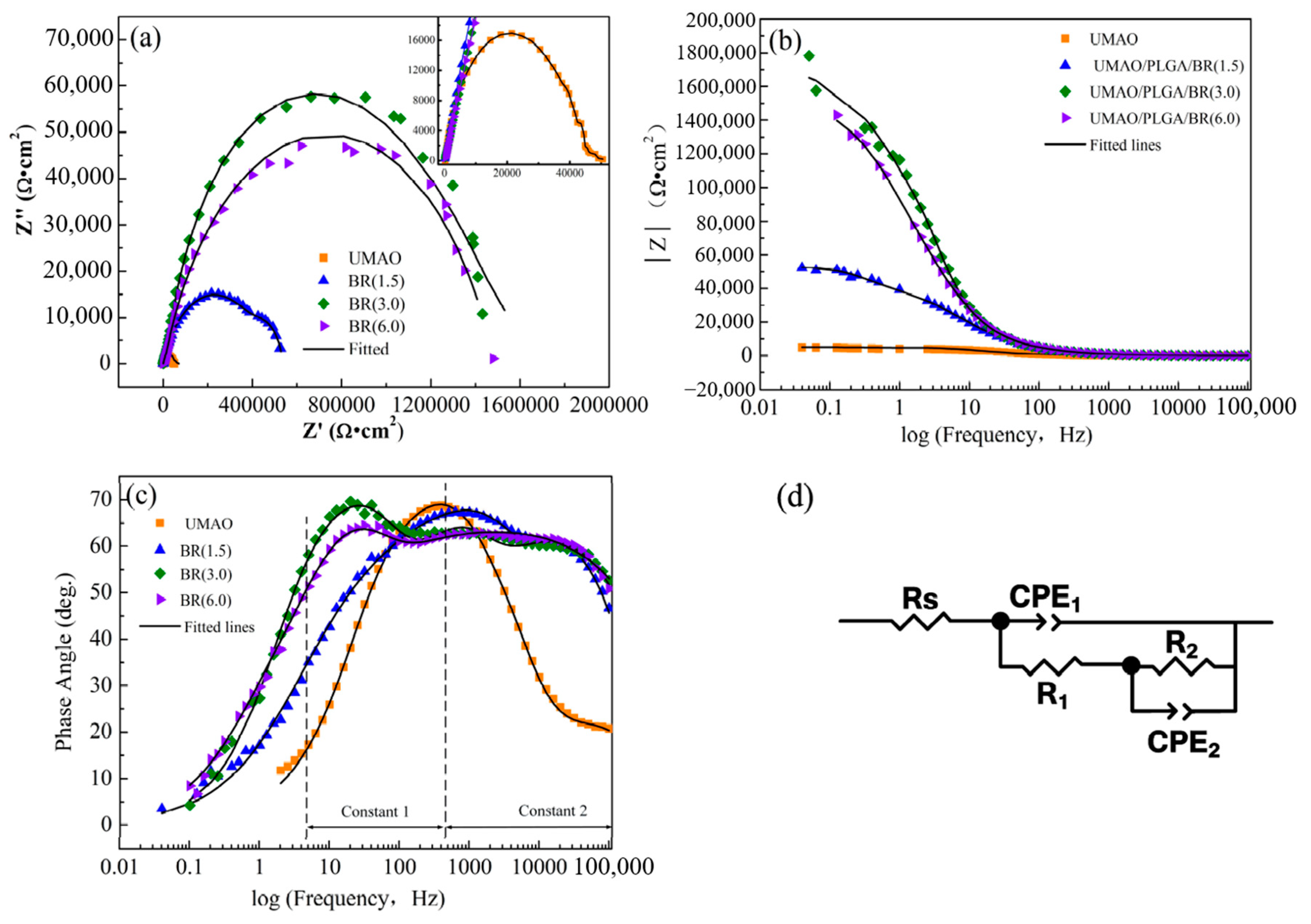
3.6. Corrosion Resistance
3.7. Surface Morphology and Phase Composition Analysis in SBF
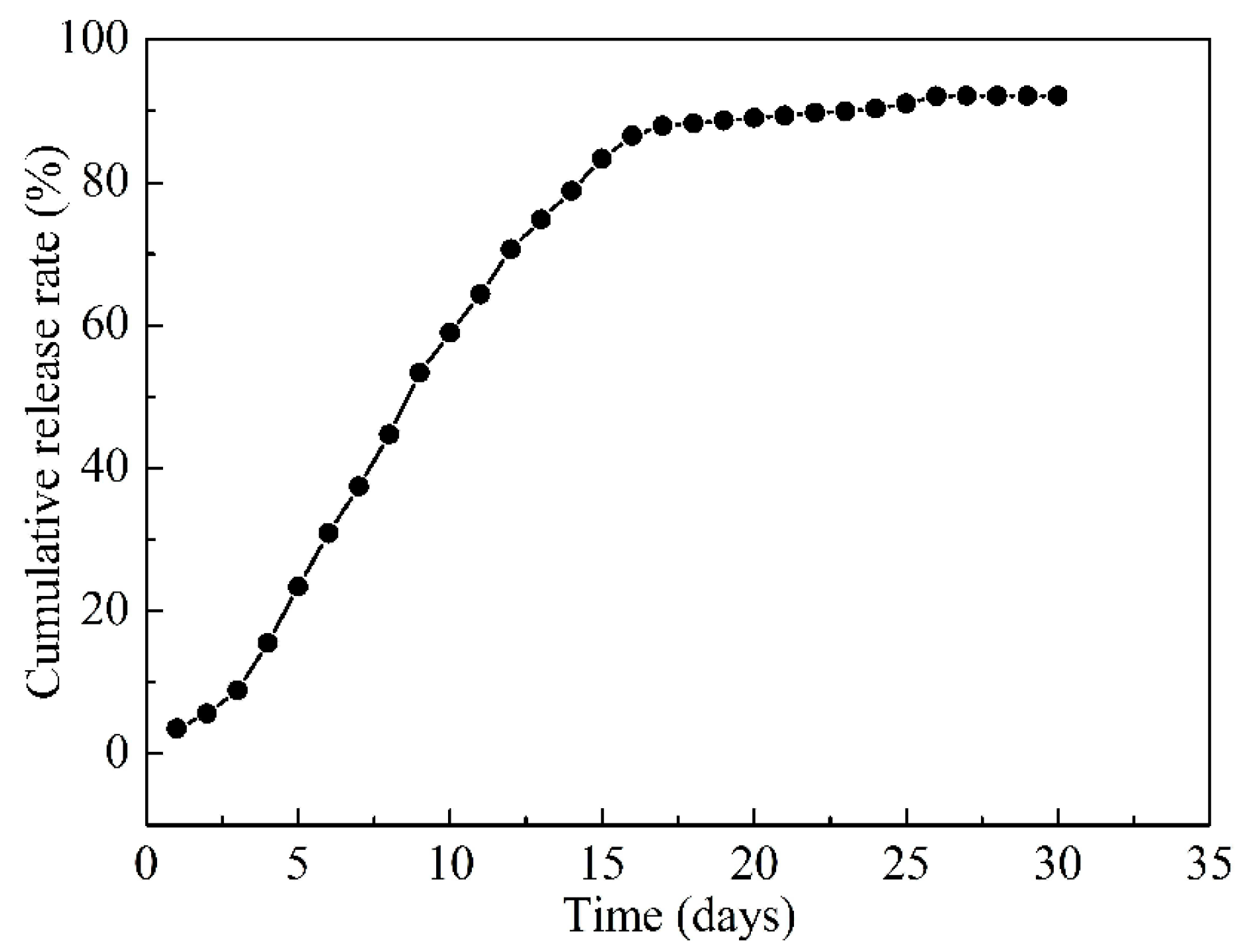
3.8. Slow-Release Drug Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis-A review. Acta Biomater 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pang, S.; Liu, Y.; Sun, L.; Liaw, P.K.; Zhang, T. Biodegradable Mg-Zn-Ca-Sr bulk metallic glasses with enhanced corrosion performance for biomedical application. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of magnesium-based biomaterials and their applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Daroonparvar, M.; Saebnoori, E.; Chami, A. In vitro degradation behavior, antibacterial activity and cyto-toxicity of TiO2-MAO/ZnHA composite coating on Mg alloy for orthopedic implants. Surf. Coat. Technol. 2018, 334, 450–460. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. Regenerative influence of nanostructured bredigite (Ca7MgSi4O16)/anodic spark coating on biodegradable AZ91 magnesium alloy implants for bone healing. Mater. Lett. 2015, 155, 97–111. [Google Scholar] [CrossRef]
- Fierascu, I.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Anuta, V.; Velescu, B.S.; Jinga, M.; Jinga, V. A Short overview of recent developments on antimicrobial coatings based on phytosynthesized metal nanoparticles. Coatings 2019, 9, 787. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, Y.; Meng, L.; Chen, P.; Shi, T.; Liu, X.; Zheng, Y. Electrophoretic deposition of colloidal particles on Mg with cytocompatibility, antibacterial performance, and corrosion resistance. Acta Biomater. 2016, 45, 387–398. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Liang, J.; Chen, J. Thermal control coatings on magnesium alloys prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2013, 280, 151–155. [Google Scholar] [CrossRef]
- Ma, C.X.; Lu, Y.; Sun, P.P.; Yuan, Y.; Jing, X.Y.; Zhang, M.L. Characterization of plasma electrolytic oxidation coatings formed on Mg-Li alloy in an alkaline polyphosphate electrolyte. Surf. Coat. Technol. 2011, 206, 287–294. [Google Scholar] [CrossRef]
- Imwinkelried, T.; Beck, S.; Iizuka, T.; Schaller, B. Effect of a plasma electrolytic coating on the strength retention of in vivo and in vitro degraded magnesium implants. Acta Biomater. 2013, 9, 8643–8649. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Liu, H.P.; Zhang, W.L.; Han, Z.Z.; Deng, M.X.; Zeng, R.C.; Li, S.Q.; Wang, Z.L. Corrosion resistance of a super hydrophobic micro-arc oxidation coating on mg-4li-1ca alloy. J. Mater. Sci. Technol. 2017, 33, 1263–1271. [Google Scholar] [CrossRef]
- Zeng, R.; Zhang, F.; Song, L. Corrosion resistance of a super-hydrophobic surface on micro-arc oxidation coated mg-li-ca alloy. J. Alloy. Compd. 2017, 728, 815–826. [Google Scholar] [CrossRef]
- Kong, K.; Hiraishi, N.; Nassar, M. Effect of phytic acidetchant on resin-dentin bonding: Monomer penetration and stability of dentin collagen. J. Prosthodont Res. 2016, 61, 251–258. [Google Scholar] [CrossRef]
- Li, M.Q.; Liu, J.; Li, J.G. The enhanced corrosion resistance of UMAO coatings on Mg by silane treatment. Mater. Int. 2014, 5, 486–491. [Google Scholar] [CrossRef]
- Peng, S.h.; Li, M.Q.; Wang, J.Y. Corrosion behavior and biological activity of micro-arc oxidation coating with puerarin on pure magnesium surface. Results Phys. 2019, 12, 1481–1489. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, M.Q.; Zhang, D.Q. Corrosion resistance and cell compatibility in vitro of Chinese herbal extract coating on magnesium. Results Phys. 2019, 12, 1465–1474. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Liu, J. A mesoporous silica nanoparticu-late/β-TCP/BG composite drug delivery system for osteoarticular tuberculosis therap. Biomaterials 2011, 32, 1986–1995. [Google Scholar] [CrossRef]
- Martínez-Vázquez, F.J.; Perera, F.H.; Miranda, P. Improving the compressive strength of bioceramic robocast scaffolds by poly-mer infiltration. Acta Biomater. 2010, 6, 4361–4368. [Google Scholar] [CrossRef] [PubMed]
- Karacan, I.; Ben-Nissan, B.; Wang, H.A.; Juritza, A.; Swain, M.; Mueller, W.; Chu, J.; Stamboulis, A.; Macha, I.; Taraschi, V. Mechanical testing of antimicrobial biocomposite coating on metallic medical implants as drugdelivery system. Mater. Sci. Eng. C 2019, 104, 749–757. [Google Scholar] [CrossRef]
- Colceriu, B.L.; Prejmerean, C.; Prodan, D.; Baldea, I.; Vlassa, M.; Filip, M.; Moldovan, M.; Moldovan, M.-A.; Antoniac, A.; Prejmerean, V.; et al. New pre-reacted glass containing dental composites (giomers) with improved fluoride release and biocompatibility. Materials 2019, 12, 4021. [Google Scholar] [CrossRef]
- Borcherding, K.; Marx, D.; Gätjen, L.; Bormann, N.; Wildemann, B.; Specht, U.; Salz, D.; Thiel, K.; Grunwald, I. Burst release of antibiotics combined with long-term release of silver targeting implant-associated infections: Design, characterization and in vitro evaluation of novel implant hybrid surface. Materials 2019, 12, 3838. [Google Scholar] [CrossRef]
- Trujillo-Nolasco, R.M.; Morales-Avila, E.; Ocampo-García, B.E.; Ferro-Flores, G.; Gibbens-Bandala, B.V.; Escudero-Castellanos, A.; Isaac-Olive, K. Preparation and in vitro evaluation of radiolabeled HA-PLGA nanoparticles as novel MTX delivery system for local treatment of rheumatoid arthritis. Mater. Sci. Eng. C 2019, 103, 109766. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Baikin, A.S.; Sergienko, K.V. Biodegradable stent coatings on the basis of PLGA polymers of different molecular mass, sustaining a steady release of the thrombolityc enzyme streptokinase. React. Funct. Polym. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Qian, J.M.; Xu, W.J.; Yong, X.Q.; Jin, X.X.; Zhang, W. Fabrication and in vitro biocompatibility of biomorphic PLGA/nHA composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2014, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, N.; Cheng, Y. In vitro Study on Biodegradable AZ31 Magnesium Alloy Fibers Reinforced PLGA Composite. J. Mater. Sci. Technol. 2013, 6, 545–550. [Google Scholar] [CrossRef]
- Pang, W.Y.; Wang, X.L.; Mok, S.K.; Lai, W.P.; Chow, H.K.; Leung, P.C.; Yao, X.S.; Wong, W.S. Naringin improves bone properties in ovariectomized mice and exerts oestrogenlike activities in rat osteoblast-like (UMR106) cells. Br. J. Pharmacol. 2010, 159, 1693–1703. [Google Scholar] [CrossRef]
- Wu, J.-B.; Fong, Y.-C.; Tsai, H.-Y.; Chen, Y.-F.; Tsuzuki, M.; Tang, C. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur. J. Pharmacol. 2008, 588, 333–341. [Google Scholar] [CrossRef]
- Afewerki, S.; Bassous, N.; Harb, S.; Palo-Nieto, C.; Ruiz-Esparza, G.U.; Marciano, F.R.; Webster, T.J.; Furtado, A.S.A.; Lobo, A.O. Advances in dual functional antimicrobial and osteoinductive biomaterials for orthopaedic applications. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Waterman, J.; Pietak, A.; Birbilis, N.; Woodfield, T.; Dias, G.; Staiger, M.P. Corrosion resistance of biomimetic calcium phosphate coatings on magnesium due to varying pretreatment time. Mater. Sci. Eng. B 2011, 20, 1756–1760. [Google Scholar] [CrossRef]
- Cui, L.Y.; Gao, S.D.; Li, P.P.; Zeng, R.C.; Zhang, F.; Li, S.Q. Corrosion resistance of a self-healing micro-arc oxidation/poly methyltrimeth oxysilane composite coating on magnesium alloy az31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Wang, Y.M.; Guo, J.W.; Wu, Y.F. Biocorrosion resistance of coated magnesium alloy by microarc oxidation in electrolyte containing zirconium and calcium salts. Front. Mater. Sci. 2014, 20, 256–366. [Google Scholar] [CrossRef]
- Li, J.N.; Cao, P.; Zhang, X.N.; Zhang, S.X.; He, Y.H. In vitro degradation and cell attachment of a PLGA coated biodegradable Mg–6Zn based alloy. J. Mater. Sci. 2010, 45, 6038–6045. [Google Scholar] [CrossRef]
- Batra, U.; Kapoor, S.; Sharma, S. Influence of Magnesium Ion Substitution on Structura land Thermal Behavior of Nanodimensional Hydroxyapatite. J. Mater. Eng. Perform. 2013, 22, 1798–1806. [Google Scholar] [CrossRef]
- Diana, M.V.; Ionut, C.I.; Elena, U. Magnesium Doped Hydroxyapatite-Based Coatings Obtained by Pulsed Galvanostatic Electrochemical Deposition with Adjustable Electrochemical Behavior. Coatings 2020, 10, 2–17. [Google Scholar]
- Zhang, F.; Zhang, C.; Song, L.; Zeng, R.-C.; Li, S.; Cui, H. Fabrication of the Superhydrophobic Surface on Magnesium Alloy and Its Corrosion Resistance. J. Mater. Sci. Technol. 2015, 31, 1139–1143. [Google Scholar] [CrossRef]
- Taheri, M.; Kish, J.; Birbilis, N.; Danaie, M.; McNally, E.; McDermid, J. Towards a Physical Description for the Origin of Enhanced Catalytic Activity of Corroding Magnesium Surfaces. Electrochim. Acta 2014, 116, 396–403. [Google Scholar] [CrossRef]
- Mousa, H.M.; Lee, D.H.; Kim, C.S.; Kim, C.S. A novel simple strategy for in situ deposition of apatite layer on AZ31B magnesium alloy for bone tissue regeneration. Appl. Surf. Sci. 2015, 351, 55–65. [Google Scholar] [CrossRef]
- Vaz, J.M.; Taketa, T.B.; Hernã¡ndez-Montelongo, J.; Chevallier, P.; Cotta, M.A.; Mantovani, D.; Beppu, M.M. Antibacterial properties of chitosan-based coatings are affected by spacer-length and molecular weight. Appl. Surf. Sci. 2018, 445, 478–487. [Google Scholar] [CrossRef]
- Pozzo, L.D.Y.; Conceição, T.; Spinelli, A.; Scharnagl, N.; Pires, A.T. Chitosan coatings crosslinked with genipin for corrosion protection of AZ31 magnesium alloy sheets. Carbohydr. Polym. 2018, 181, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Toorani, M.; Aliofkhazraei, M. Review of electrochemical properties of hybrid coating systems on Mg withplasma electrolytic oxidation process as pretreatment. Surf. Interfaces 2019, 14, 262–295. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhao, Z.P.; Wu, F.M.; Wang, Y.L.; Wu, J. Corrosion and wear resistance of AZ91D magnesium alloy with and without microarc oxidation coating in Hank’s solution. J. Mater. Sci. 2007, 42, 8523–8528. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Beni, B.H.; Vashaee, D.; Tayebi, L. Surface microstructure and in vitro analysis of nanostructured akermanite (Ca2MgSi2O7) coating on biodegradable magnesium alloy for biomedical applications. Colloids Surfaces B: Biointerfaces 2014, 117, 432–440. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Han, E.; Dong, J.; Ke, W. AC impedance spectroscopy study of the corrosion behavior of an AZ91 magnesium alloy in 0.1M sodium sulfate solution. Electrochim. Acta 2007, 52, 3299–3309. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Uggowitzer, P.J.; Song, G.-L.; Atrens, A. The influence of yttrium (Y) on the corrosion of Mg–Y binary alloys. Corros. Sci. 2010, 52, 3687–3701. [Google Scholar] [CrossRef]
- Niu, B.; Shi, P.; Shanshan, E.; Wei, D.; Li, Q.; Chen, Y. Preparation and characterization of HA sol–gel coating on MAO coated AZ31 alloy. Surf. Coat. Technol. 2016, 286, 42–48. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, S.; Zhang, F.; Xu, G.; Wang, F.; Yu, N.; Wu, X. Preparation and corrosion resistance of magnesium phytic acid/hydroxyapatite composite coatings on biodegradable AZ31 magnesium alloy. J. Mater. Sci. Mater. Electron. 2017, 28, 82. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Narayanan, T.S. Electrode position of Hydroxyapatite Coating on Magnesium for Biomedical Applications. J. Coat. Technol. Res. 2012, 9, 495–502. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gupta, A.; Verma, M.; Anand, M.P.; Sengupta, P.; Saravanan, M.; Manna, I.; Balani, K. Antibacterial and magnetic response of site-specific cobalt incorporated hydroxyapatite. Ceram. Int. 2020, 46, 513–522. [Google Scholar] [CrossRef]
- Wang, H.; Eliaz, N.; Xiang, Z.; Hsu, H.P.; Spector, M.; Hobbs, L.W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 2006, 27, 4192–4203. [Google Scholar] [CrossRef] [PubMed]
- Cheon, K.H.; Gao, C.; Kang, M.H.; Jung, H.D.; Jang, T.S.; Kim, H.E. A crack-free anti-corrosive coating strategy for magnesium implants under deformation. Corros. Sci. 2017, 11, 6–24. [Google Scholar] [CrossRef]
- Müller, L.; Müller, F.A. Preparation of SBF with different HCO3- content and its influence on the composition of biomimetic apatites. Acta Biomater. 2006, 2, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Maczynska, B.; Secewicz, A.; Smutnicka, D.; Szymczyk, P.; Dudek-Wicher, R.; Junka, A.; Bartoszewicz, M. In vitro efficacy of gentamicin released from collagen sponge in eradication of bacterial biofilm preformed on hydroxyapatite surface. PLoS ONE 2019, 14, 217–229. [Google Scholar] [CrossRef]
- Llorens, E.; Calderon, S.; Del Valle, L.J.; Puiggalí, J. Polybiguanide (PHMB) loaded in PLA scaffolds displaying high hydrophobic, biocompatibility and antibacterial properties. Mater. Sci. Eng. C 2015, 50, 74–84. [Google Scholar] [CrossRef]


| No. | Chemical Formula | Amount | Reagent Grade | Purity | Manufacturer |
|---|---|---|---|---|---|
| 1 | NaCl | 8.035 g/L | ACS reagent | ≥99.90% | Comeo Co., Ltd., Tianjin, China |
| 2 | NaHCO3 | 0.355 g/L | Bio Reagent | ≥99.50% | |
| 3 | KCl | 0.225 g/L | ACS reagent | ≥99.50% | |
| 4 | K2HPO4·3H2O | 0.231 g/L | ACS reagent | ≥99.90% | |
| 5 | MgCl2·6H2O | 0.311 g/L | ACS reagent | ≥98.00% | |
| 6 | 1.0M-HCl | 39 mL | ACS reagent | ≥37.00% | |
| 7 | CaCl2 | 0.292 g/L | ACS reagent | ≥99.90% | |
| 8 | Na2SO4 | 0.072 g/L | ACS reagent | ≥99.90% | |
| 9 | (CH2OH)3CNH2 | 6.118 g/L | Standard & Buffer | ≥99.90% |
| Sample | UMAO | BR (1.5 g/L) | BR (3.0 g/L) | BR (6.0 g/L) |
|---|---|---|---|---|
| contact angle 1 | 20.13° | 67.42° | 70.23° | 71.35° |
| contact angle 2 | 20.07° | 69.08° | 69.53° | 69.64° |
| contact angle 3 | 20.93° | 68.42° | 68.90° | 71.12° |
| contact angle 4 | 21.19° | 67.16° | 69.43° | 70.62° |
| contact angle 5 | 20.83° | 68.52° | 70.56° | 69.57° |
| average value | 20.63 ± 0.56° | 68.12 ± 0.96° | 69.73 ± 0.83° | 70.46 ± 0.89° |
| Sample | UMAO | BR (1.5 g/L) | BR (3.0 g/L) | BR (6.0 g/L) |
|---|---|---|---|---|
| Roughnesses (nm) | 246 | 92.4 | 190 | 145 |
| Sample | UMAO | BR (1.5 g/L) | BR (3.0 g/L) | BR (6.0 g/L) |
|---|---|---|---|---|
| Ecorr (V) | −1.834 ± 0.007 | −1.741 ± 0.006 | −1.872 ± 0.007 | −1.769 ± 0.006 |
| Icorr (A/cm2) | (2.26 ± 0.02) × 10−6 | (3.33 ± 0.019) × 10−7 | (3.14 ± 0.017) × 10−8 | (1.26 ± 0.023) × 10−7 |
| CR (mm/yr) | 5.16 × 10−3 | 7.60 × 10−4 | 7.17 × 10−5 | 2.88 × 10−4 |
| Sample | Rs (Ω·cm2) | CPE1 (F·cm2) | R1(Ω·cm2) | CPE2 (F·cm2) | R2(Ω·cm2) |
| UMAO | 269.2 | 4.35 × 10−7 | 4.37 × 104 | 2.59 × 10−5 | 1.18 × 104 |
| BR1.5 | 126.9 | 1.67 × 10−7 | 4.31 × 105 | 4.16 × 10−6 | 1.17 × 105 |
| BR3.0 | 152.1 | 1.09 × 10−7 | 7.02 × 105 | 1.06 × 10−8 | 1.61 × 106 |
| BR6.0 | 117.9 | 1.46 × 10−7 | 1.95 × 105 | 7.49 × 10−9 | 1.29 × 106 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, L.; Ma, Z.; Wang, J.; Yuan, S.; Li, M. Corrosion Behavior and Biological Activity of Micro Arc Oxidation Coatings with Berberine on a Pure Magnesium Surface. Coatings 2020, 10, 837. https://doi.org/10.3390/coatings10090837
Mu L, Ma Z, Wang J, Yuan S, Li M. Corrosion Behavior and Biological Activity of Micro Arc Oxidation Coatings with Berberine on a Pure Magnesium Surface. Coatings. 2020; 10(9):837. https://doi.org/10.3390/coatings10090837
Chicago/Turabian StyleMu, Liting, Zhen Ma, Jingyan Wang, Shidan Yuan, and Muqin Li. 2020. "Corrosion Behavior and Biological Activity of Micro Arc Oxidation Coatings with Berberine on a Pure Magnesium Surface" Coatings 10, no. 9: 837. https://doi.org/10.3390/coatings10090837
APA StyleMu, L., Ma, Z., Wang, J., Yuan, S., & Li, M. (2020). Corrosion Behavior and Biological Activity of Micro Arc Oxidation Coatings with Berberine on a Pure Magnesium Surface. Coatings, 10(9), 837. https://doi.org/10.3390/coatings10090837




