Surface Modifications of Biodegradable Metallic Foams for Medical Applications
Abstract
1. Introduction
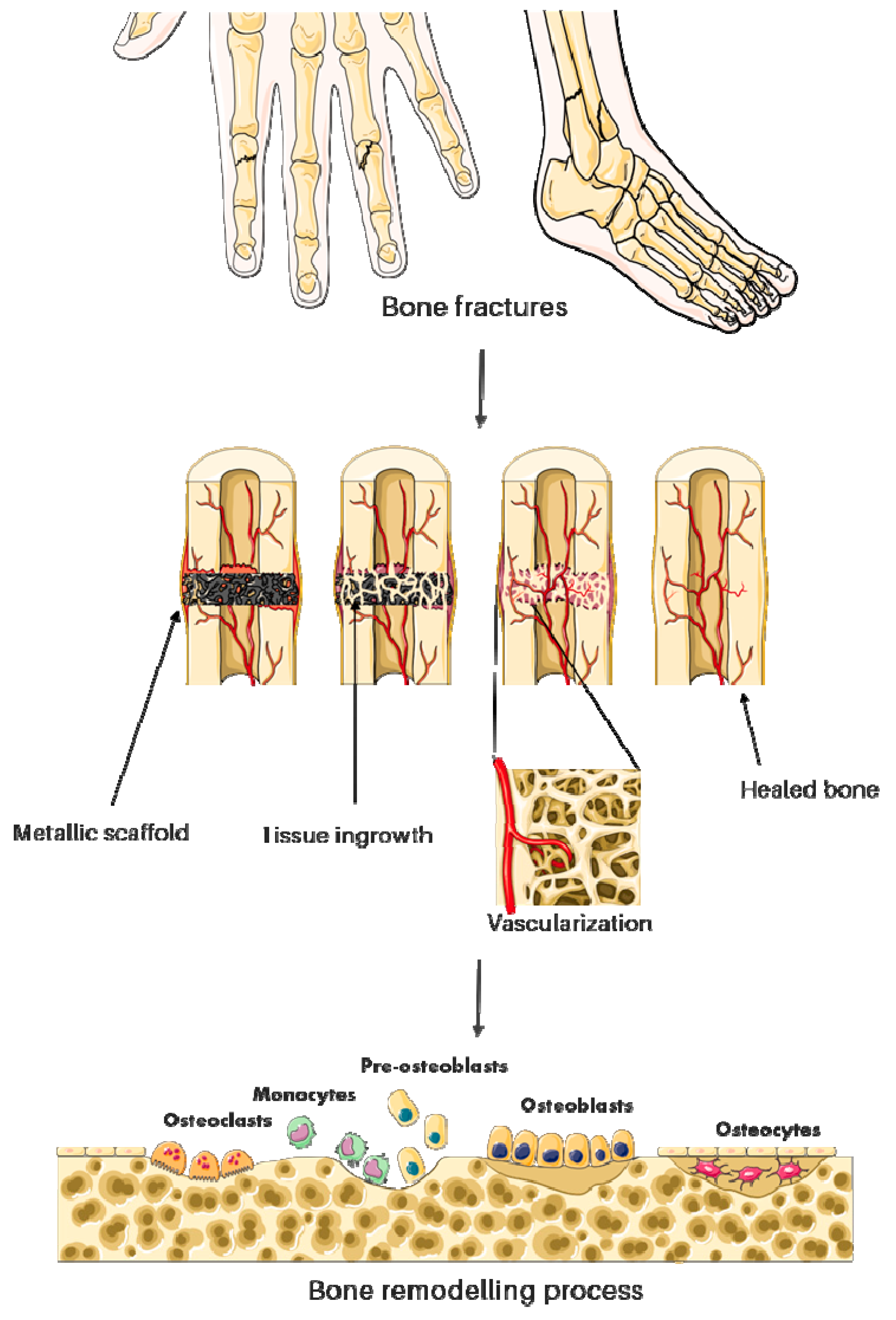
2. Medical Applications of Metallic Foams
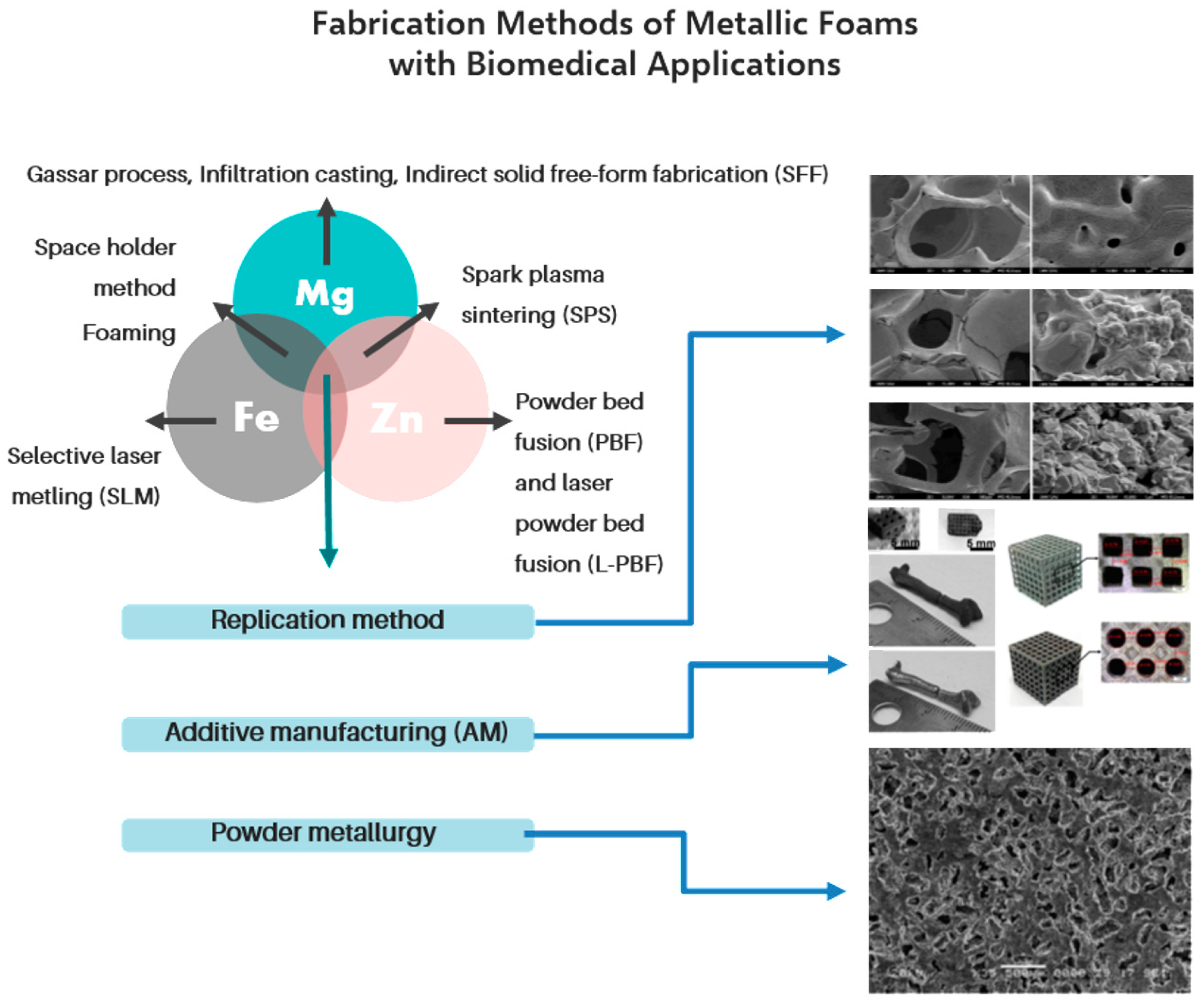
3. Fabrication Methods of Metallic Foams for Biomedical Applications
4. Biodegradable Metallic Foams
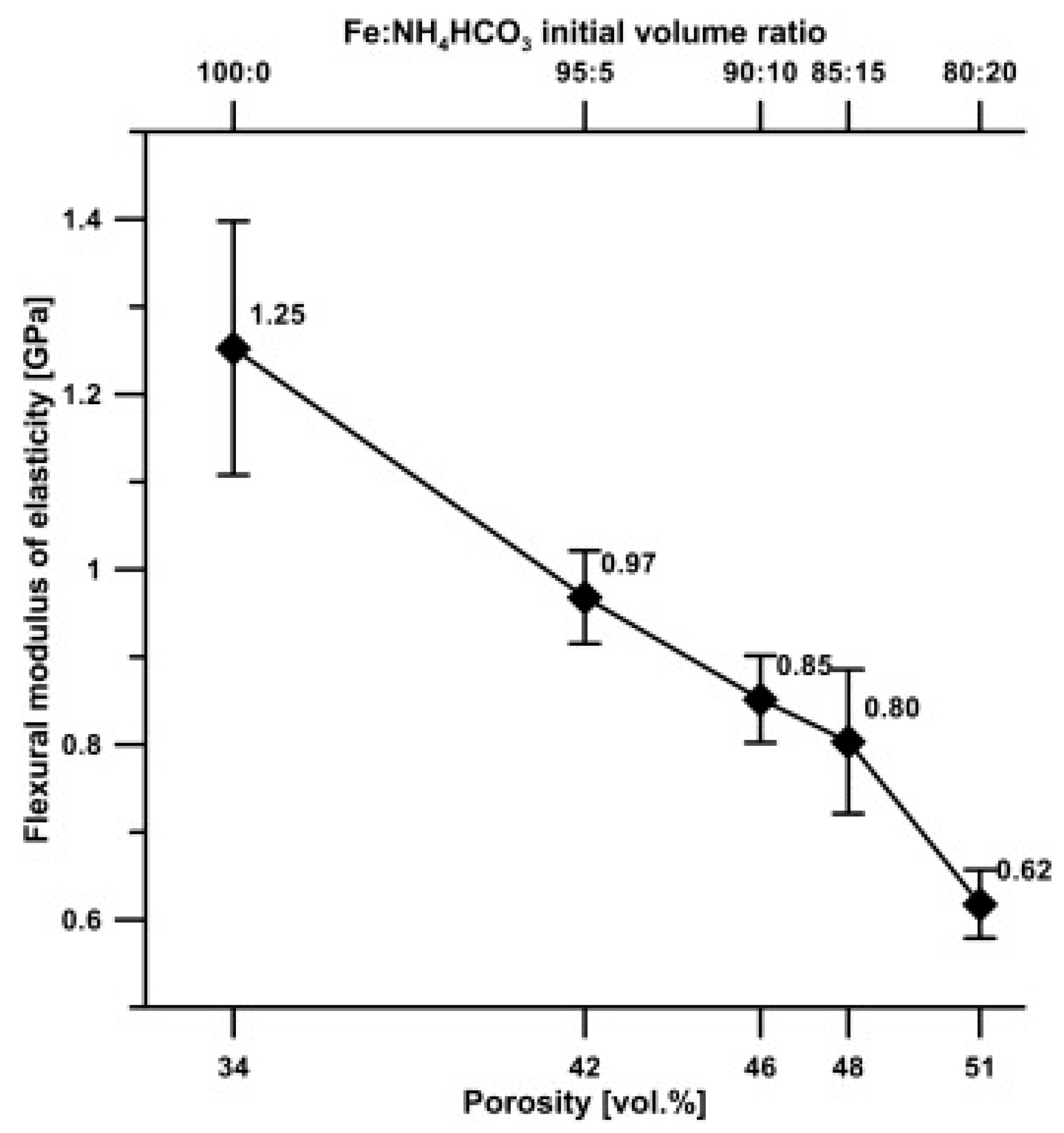
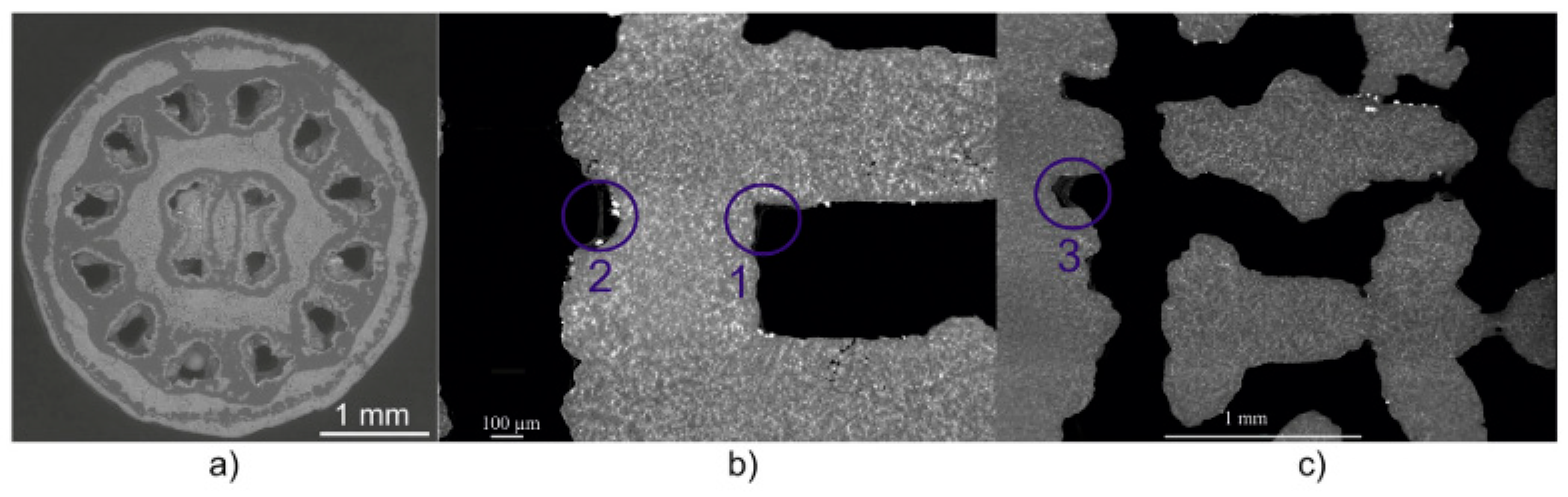
4.1. Iron and Fe-Based Biodegradable Foams
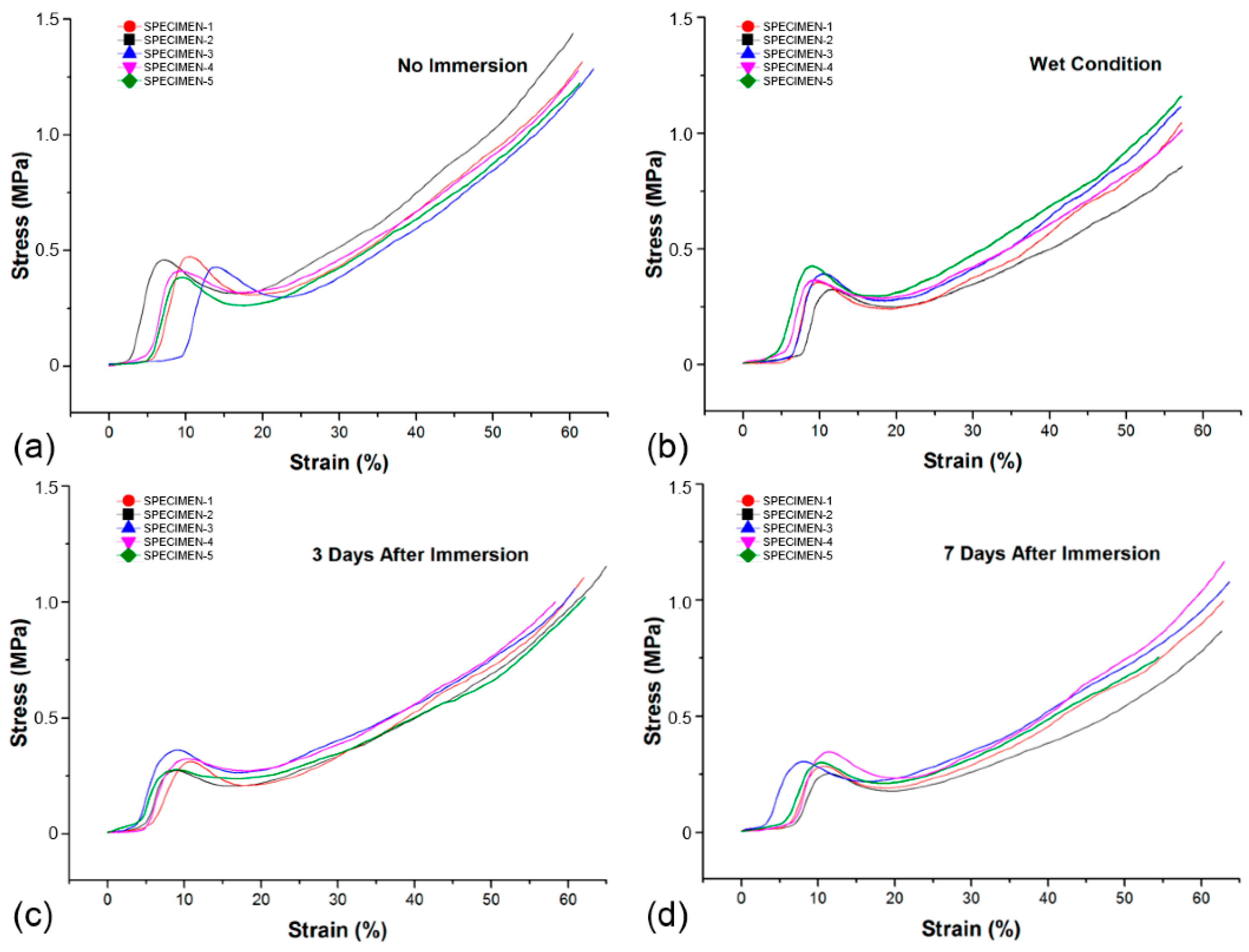
4.2. Magnesium and Mg-Based Biodegradable Foams
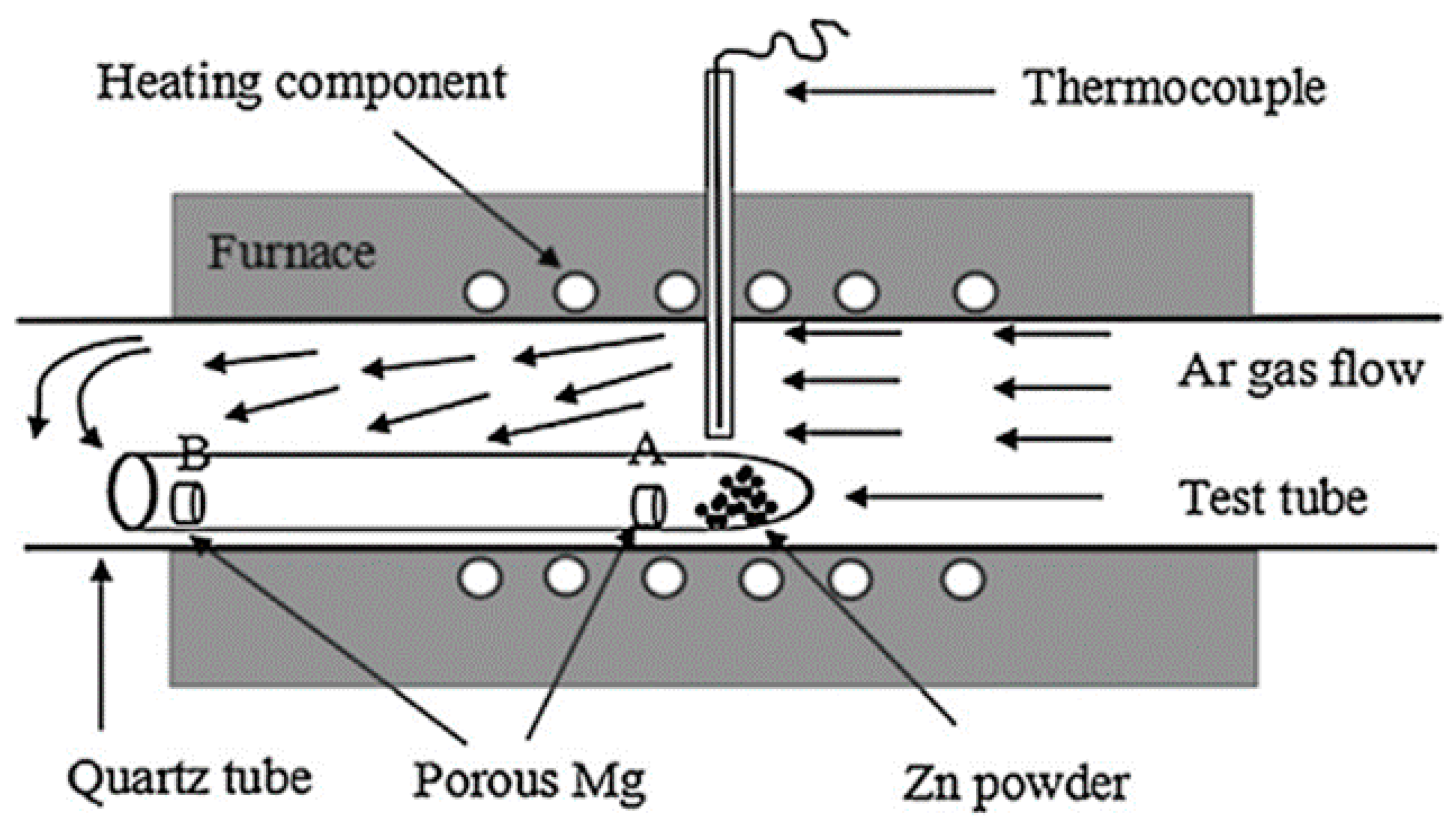
4.3. Zinc and Zn-Based Biodegradable Foams
5. Surface Modification of Metallic Foams
5.1. Methods of Surface Modification of Metallic Foams
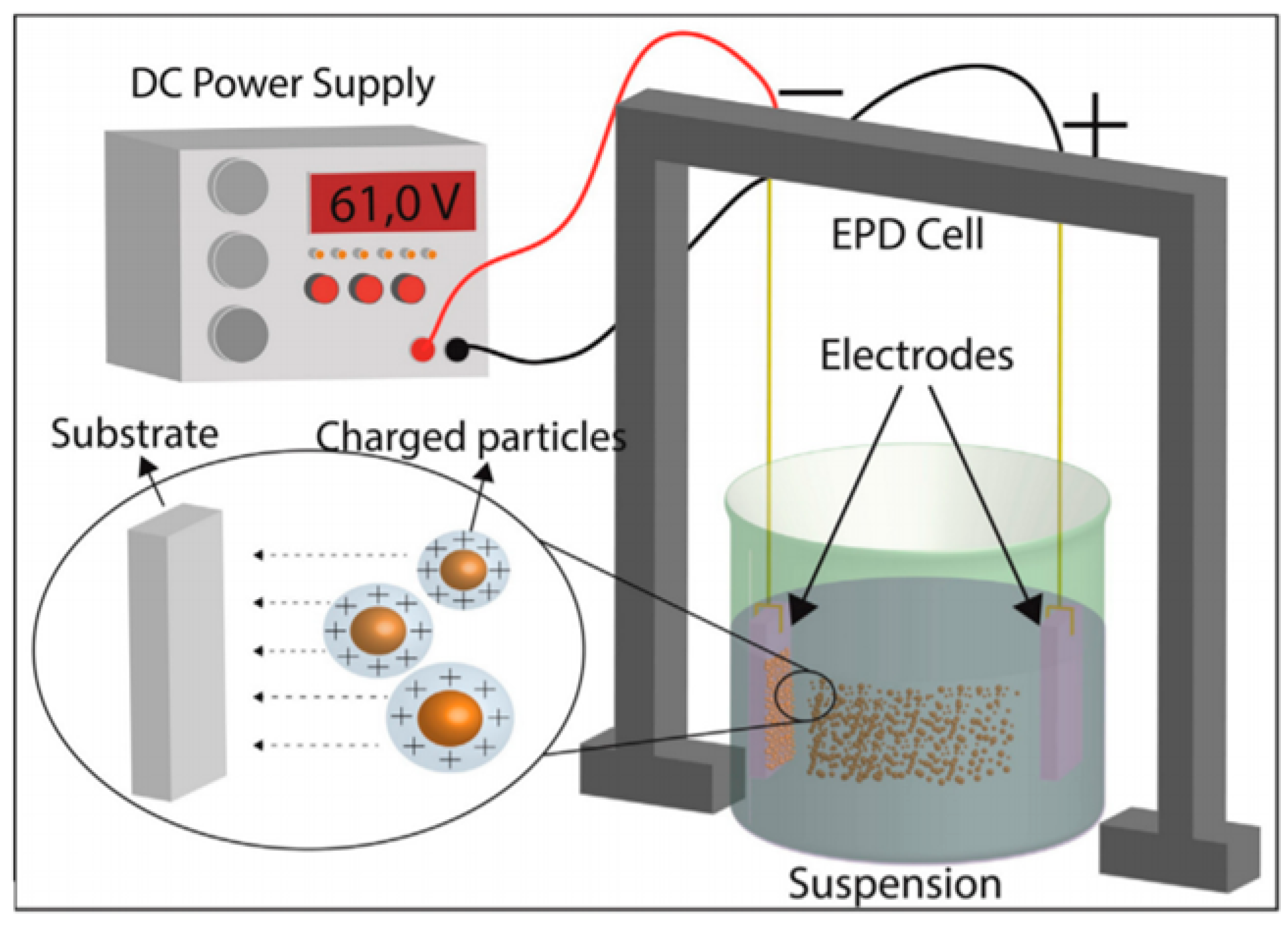
5.1.1. Electrophoretic Deposition
5.1.2. Thermal Evaporation Technique
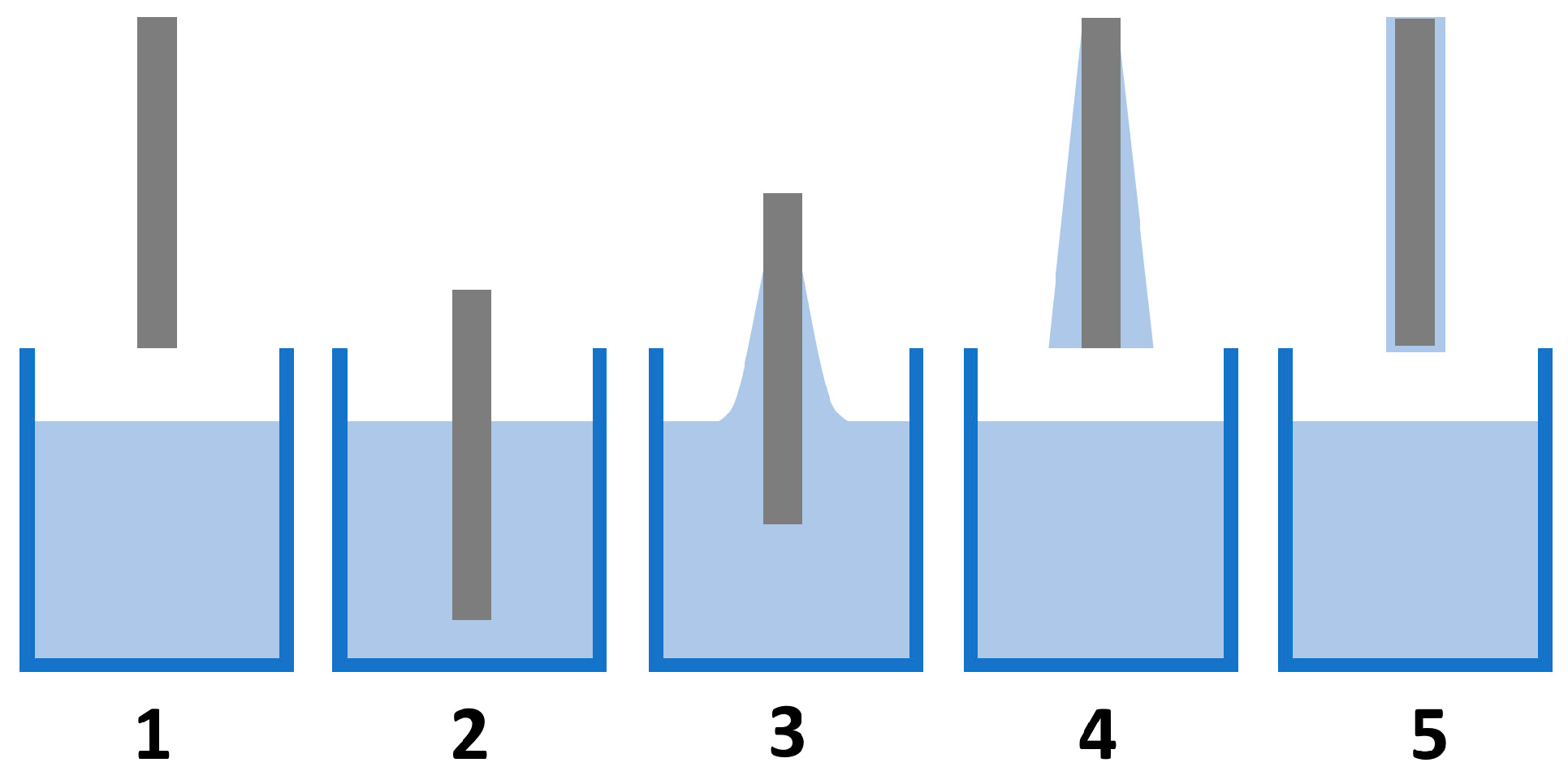
5.1.3. Dip-Coating Method
- Immersion: The substrate is dipped into the coating bath at a constant speed.
- Startup: After immersion, the substrate remains in the bath for a selected time, and then it is ready to be pulled out.
- Deposition: The deposition of the thin coating layer starts while the substrate is pulling out. The resulting thickness of the coating directly depends on the speed pulling the substrate from the coating bath. A slower pull speed causes the thinner coating of the film.
- Drainage: Excess fluid is drained in this step.
- Evaporation: The final step involves the evaporation of fluid from the substrate surface and the creation of the final thin coating. Volatile solvents are evaporated earlier in step 3 [93].
5.1.4. Vacuum Infiltration
5.1.5. Conversion Coating Method
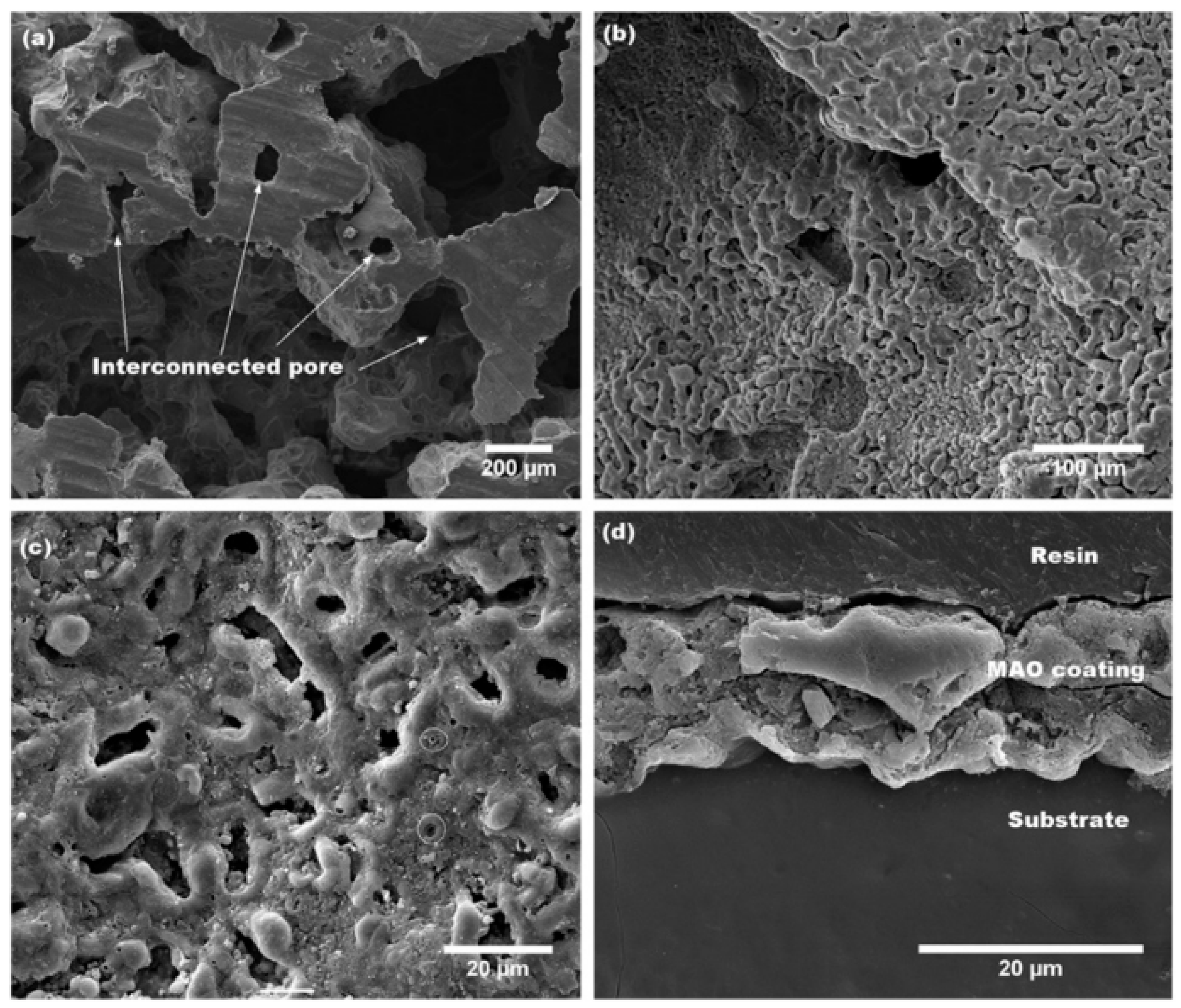
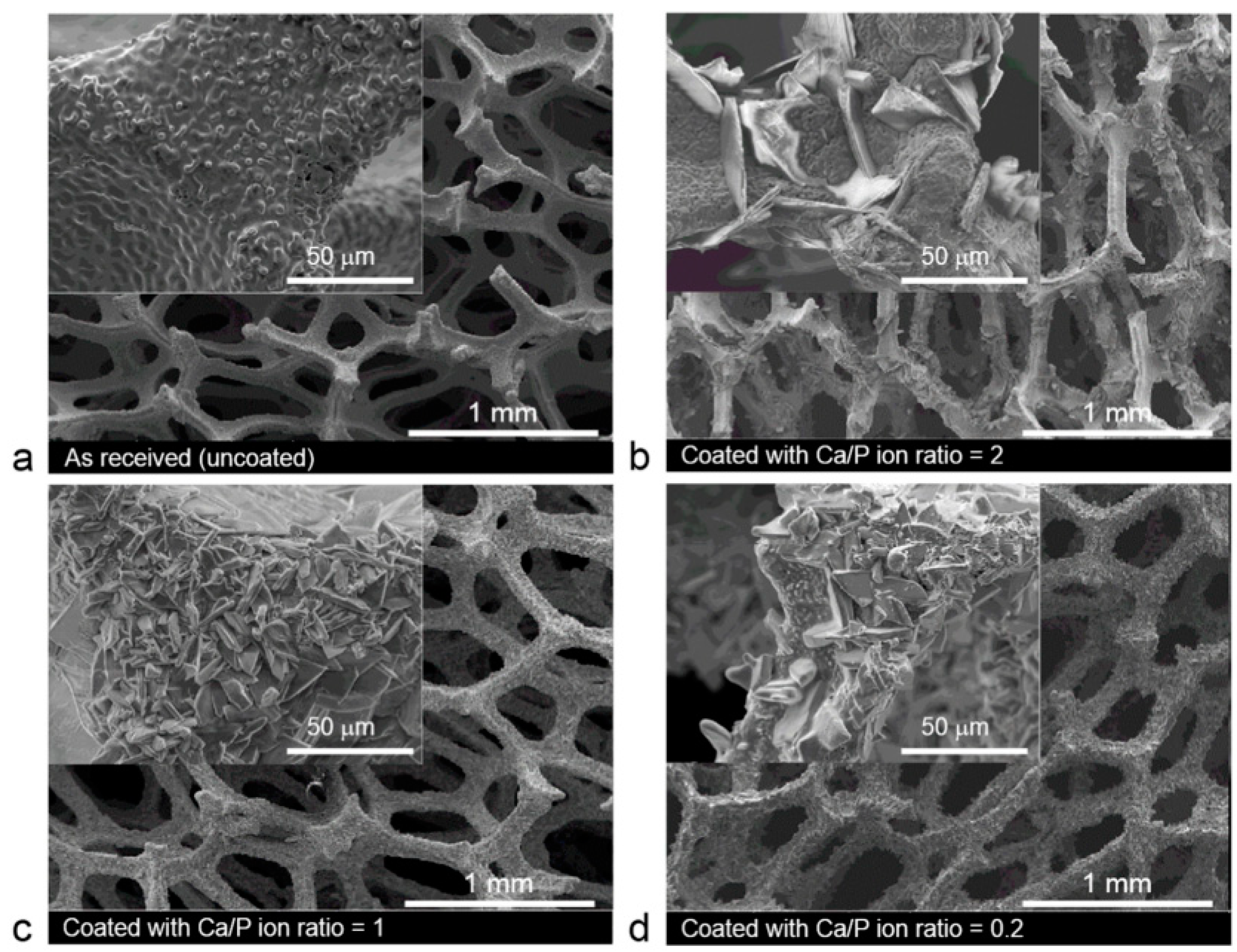
5.1.6. Micro-Arc Oxidation
5.2. Coatings
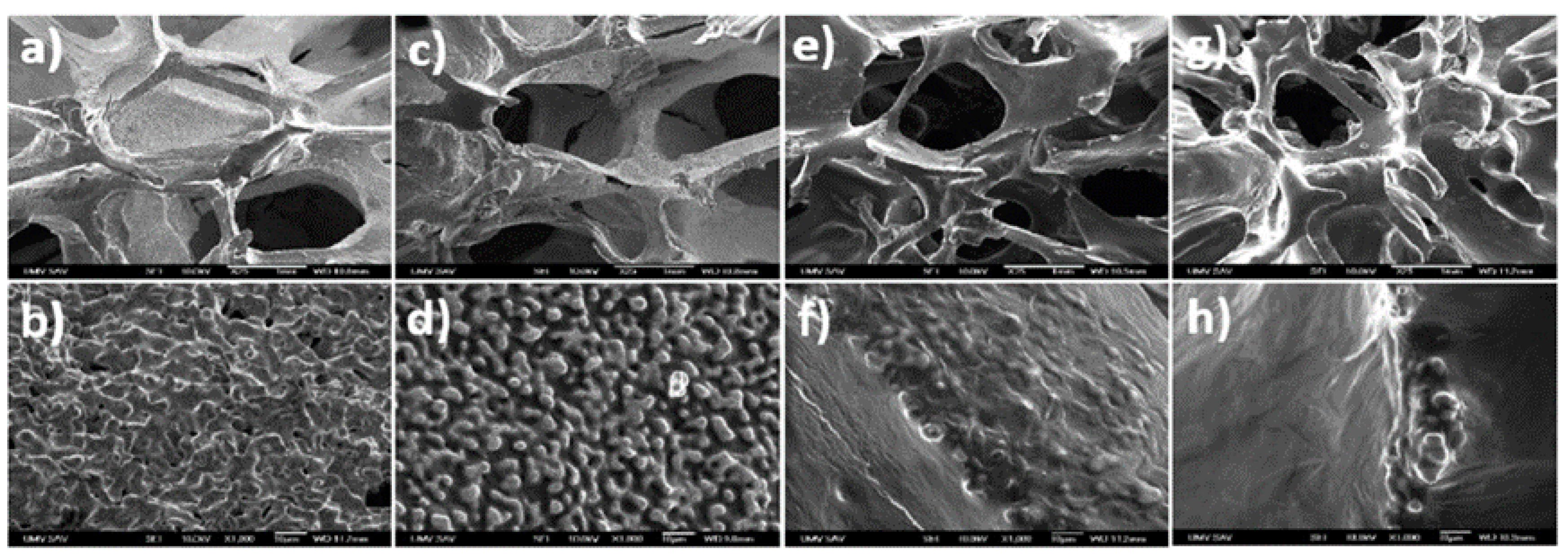
5.2.1. Polymer Coatings
Polyethylene Glycol
Polyethyleneimine
Polylactic Acid
Chitosan
5.2.2. Inorganic Ceramic Coatings
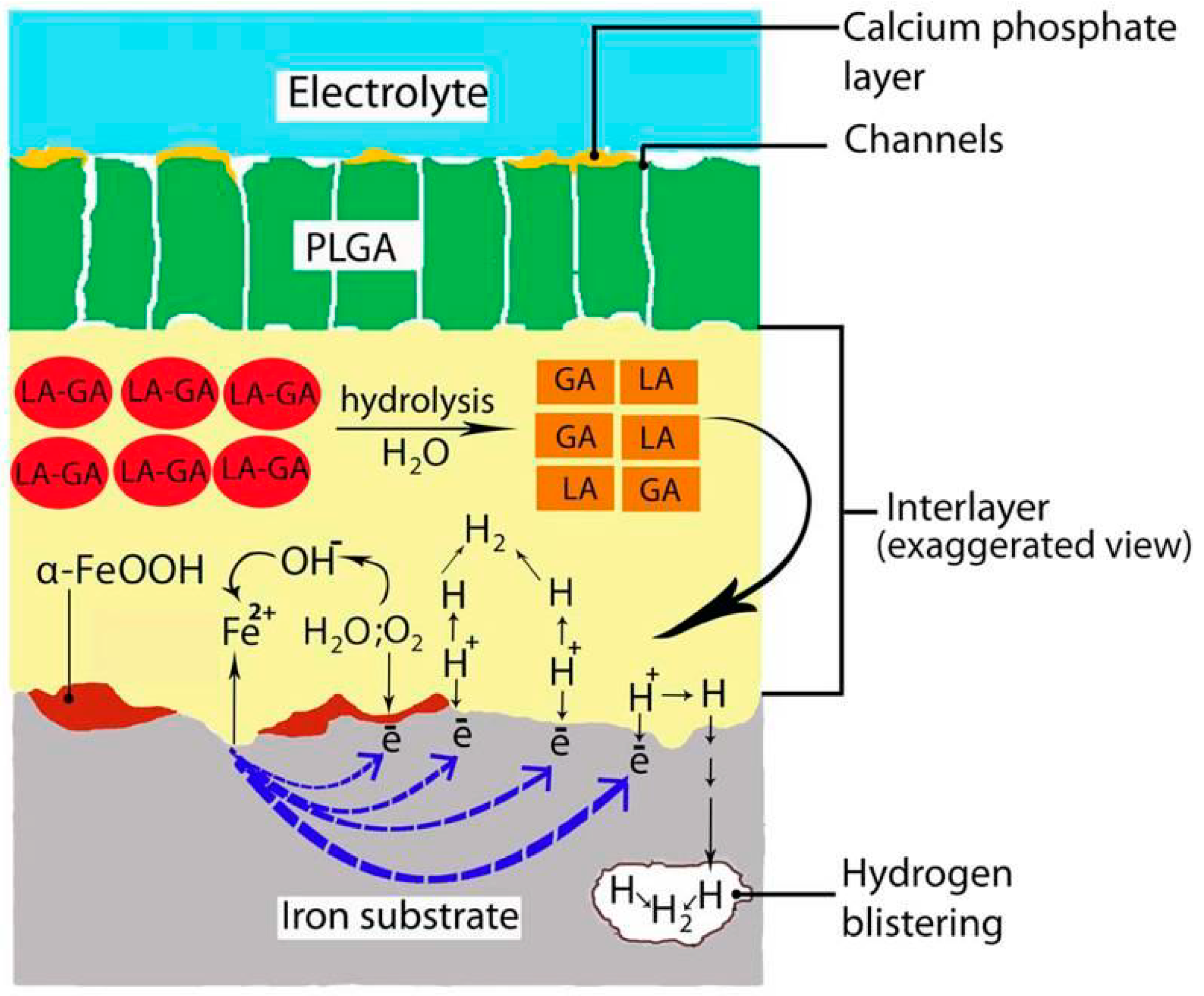
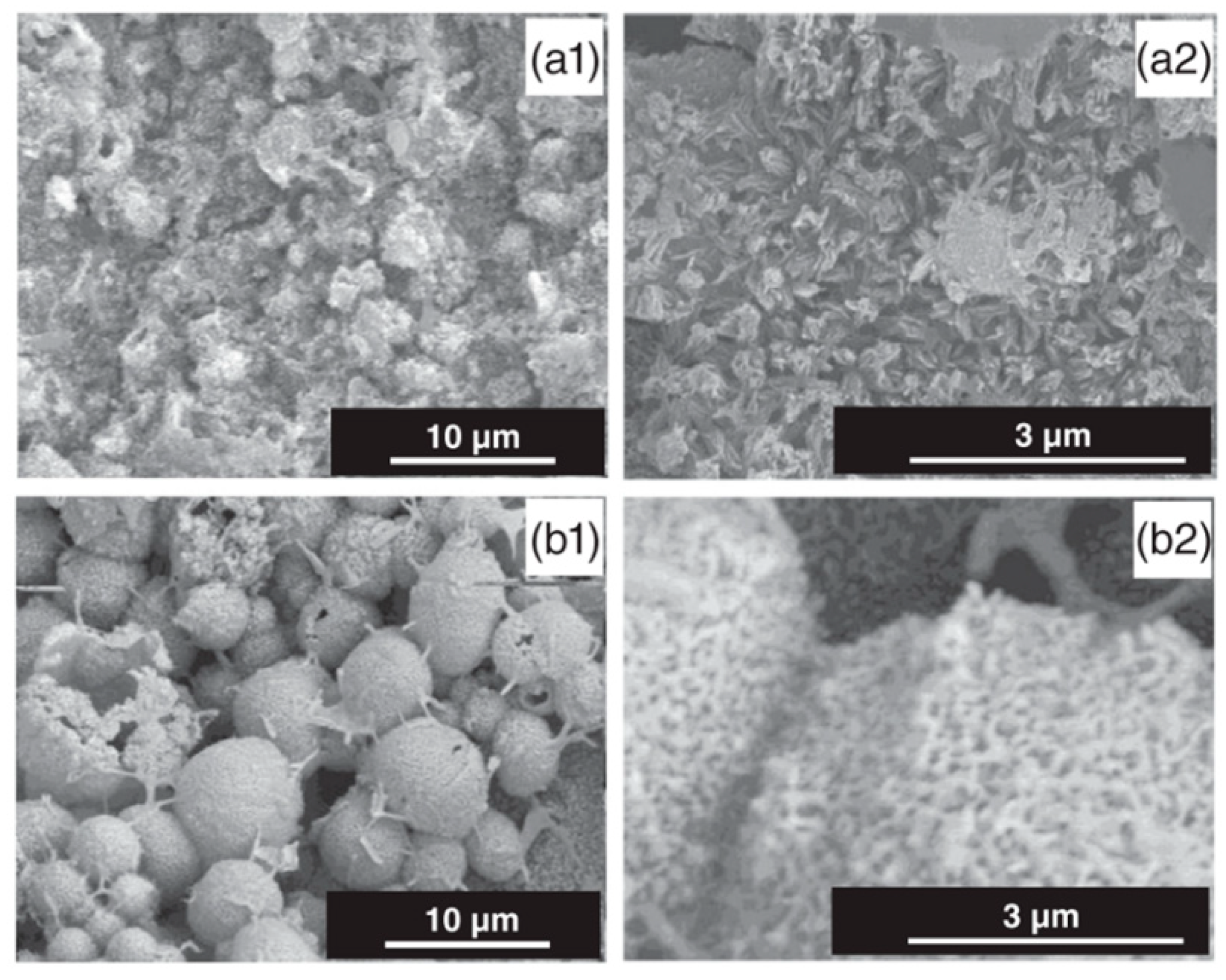
Calcium Phosphate
5.2.3. Composite Coatings
5.2.4. Metal Coatings
5.3. Influence of Coatings on Biocompatibility and Corrosion Properties of Metallic Foams
5.4. Promising Biomaterials
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef]
- Zivic, F.; Grujovic, N.; Pellicer, E.; Sort, J.; Mirovic, S.; Adamovic, D.; Vulovic, M. Biodegradable Metals as Biomaterialsfor Clinical Practice: Iron-Based Materials. In Biomaterials in Clinical Practice; Springer: Cham, Switzerland, 2017; p. 225. ISBN 978-3-319-68025-5. [Google Scholar]
- Radenkovic, G.; Petkovic, D. Metallic Biomaterials. In Biomaterials in Clinical Practice; Springer: Cham, Switzerland, 2017; p. 183. ISBN 978-3-319-68025-5. [Google Scholar]
- Adamovic, D.; Ristic, B.; Zivic, F. Review of Existing Biomaterials—Method of Material Selection for Specific Applications in Orthopedics. In Biomaterials in Clinical Practice; Springer: Cham, Switzerland, 2017; p. 47. ISBN 978-3-319-68025-5. [Google Scholar]
- Munir, K.; Wen, C.; Li, Y. Production methods and characterization of porous Mg and Mg alloys for biomedical applications. In Metallic Foam Bone: Processing, Modification and Characterization and Properties; Elsevier: Amsterdam, The Netherlands, 2017; pp. 25–82. ISBN 978-0-08-101290-1. [Google Scholar]
- Wen, Z.; Zhang, L.; Chen, C.; Liu, Y.; Wu, C.; Dai, C. A construction of novel iron-foam-based calcium phosphate/chitosan coating biodegradable scaffold material. Mater. Sci. Eng. C 2013, 33, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloy 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef]
- Oriňaková, R.; Gorejová, R.; Orságová, Z.; Haverová, L.; Oriňak, A.; Maskaľová, I.; Kupková, M.; Džupon, M.; Baláž, M.; Hrubovčáková, M.; et al. Evaluation of Mechanical Properties and Hemocompatibility of Open Cell Iron Foams with Polyethylene Glycol Coating. Appl. Surf. Sci. 2019, 505, 144634. [Google Scholar] [CrossRef]
- Oriňak, A.; Oriňaková, R.; Orságová Králová, Z.; Morovská Turoňová, A.; Kupková, M.; Hrubovčáková, M.; Radoňak, J.; Džunda, R.; Turoňová Morovská, A.; Kupková, M.; et al. Sintered metallic foams for biodegradable bone replacement materials. J. Porous Mater. 2014, 21, 131–140. [Google Scholar] [CrossRef]
- Gorejová, R.; Haverová, L.; Oriňaková, R.; Oriňak, A.; Oriňak, M. Recent advancements in Fe-based biodegradable materials for bone repair. J. Mater. Sci. 2019, 54, 1913–1947. [Google Scholar] [CrossRef]
- Čapek, J.; Vojtěch, D.; Oborná, A. Microstructural and mechanical properties of biodegradable iron foam prepared by powder metallurgy. Mater. Des. 2015, 83, 468–482. [Google Scholar] [CrossRef]
- Oriňáková, R.; Oriňák, A.; Bučková, L.M.; Giretová, M.; Medveckỳ, L.; Labbanczová, E.; Kupková, M.; Hrubovčáková, M.; Koval, K. Iron based degradable foam structures for potential orthopedic applications. Int. J. Electrochem. Sci. 2013, 8, 12451–12465. [Google Scholar]
- Li, Y.; Zhou, J.; Pavanram, P.; Leeflang, M.A.; Fockaert, L.I.; Pouran, B.; Tümer, N.; Schröder, K.U.; Mol, J.M.C.; Weinans, H.; et al. Additively manufactured biodegradable porous magnesium. Acta Biomater. 2018, 67, 378–392. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, P. Degradable porous Fe-35wt.%Mn produced via powder sintering from NH4HCO3 porogen. Mater. Chem. Phys. 2015, 163, 394–401. [Google Scholar] [CrossRef]
- Heiden, M.; Walker, E.; Stanciu, L. Magnesium, Iron and Zinc Alloys, the Trifecta of Bioresorbable Orthopaedic and Vascular Implantation—A Review. Biotechnol. Biomater. 2005, 5, 1000178. [Google Scholar]
- Meagher, P.; O’Cearbhaill, E.D.; Byrne, J.H.; Browne, D.J. Bulk Metallic Glasses for Implantable Medical Devices and Surgical Tools. Adv. Matter. 2016, 28, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Ulum, M.F.; Caesarendra, W.; Alavi, R.; Hermawan, H. In-Vivo Corrosion Characterization and Assessment. Coatings 2019, 9, 282. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.M.M. Morphological and mechanical characterization of topologically ordered open cell porous iron foam fabricated using 3D printing and pressureless microwave sintering. Mater. Des. 2018, 160, 442–454. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.M. Corrosion behaviour of the porous iron scaffold in simulated body fluid for biodegradable implant application. Mater. Sci. Eng. C 2019, 99, 838–852. [Google Scholar] [CrossRef]
- Haverová, L.; Oriňaková, R.; Oriňak, A.; Gorejová, R.; Baláž, M.; Vanýsek, P.; Kupková, M.; Hrubovčáková, M.; Mudroň, P.; Radoňák, J.; et al. An in vitro corrosion study of open cell Iron structures with PEG coating for bone replacement applications. Metals 2018, 8, 499. [Google Scholar] [CrossRef]
- He, J.; He, F.-L.; Li, D.-W.; Liu, Y.-L.; Liu, Y.-Y.; Ye, Y.-J.; Yin, D.-C. Advances in Fe-based biodegradable metallic materials. RSC Adv. 2016, 6, 112819–112838. [Google Scholar] [CrossRef]
- Lefevre, L.-P.; Banhart, J.; Dunand, D.C. Porous Metals and Metallic Foams: Current Status and Recent Developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef]
- Lietaert, K.; Weber, L.; Van Humbeeck, J.; Mortensen, A.; Luyten, J.; Schrooten, J. Open cellular magnesium alloys for biodegradable orthopaedic implants. J. Magnes. Alloy 2013, 1, 303–311. [Google Scholar] [CrossRef]
- Ray, S.; Thormann, U.; Eichelroth, M.; Budak, M.; Biehl, C.; Rupp, M.; Sommer, U.; El Khassawna, T.; Alagboso, F.I.; Kampschulte, M.; et al. Strontium and bisphosphonate coated iron foam scaffolds for osteoporotic fracture defect healing. Biomaterials 2018, 157, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alavi, R.; Trenggono, A.; Champagne, S.; Hermawan, H. Investigation on Mechanical Behavior of Biodegradable Iron Foams under Different Compression Test Conditions. Metals 2017, 7, 202. [Google Scholar] [CrossRef]
- Pellicer, E.; Lorenzetti, M.; Fornell, J.; Baró, M.D.; Novak, S.; Sort, J. Progress Beyond the State-of-the-Art in the Field of Metallic Materials for Bioimplant Applications. In Biomaterials in Clinical Practice; Springer: Cham, Switzerland, 2017; p. 25. ISBN 978-3-319-68025-5. [Google Scholar]
- Erryani, A.; Pramuji, F.; Annur, D.; Kartika, I.; Amal, M.I. Microstructures and Mechanical Study of Mg Alloy Foam Based on Mg-Zn-Ca-CaCO3 System. OP Conf. Ser. Mater. Sci. 2017, 202, 012028. [Google Scholar] [CrossRef]
- Malladi, L.; Mahapatro, A.; Gomes, A.S.; Steffin, A. Fabrication of magnesium-based metallic scaffolds for bone tissue engineering. Mater. Technol. 2018, 33, 173–182. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Čapek, J.; Msallamová, Š.; Jablonská, E.; Lipov, J.; Vojtěch, D. A novel high-strength and highly corrosive biodegradable Fe-Pd alloy: Structural, mechanical and in vitro corrosion and cytotoxicity study. Mater. Sci. Eng. C 2017, 9, 550–562. [Google Scholar] [CrossRef]
- Lietaert, K.; Wauthle, R.; Schrooten, J. Porous Metals in Orthopedics. In Biomaterials in Clinical Practice; Springer: Cham, Switzerland, 2017; p. 281. ISBN 978-3-319-68025-5. [Google Scholar]
- Sharma, P.; Pandey, P.M. A novel manufacturing route for the fabrication of topologically-ordered open-cell porous iron scaffold. Mater. Lett. 2017, 222, 160–163. [Google Scholar] [CrossRef]
- Su, Y.; Champagne, S.; Trenggono, A.; Tolouei, R.; Mantovani, D.; Hermawan, H. Development and characterization of silver containing calcium phosphate coatings on pure iron foam intended for bone scaffold applications. Mater. Des. 2018, 148, 124–134. [Google Scholar] [CrossRef]
- Liao, Y.; Qiu, G.; Yang, Y.; Lv, X.; Bai, C. Preparation and compressive properties of magnesium foam. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2016, 45, 2498–2502. [Google Scholar]
- Chen, Q.Z. Foaming technology of tissue engineering scaffolds—A review. Eng. Technol. 2011, 3, 34–47. [Google Scholar] [CrossRef]
- Trinidad, J.; Marco, I.; Arruebarrena, G.; Wendt, J.; Letzig, D.; Sáenz De Argandoña, E.; Goodall, R. Processing of magnesium porous structures by infiltration casting for biomedical applications. Adv. Eng. Mater. 2014, 16, 241–247. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Salerno, A.; Netti, P.A. Introduction to Biomedical Foams. In Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing: Sawston/Cambridge, UK, 2014; ISBN 9780857096968. [Google Scholar]
- Guarino, V.; Ambrosio, L. 2—Properties of biomedical foams for tissue engineering applications. In Biomedical Foams for Tissue Engineering Applications; Woodhead Publishing: Sawston/Cambridge, UK, 2014; pp. 40–70. ISBN 9780857096968. [Google Scholar]
- Servier Medical Art to Illustrate your Publications and Powerpoint Presentations. Available online: https://smart.servier.com/ (accessed on 10 July 2020).
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Čapek, J.; Vojtěch, D. Microstructural and mechanical characteristics of porous iron prepared by powder metallurgy. Mater. Sci. Eng. C 2014, 43, 494–501. [Google Scholar] [CrossRef]
- Oriňaková, R.; Oriňak, A.; Markušová, L.; Labbanczová, E.; Kupková, M.; Hrubovčaková, M.; Fedorková, A. Biodegradable Open Cell Iron Foams for Potential Skeletal Application. Powder Metall. Prog. 2012, 12, 219–223. [Google Scholar]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Aghion, E.; Yered, T.; Perez, Y.; Gueta, Y. The prospects of carrying and releasing drugs via biodegradable magnesium foam. Adv. Eng. Mater. 2010, 12, 374–379. [Google Scholar] [CrossRef]
- Oriňaková, R.; Gorejová, R.; Macko, J.; Oriňak, A.; Kupková, M.; Hrubovčáková, M.; Ševc, J.; Smith, R.M. Evaluation of in vitro biocompatibility of open cell iron structures with PEG coating. Appl. Surf. Sci. 2019, 475, 515–518. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Leeflang, M.; Pouran, B.; Gonzalez-Garcia, Y.; Weinans, H.; Mol, J.M.C.; et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393. [Google Scholar] [CrossRef]
- Banhart, J. Manufacturing Routes for Metallic Foams. JOM 2000, 52, 22–27. [Google Scholar] [CrossRef]
- Chou, D.T.; Wells, D.; Hong, D.; Lee, B.; Kuhn, H.; Kumta, P.N. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 2013, 9, 8593–8603. [Google Scholar] [CrossRef]
- Quadbeck, P.; Stephani, G.; Kümmel, K.; Adler, J.; Standke, G. Synthesis and Properties of Open-Celled Metal Foams. Mater. Sci. Forum 2007, 534–536, 1005–1008. [Google Scholar] [CrossRef]
- Posada, V.M.; Orozco, C.; Ramirez Patino, J.F.; Fernandez-Morales, P. Human Bone Inspired Design of an Mg Alloy-Based Foam. Mater. Sci. Forum 2018, 933, 291–296. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Li, Y.; Liu, H.; Bai, J.; Bao, N.R.; Zhang, Y.; He, P.; Zhao, J.N.; Tao, L.; Xue, F.; et al. Mechanical and Biological Properties of a Biodegradable Mg-Zn-Ca Porous Alloy. Orthop. Surg. 2018, 10, 160–168. [Google Scholar] [CrossRef]
- Hou, Y.; Jia, G.; Yue, R.; Chen, C.; Pei, J.; Zhang, H.; Huang, H.; Xiong, M.; Yuan, G. Synthesis of biodegradable Zn-based scaffolds using NaCl templates: Relationship between porosity, compressive properties and degradation behavior. Mater. Charact. 2018, 137, 162–169. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Y.; Zhang, H.; Chen, X.; Li, Y. Compressive and Corrosion Properties of Lotus-Type Porous Mg-Mn Alloys Fabricated by Unidirectional Solidification. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2020, 51, 3238–3247. [Google Scholar] [CrossRef]
- Kang, M.H.; Lee, H.; Jang, T.S.; Seong, Y.J.; Kim, H.E.; Koh, Y.H.; Song, J.; Jung, H. Do Biomimetic porous Mg with tunable mechanical properties and biodegradation rates for bone regeneration. Acta Biomater. 2019, 84, 453–467. [Google Scholar] [CrossRef]
- Čapek, J.; Jablonská, E.; Lipov, J.; Kubatík, T.F.; Vojtěch, D. Preparation and characterization of porous zinc prepared by spark plasma sintering as a material for biodegradable scaffolds. Mater. Chem. Phys. 2018, 203, 249–258. [Google Scholar] [CrossRef]
- Yusop, A.H.; Bakir, A.A.; Shaharom, N.A.; Abdul Kadir, M.R.; Hermawan, H. Porous Biodegradable Metals for Hard Tissue Scaffolds: A Review. Int. J. Biomater. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Popescu, I.N.; Vidu, R.; Bratu, V. Porous Metallic Biomaterials Processing (Review) Part 1: Compaction, Sintering Behavior, Properties and Medical Applications. Sci. Bull. Valahia Univ.-Mater. Mech. 2017, 15, 28–40. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Shimojima, K.; Chino, Y.; Hosokawa, H.; Mabuchi, M. Compressibility of porous magnesium foam: Dependency on porosity and pore size. Mater. Lett. 2004, 58, 357–360. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Puggi, U.; Zhang, X.Y.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; et al. Additively manufactured functionally graded biodegradable porous iron. Acta Biomater. 2019, 96, 646–661. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Dianyu, E.; Yang, W.; Qi, F.; Xie, D.; Shen, L.; Peng, S.; Shuai, C. Mg bone implant: Features, developments and perspectives. Mater. Des. 2020, 185, 108259. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Bobbert, F.S.L.; Kubo, Y.; Leeflang, M.A.; Jahr, H.; Zadpoor, A.A. Additively manufactured functionally graded biodegradable porous zinc. Biomater. Sci. 2020, 96, 646–661. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Bobbert, F.S.L.; Lietaert, K.; Dong, J.H.; Leeflang, M.A.; Zhou, J.; Zadpoor, A.A. Corrosion fatigue behavior of additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 106, 439–449. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Voshage, M.; Chen, Y.; Schückler, P.G.; Jauer, L.; Xia, D.; Guo, H.; Zheng, Y.; Schleifenbaum, J.H. Additive manufacturing of biodegradable Zn-xWE43 porous scaffolds: Formation quality, microstructure and mechanical properties. Mater. Des. 2019, 181, 107973. [Google Scholar] [CrossRef]
- Markušová-Bučková, L.; Oriňaková, R.; Oriňak, A.; Gorejová, R.; Kupková, M.; Hrubovčáková, M.; Baláž, M.; Kováľ, K. Static corrosion test of porous iron material with polymer coating. Powder Metall. Prog. 2016, 16, 99–106. [Google Scholar] [CrossRef]
- Oriňaková, R.; Oriňak, A.; Giretová, M.; Medvecký, L.; Kupková, M.; Hrubovčáková, M.; Maskal’ová, I.; Macko, J.; Kal’avský, F. A study of cytocompatibility and degradation of iron-based biodegradable materials. J. Biomater. Appl. 2016, 30, 1060–1070. [Google Scholar] [CrossRef]
- Oriňaková, R.; Oriňak, A.; Kupková, M.; Hrubovčáková, M.; Markušová-Bučková, L.; Giretová, M.; Medvecký, L.; Dobročka, E.; Petruš, O.; Kaľavský, F. In vitro degradation and cytotoxicity evaluation of iron biomaterials with hydroxyapatite film. Int. J. Electrochem. Sci. 2015, 10, 8158–8174. [Google Scholar]
- Wegener, B.; Sievers, B.; Utzschneider, S.; Müller, P.; Jansson, V.; Rößler, S.; Nies, B.; Stephani, G.; Kieback, B.; Quadbeck, P. Microstructure, cytotoxicity and corrosion of powder-metallurgical iron alloys for biodegradable bone replacement materials. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 1789–1796. [Google Scholar] [CrossRef]
- Vahidgolpayegani, A.; Wen, C.; Hodgson, P.; Li, Y. Production Methods and Characterization of Porous Mg and Mg Alloys for Biomedical Applications; Woodhead Publishing: Sawston/Cambridge, UK, 2016; ISBN 9780081012901. [Google Scholar]
- Zhuang, H.; Han, Y.; Feng, A. Preparation, mechanical properties and in vitro biodegradation of porous magnesium scaffolds. Mater. Sci. Eng. C 2008, 28, 1462–1466. [Google Scholar] [CrossRef]
- Song, Y.H.; Park, S.H.; Kim, S.Y.; Seo, C.H.; Hur, B.Y. A Study on Al-Mg Alloy Foams by Melt Foaming Method. Solid State Phenom. 2009, 124–126, 1841–1844. [Google Scholar]
- Wang, X.; Lu, H.M.; Li, X.L.; Li, L.; Zheng, Y.F. Effect of cooling rate and composition on microstructures and properties of Zn-Mg alloys. Trans. Nonferrous Met. Soc. China 2007, 17, 122–125. [Google Scholar]
- Zadpoor, A.A. Additively manufactured porous metallic biomaterials. J. Mater. Chem. B 2019, 7, 4088–4117. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontol 2000 2019, 79, 178–189. [Google Scholar] [CrossRef]
- Saleh, M.M.; Saleh, M.M.; Touny, A.H.; Al-Omair, M.A. Biodegradable/biocompatible coated metal implants for orthopedic applications. Biomed. Mater. Eng. 2016, 27, 87–99. [Google Scholar] [CrossRef]
- Van der Biest, O.O.; Vandeperre, L. Electrophoretic Deposition of Materials. Annu. Rev. Mater. Sci. 1999, 29, 327–352. [Google Scholar] [CrossRef]
- Amrollahi, P.; Krasinski, J.S.; Vaidyanathan, R.; Tayebi, L.; Vashaee, D.; Advanced, H.; Advanced, H.; State, O. Electrophoretic deposition (EPD): Fundamentals and applications from nano- to micro- scale structures. In Handbook of Nanoelectrochemistry; Springer: Cham, Switzerland, 2015; pp. 1–27. ISBN 9783319152073. [Google Scholar]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Gunputh, U.; Le, H. Composite coatings for implants and tissue engineering scaffolds. Biomed. Compos. 2017, 2017, 111–138. [Google Scholar]
- Mesquita-Guimarães, J.; Henriques, B.H.; Silva, F.S. Bioactive Glass Coatings, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081009369. [Google Scholar]
- Ding, D. Processing, Properties and Applications of Ceramic Matrix Composites, SiCf/SiC: An Overview; Woodhead Publishing: Sawston/Cambridge, UK, 2014; Volume 2, ISBN 9780081021675. [Google Scholar]
- Amini, A.R.; Laurencin, C.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Pishbin, F.; Simchi, A.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition of chitosan/45S5 Bioglass® composite coatings for orthopaedic applications. Surf. Coat. Technol. 2011, 205, 5260–5268. [Google Scholar] [CrossRef]
- Koskinen, J. Cathodic-Arc and Thermal-Evaporation Deposition; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, ISBN 9780080965338. [Google Scholar]
- Antunes, R.A.; de Oliveira, M.C.L. Effect of Surface Treatments on the Fatigue Life of Magnesium and Its Alloys for Biomedical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 1, ISBN 9781782420828. [Google Scholar]
- Kang, C.W.; Fang, F.Z. State of the art of bioimplants manufacturing: Part II. Adv. Manuf. 2018, 6, 137–154. [Google Scholar] [CrossRef]
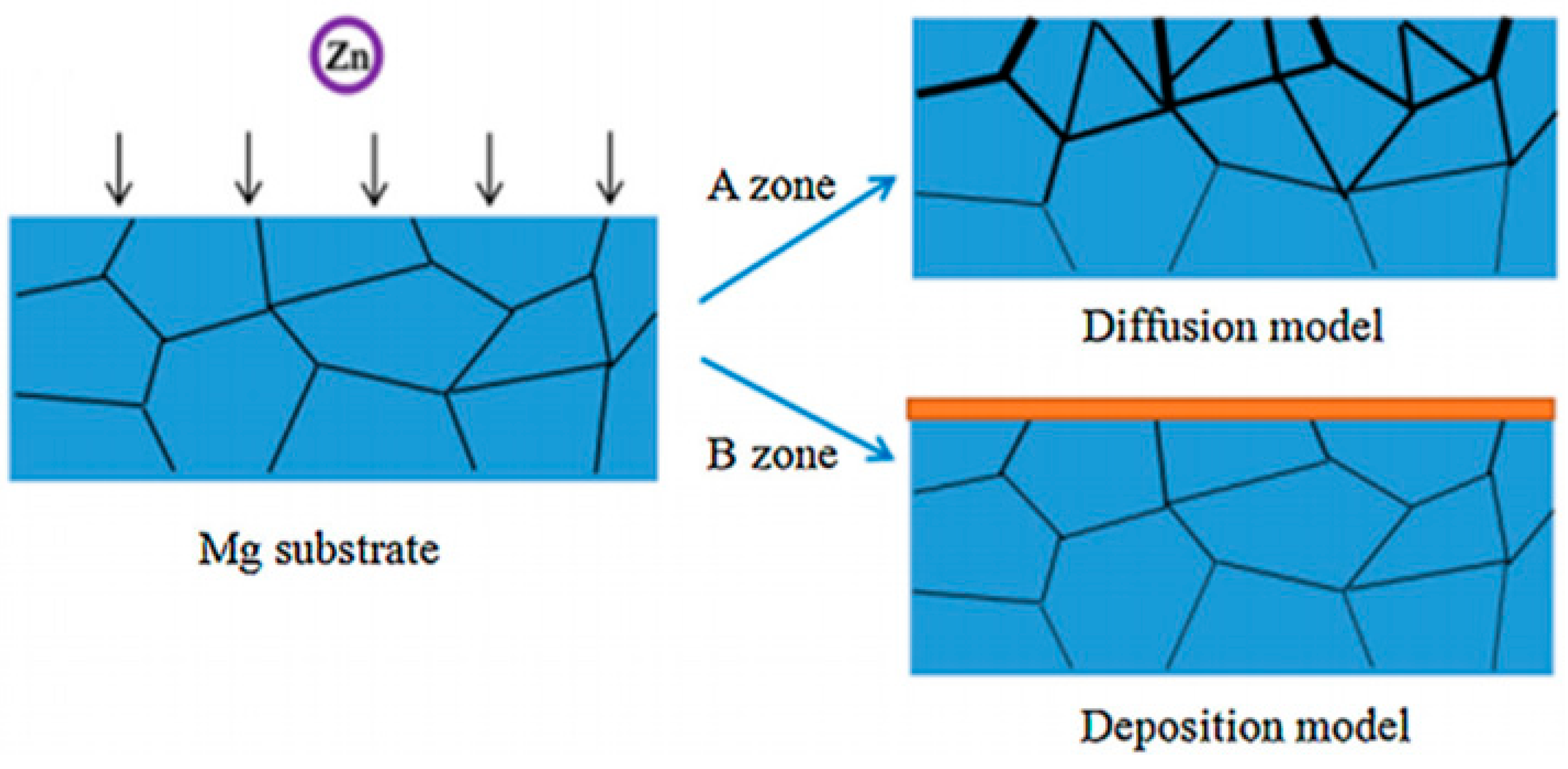
- Wang, X.; Wang, X.; Wang, D.; Zhao, M.; Han, F. A novel approach to fabricate Zn coating on Mg foam through a modified thermal evaporation technique. J. Mater. Sci. Technol. 2018, 34, 1558–1563. [Google Scholar] [CrossRef]
- Neacşu, I.A.; Nicoară, A.I.; Vasile, O.R.; Vasile, B.Ş. Inorganic micro- and nanostructured implants for tissue engineering. In Nanobiomaterials in Hard Tissue Engineering: Applications of Nanobiomaterials; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 271–295. ISBN 9780323428620. [Google Scholar]
- Kakaei, K.; Esrafili, M.D.; Ehsani, A. Graphene and Anticorrosive Properties; Elsevier: Amsterdam, The Netherlands, 2019; Volume 27, ISBN 9780128145234. [Google Scholar]
- Brinker, C.J.; Hurd, A.J. Fundamentals of sol-gel dip-coating. J. Phys. III 1994, 4, 1231–1242. [Google Scholar] [CrossRef]
- Faustini, M.; Louis, B.; Albouy, P.A.; Kuemmel, M.; Grosso, D. Preparation of sol-gel films by dip-coating in extreme conditions. J. Phys. Chem. C 2010, 114, 7637–7645. [Google Scholar] [CrossRef]
- Oriňaková, R.; Gorejová, R.; Králová, Z.O.; Oriňak, A.; Shepa, I.; Hovancová, J.; Kovalčíková, A.; Bujňáková, Z.L.; Király, N.; Kaňuchová, M.; et al. Influence of albumin interaction on corrosion resistance of sintered iron biomaterials with polyethyleneimine coating. Appl. Surf. Sci. 2020, 509, 145379. [Google Scholar] [CrossRef]
- Gorejová, R.; Oriňaková, R.; Králová, Z.O.; Baláž, M.; Kupková, M.; Hrubovčáková, M.; Haverová, L.; Džupon, M.; Oriňak, A.; Kal’avskỳ, F.; et al. In vitro corrosion behavior of biodegradable iron foams with polymeric coating. Materials 2020, 13, 184. [Google Scholar] [CrossRef]
- Hrubovčáková, M.; Kupková, M.; Džupon, M.; Giretová, M.; Medvecký, L.; Džunda, R. Biodegradable polylactic acid and polylactic acid/hydroxyapatite coated iron foams for bone replacement materials. Int. J. Electrochem. Sci. 2017, 12, 11122–11136. [Google Scholar] [CrossRef]
- Julmi, S.; Krüger, A.K.; Waselau, A.C.; Meyer-Lindenberg, A.; Wriggers, P.; Klose, C.; Maier, H.J. Processing and coating of open-pored absorbable magnesium-based bone implants. Mater. Sci. Eng. C 2019, 98, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Gerhardt, R.A.; Szutkowska, M. Advanced Ceramic Materials; John Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Yusop, A.H.M.; Daud, N.M.; Nur, H.; Kadir, M.R.A.; Hermawan, H.; Hakim, A.; Yusop, M.; Daud, N.M.; Nur, H.; Rafiq, M.; et al. Controlling the degradation kinetics of porous iron by poly(lactic-co-glycolic acid) infiltration for use as temporary medical implants. Sci. Rep. 2015, 5, 11194. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.E. Conversion Coatings; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128098943. [Google Scholar]
- Pfeifer, M. Manufacturing Process Considerations. In Mater. Enabled Des; Butterworth-Heinemann: Oxford, UK, 2009; pp. 115–160. ISBN 9780750682879. [Google Scholar]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Ly, X.N.; Yang, S. Influence of current mode on microstructure and corrosion behavior of micro-arc oxidation (MAO) biodegradable Mg-Zn-Ca alloy in Hank’s solution. Surf. Coat. Technol. 2019, 358, 331–339. [Google Scholar] [CrossRef]
- Rúa, J.M.; Zuleta, A.A.; Ramírez, J.; Fernández-Morales, P. Micro-arc oxidation coating on porous magnesium foam and its potential biomedical applications. Surf. Coat. Technol. 2019, 360, 213–221. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Neogi, S. Techniques for Deposition of Coatings with Enhanced Adhesion to Bio-Implants. In Adhes. Pharm. Biomed. Dent. Fields; John Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 235–255. ISBN 9781119323501. [Google Scholar]
- Pacelli, S.; Manoharana, V.; Desalvo, A.; Lomis, N.; Jodhaa, K.S.; Prakash, S.; Paul, A. Tailoring biomaterial surface properties to modulate host-implant interactions: Implication in cardiovascular and bone therapy. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.Y.; Perinpanayagam, H.; Mozumder, M.S.; Zhu, J. Novel development of biocompatible coatings for bone implants. Coatings 2015, 5, 737–757. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R.; Jafari, S.H.; Mozafari, M. Application of Compatibilized Polymer Blends in Biomedical Fields; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128160060. [Google Scholar]
- Boussif, O.; Lezoualch, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Fang, Z.; Niu, Y.; Lou, J.; Wu, Y.; Zou, S.; Xia, S.; Sun, M.; Du, F. Fabrication of HA/PEI-functionalized carbon dots for tumor targeting, intracellular imaging and gene delivery. RSC Adv. 2017, 7, 3369–3375. [Google Scholar] [CrossRef]
- Yao, X.; Zhou, N.; Wan, L.; Su, X.; Sun, Z.; Mizuguchi, H.; Yoshioka, Y.; Nakagawa, S.; Zhao, R.C.; Gao, J.Q. Polyethyleneimine-coating enhances adenoviral transduction of mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014, 447, 383–387. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Pasha, S.K.K.; Bhagat, P.R. Biopolymer Composites in Electronics 3—Biopolymer Composites with High Dielectric Performance: Interface; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128092613. [Google Scholar]
- Hagen, R. PLA (Polylactic Acid). In Reference Module in Materials Science and Materials Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 1–7. ISBN 9780128035818. [Google Scholar]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J. Biomimetic Phosphate Nanocomposites for Bone-Tissue Regeneration; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9781782424758. [Google Scholar]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef]
- Ferguson, J.; Diefenbeck, M.; McNally, M. Ceramic Biocomposites as Biodegradable Antibiotic Carriers in the Treatment of Bone Infections. J. Bone Jt. Infect. 2016, 2, 38–51. [Google Scholar] [CrossRef]
- Zafar, M.S.; Ullah, R.; Qamar, Z.; Fareed, M.A.; Amin, F.; Khurshid, Z.; Sefat, F. Properties of dental biomaterials. In Advanced Dental Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 7–35. ISBN 9780081024768. [Google Scholar]
- Cohn, M.R.; Unnanuntana, A.; Pannu, T.J.; Warner, S.J.; Lane, J.M. 7.16 Materials in fracture fixation. In Comprehensive Biomaterials II; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 7, pp. 278–297. ISBN 9780081006924. [Google Scholar]
- Cheng, J.; Liu, B.; Wu, Y.H.; Zheng, Y.F. Comparative invitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013, 29, 619–627. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
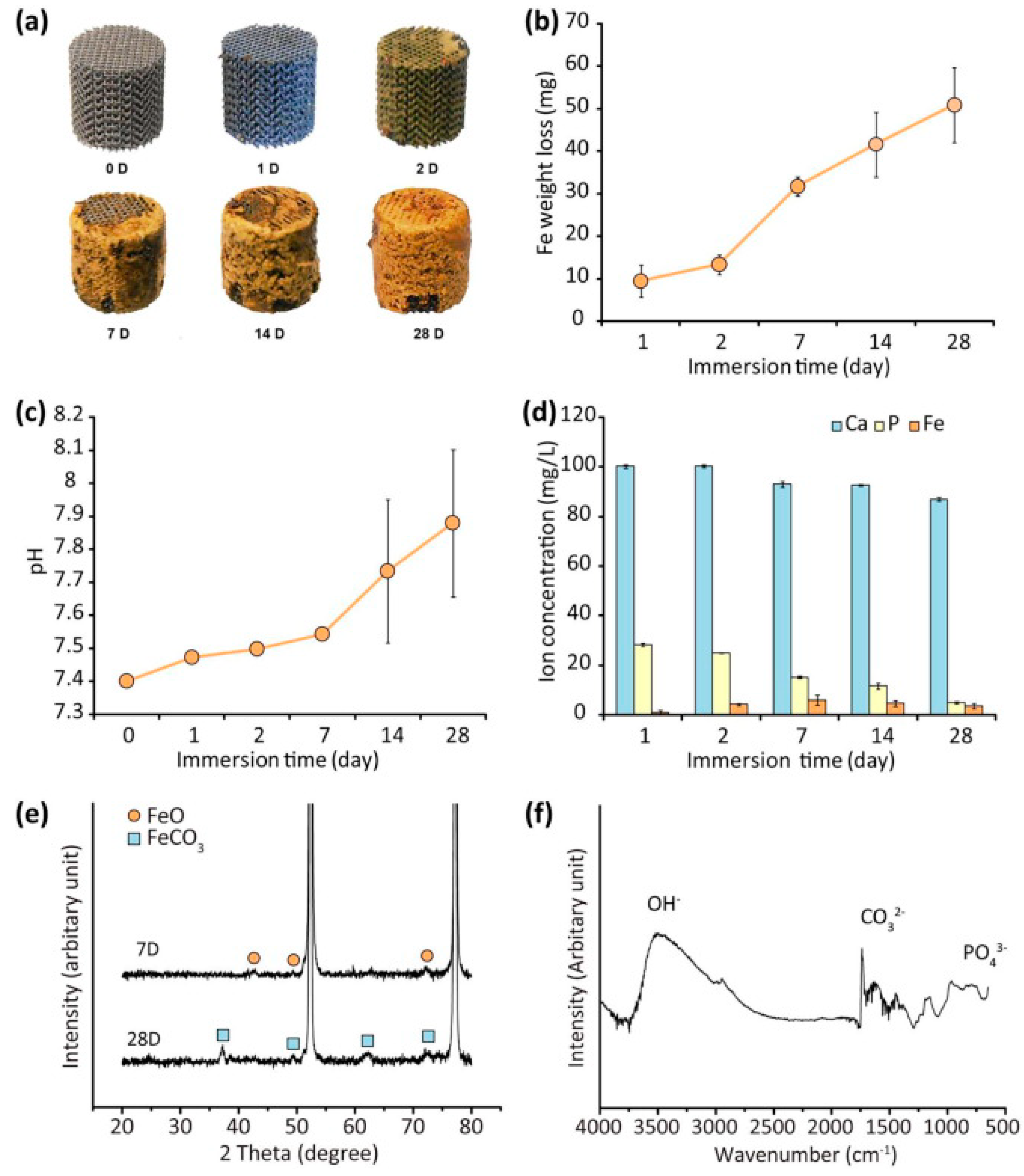
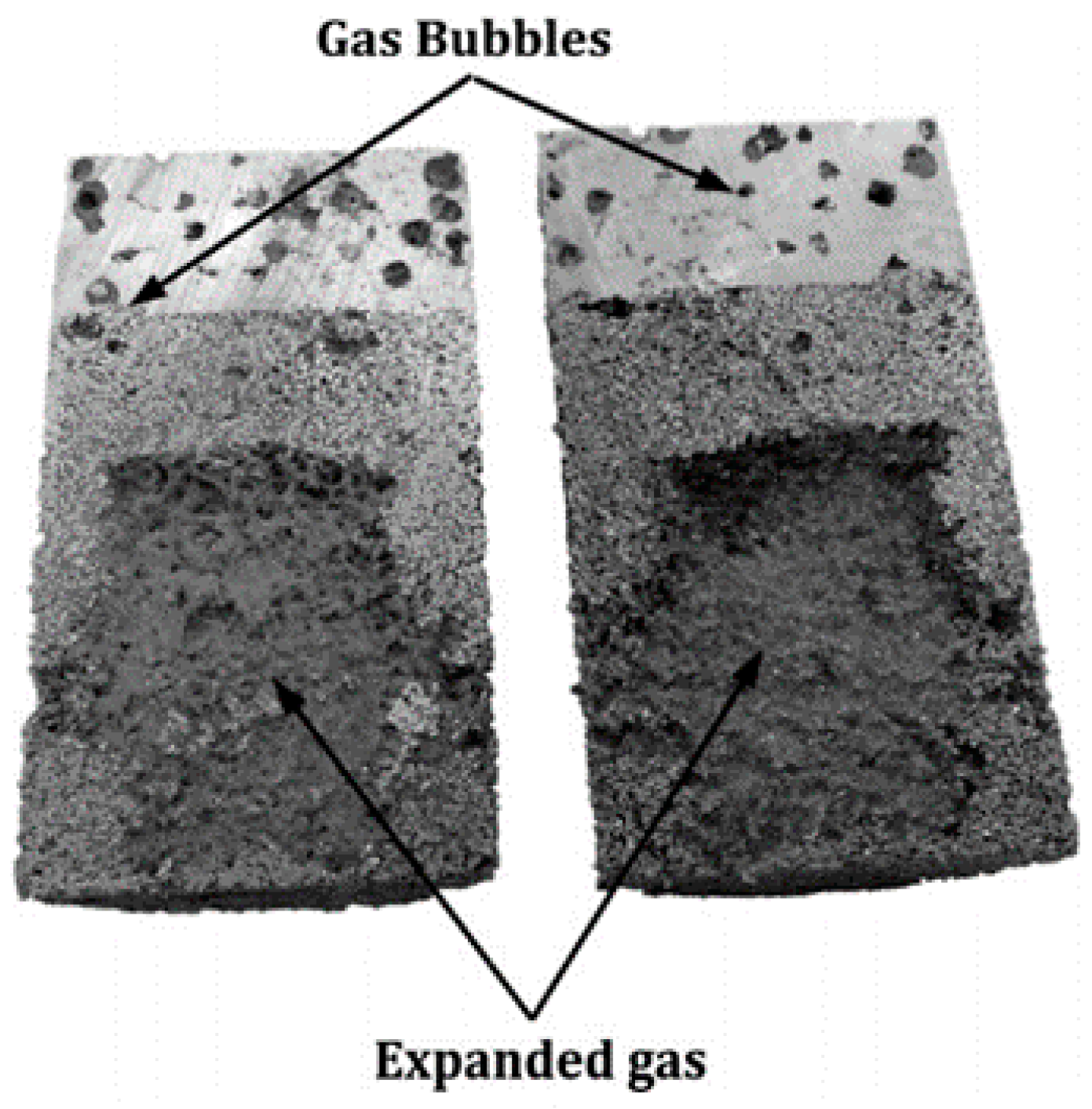
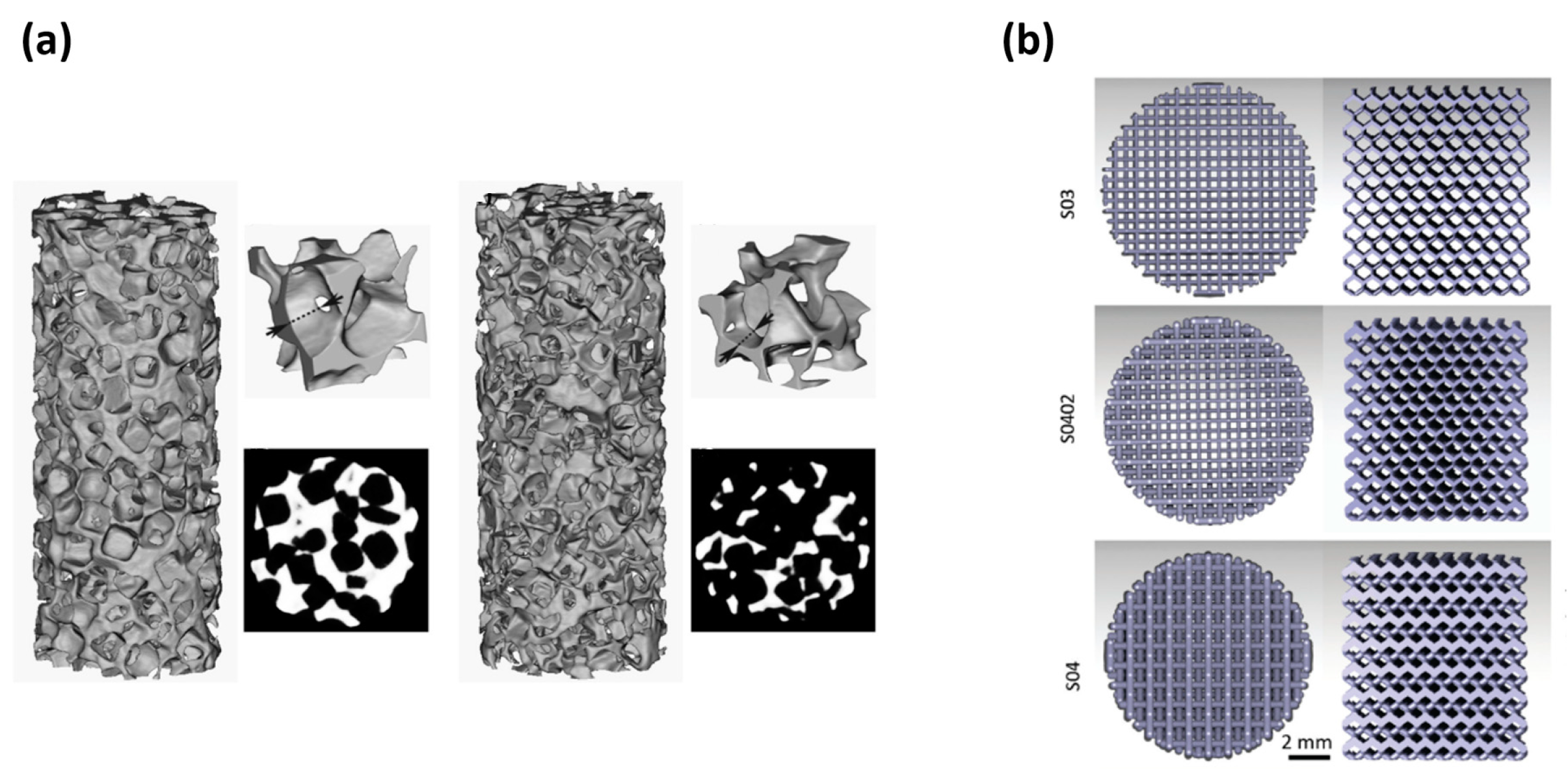

| Methods | Produced Porous Material | Advantage | Disadvantage | Ref. | |
|---|---|---|---|---|---|
| Casting | IC | Mg based foams, Zn based foams | -Simple and low-cost devices, -Pure materials, tailored pore size and distribution, -Complex configurations in nearly final shape, -Elimination of intermediate treatment | -Random pore architecture, -Slow fabrication time | [25,54,55,56] |
| Foaming | MPBA | Mg and Mg based foams, | [5,29,30,51] | ||
| GP | Mg and Mg based foams | [57] | |||
| Sintering | SSHP | Mg and Mg based foams, Fe and Fe based foams | [12,60] | ||
| RMPUT | Fe and Fe based foams | [10,13,22,26] | |||
| SPS | Mg porous materials, Zn porous materials | [58,59] | |||
| AM | LAM | Mg and Mg based scaffolds, Fe and Fe based scaffolds | -Customized shape, complicated structure, -High precision and accuracy, -Fast fabrication | -Safety concerns, -High machine and processing cost, -Limited number of available materials | [2,14,55,63] |
| 3DP | Fe and Fe based scaffolds | [2,23,50,52] | |||
| PBF, L-PBF | TOPZS | [65,66,67] | |||
| Combination | 3DP and casting | TOPM | -Precisely controlled topological parameters, -Flexibility in production of various types of unit cell structures and porosity | [44] | |
| 3DP and PMS | TOPIS | [20,34] | |||
| Coatings | Coating Method | Advantage | Disadvantage | Ref. | |
|---|---|---|---|---|---|
| Polymer | PEG | DC | -Improvement of biocompatibility and cell adhesion -Non-toxic -Suppression of platelet adhesion, and tissue damage -Adjustability of corrosion rate and mechanical properties | -Non-homogenous final coating layers -Rapidly soluble in polar solutions | [9,22,110,111] |
| PEI | DC | -Protection against nuclease-mediated cell degradation -pH buffering -Improvement of acceptance of coated equipment | -Cytotoxicity dependence on polymer concentration and layer thickness | [96,97,112,114] | |
| PLA PLA/HA PLGA | DC, VI | -Drug carrier -Corrosion enhancement -Natural origin -Low production cost -Excellent biocompatibility -Good processability -Reduction of degradation process | -Crystalline form -Induction of hydrogen evolution | [98,101,115,116] | |
| Chitosan | EPD | -Biopolymer naturally occurring in crustaceans -Biodegradable -Biocompatible, non-toxic -Corrosion enhancement -Imitation of structure of extracellular matrix -Stimulation of adhesion and proliferation of cells and osteoinduction | -Demanding solubility -Problem with purity of biopolymer | [6,7,117,118] | |
| Inorganic ceramic | Calcium phosphate (HA, bisphosphonates-Sr) | MAO, ED | -Mineral composition similar to bone -Highly biocompatible -Drug carrier -Binder agent -Increasing of implant osteointegration -Bone defect reparation ability | -Brittleness -Low tensile strength -Fracture toughness | [26,70,106,119,120,121] |
| Composites | nHA/chitosan Ag/CaP | EPD, CC | -Improvement of surface bioactivity -Enhanced mineralization ability -Promoting of osteoblast adhesion, migration, differentiation and proliferation -Bone repair and regeneration ability -Antibacterial potential | [6,35] | |
| Metals | Zn | PVD | -Improvement of mechanical properties -Possibility of degradation modification -Anti-corrosion properties | -Need for complex instrumentation | [91] |
| Material | Coating | Degradation Rate [mm y−1] | Corrosive Medium | Ref. |
|---|---|---|---|---|
| Fe (plate) | - | 0.105 * | Hanks’solution | [125] |
| Fe (RMPUT) | 0.678–0.972 * | Hanks’ solution | [13] | |
| Fe (AM) | 1.18 ± 0.22 * | r-SBF | [50] | |
| Mg (plate) | 1.94 * | Hanks’ solution | [125] | |
| Mg (disk) | 0.20 * | SBF | [126] | |
| Zn (plate) | 0.325 * | Hanks’ solution | [125] | |
| Zn (AM) | 0.06–0.07 s | r-SBF r-SBF | [65] | |
| 0.13–0.17 d | ||||
| Fe | PEG | 0.536–0.703 * | Hanks’ solution | [22] |
| PEI | 1.738 * | [96] | ||
| PLA | 0.650 * | [98] | ||
| PLA/HA | 0.480 * | |||
| PLGA | 0.420 * | PBS | [101] | |
| HAP | 0.1578 * | Hanks’ solution | [70] | |
| Mn-HAP | 0.2762 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oriňaková, R.; Gorejová, R.; Orságová Králová, Z.; Oriňak, A. Surface Modifications of Biodegradable Metallic Foams for Medical Applications. Coatings 2020, 10, 819. https://doi.org/10.3390/coatings10090819
Oriňaková R, Gorejová R, Orságová Králová Z, Oriňak A. Surface Modifications of Biodegradable Metallic Foams for Medical Applications. Coatings. 2020; 10(9):819. https://doi.org/10.3390/coatings10090819
Chicago/Turabian StyleOriňaková, Renáta, Radka Gorejová, Zuzana Orságová Králová, and Andrej Oriňak. 2020. "Surface Modifications of Biodegradable Metallic Foams for Medical Applications" Coatings 10, no. 9: 819. https://doi.org/10.3390/coatings10090819
APA StyleOriňaková, R., Gorejová, R., Orságová Králová, Z., & Oriňak, A. (2020). Surface Modifications of Biodegradable Metallic Foams for Medical Applications. Coatings, 10(9), 819. https://doi.org/10.3390/coatings10090819






