Microstructure and Properties of Fe-Based Alloy Coating on Gray Cast Iron Fabricated Using Induction Cladding
Abstract
1. Introduction
2. Experiment Procedures
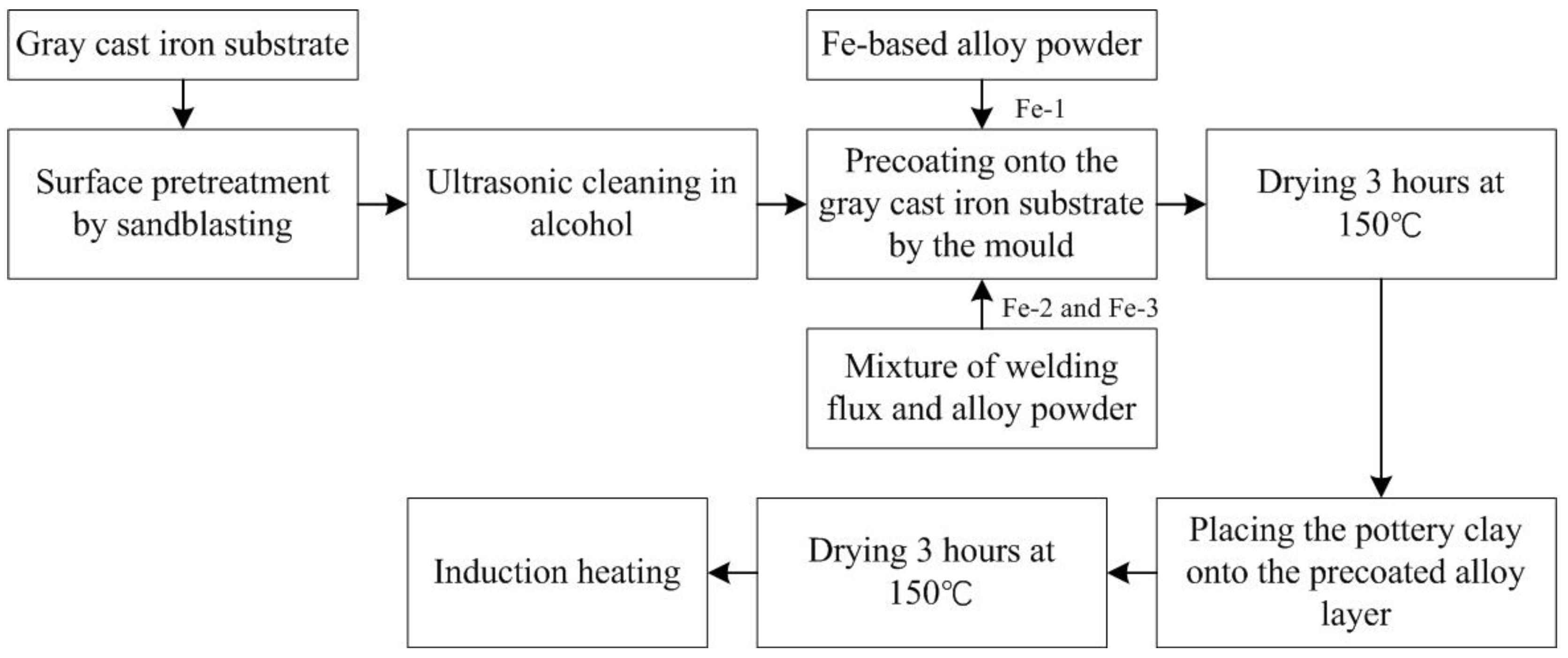
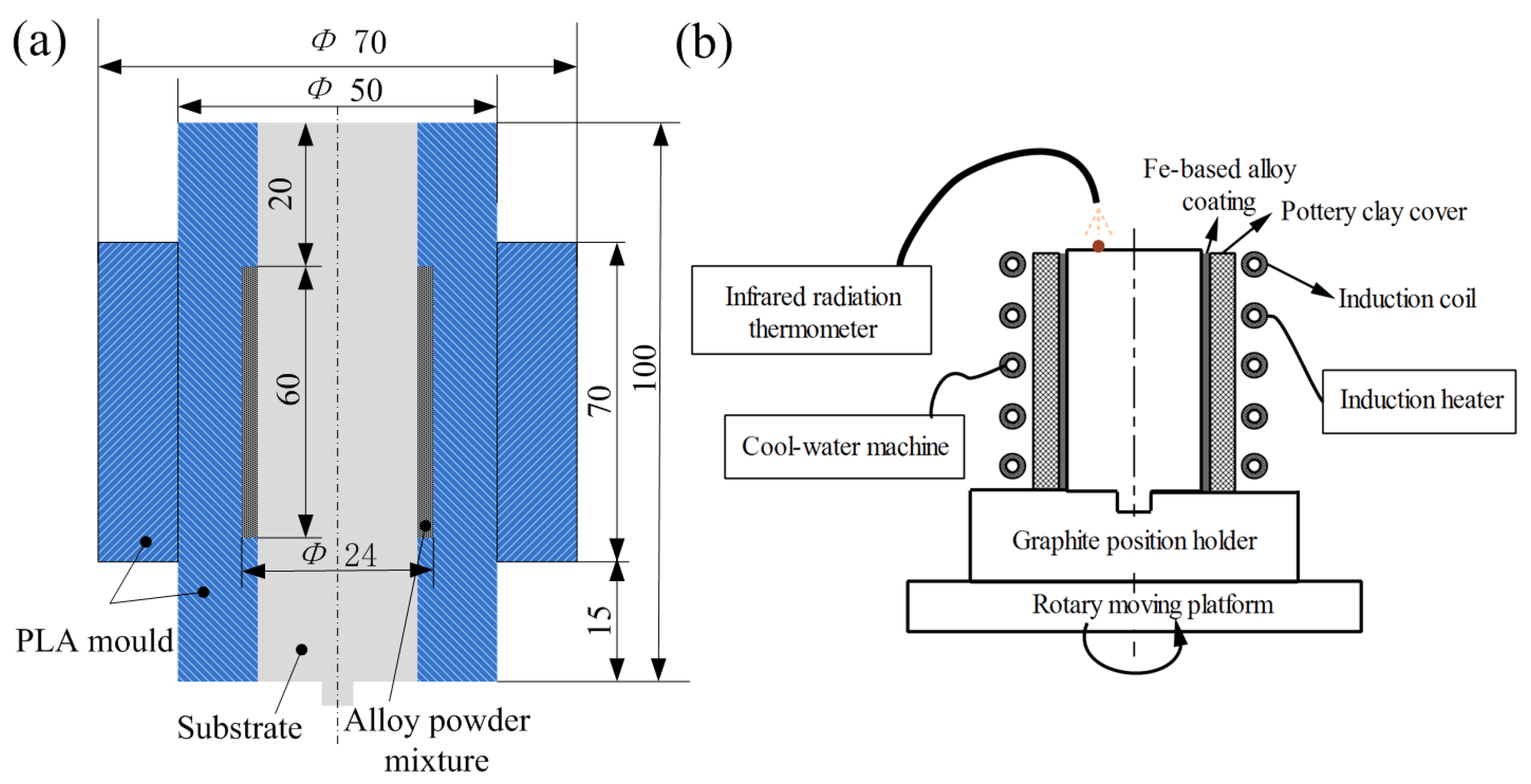
2.1. Materials and Induction Cladding Process
2.2. Characterization of the Coating
2.3. Electrochemical Corrosion Test
2.4. Wear Test
3. Results and Discussion
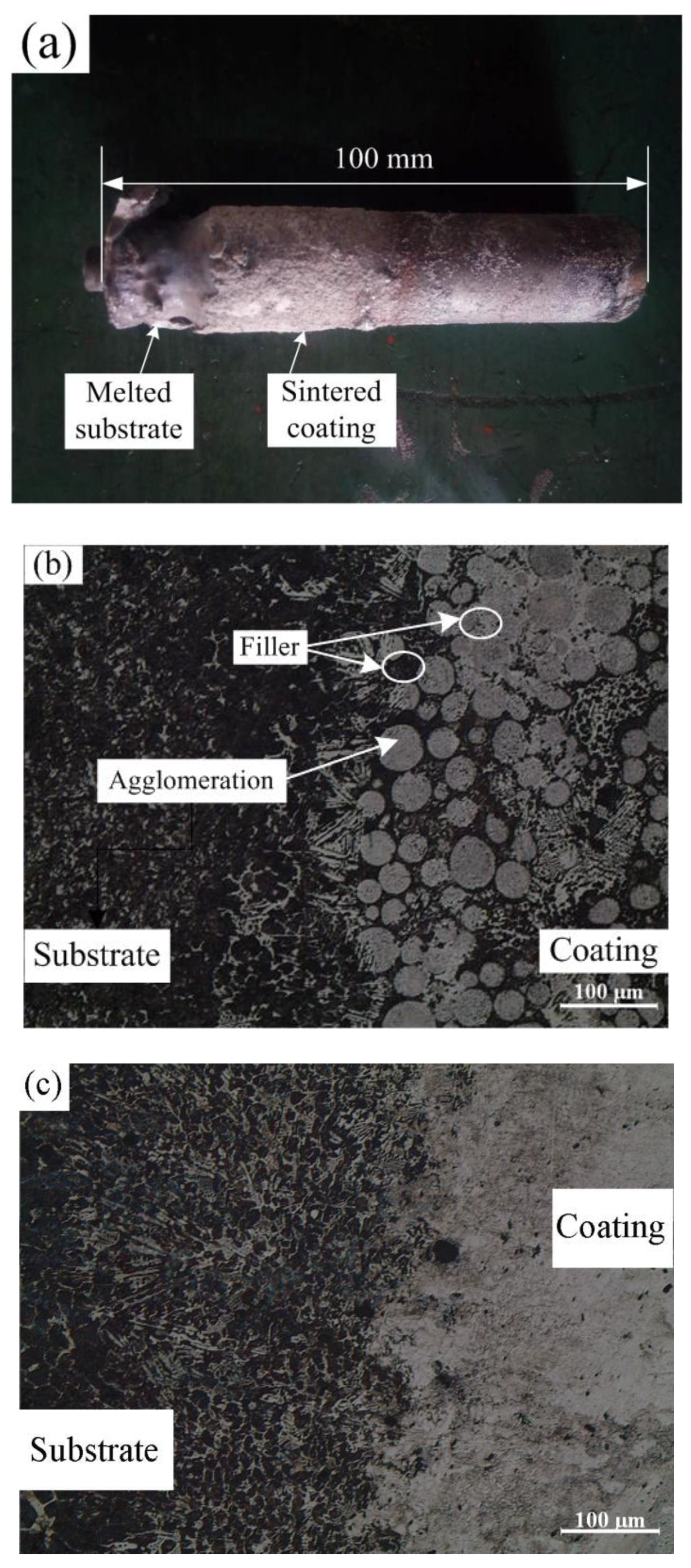
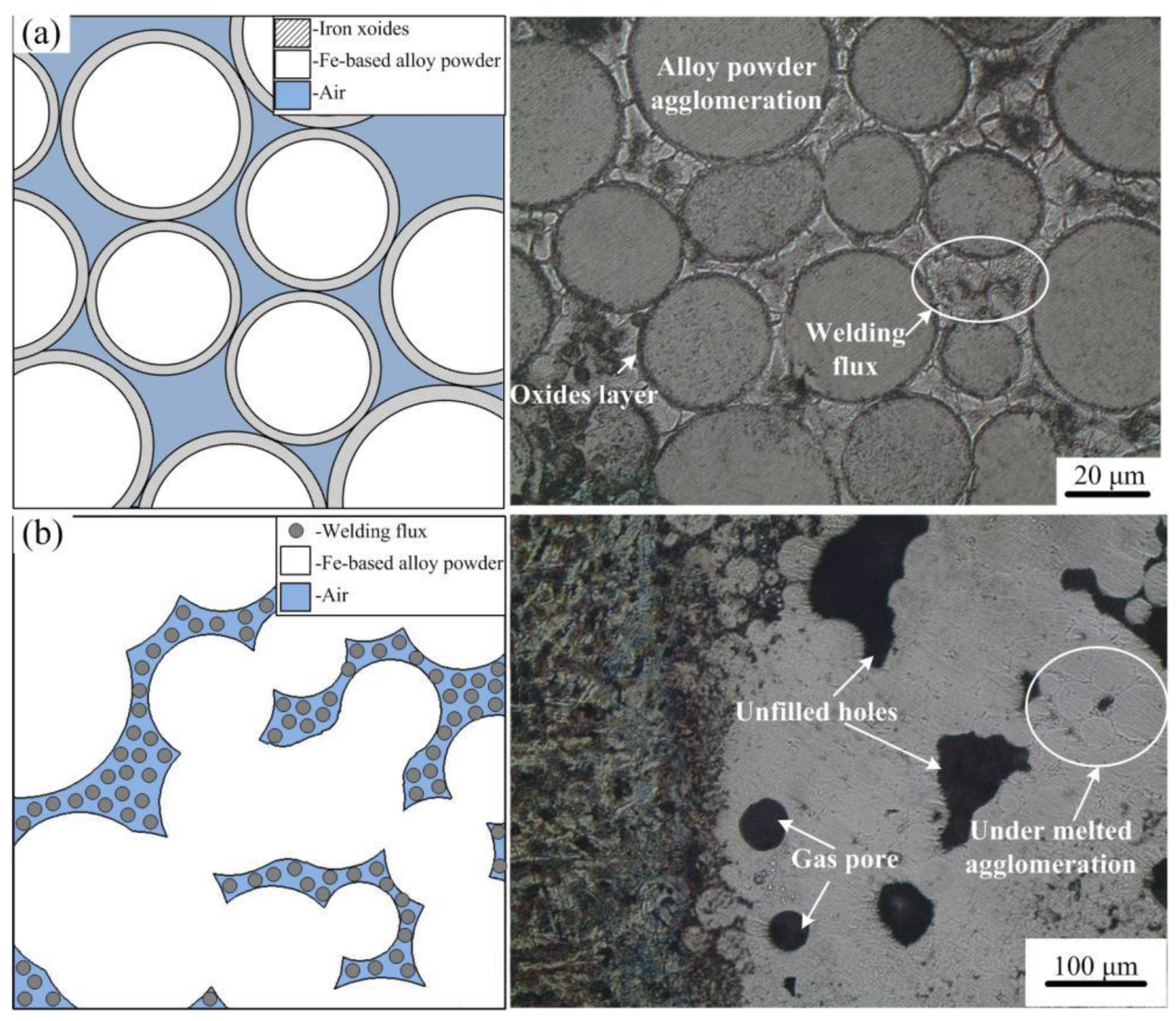
3.1. Forming Mechanism of Fe-Based Coating
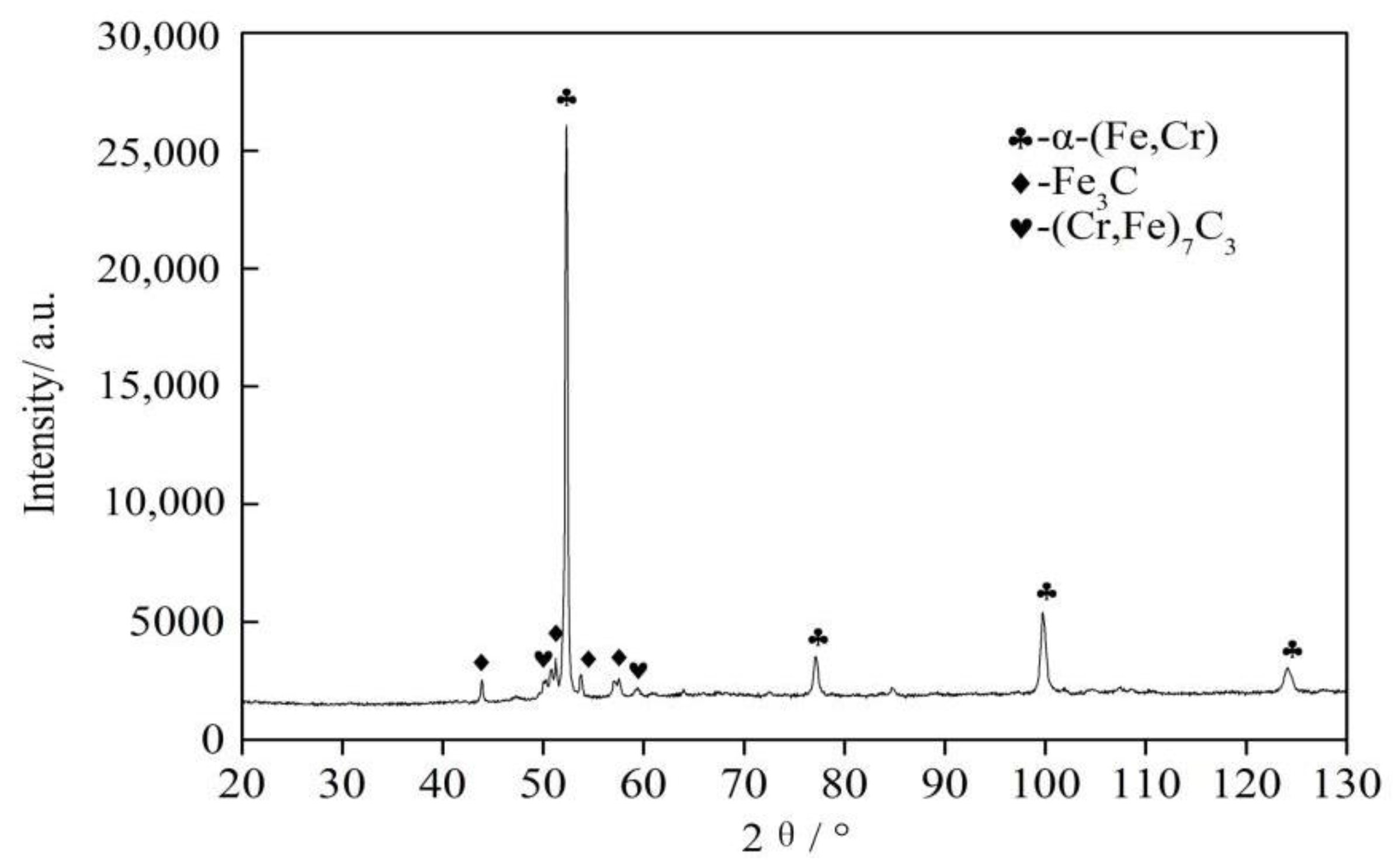
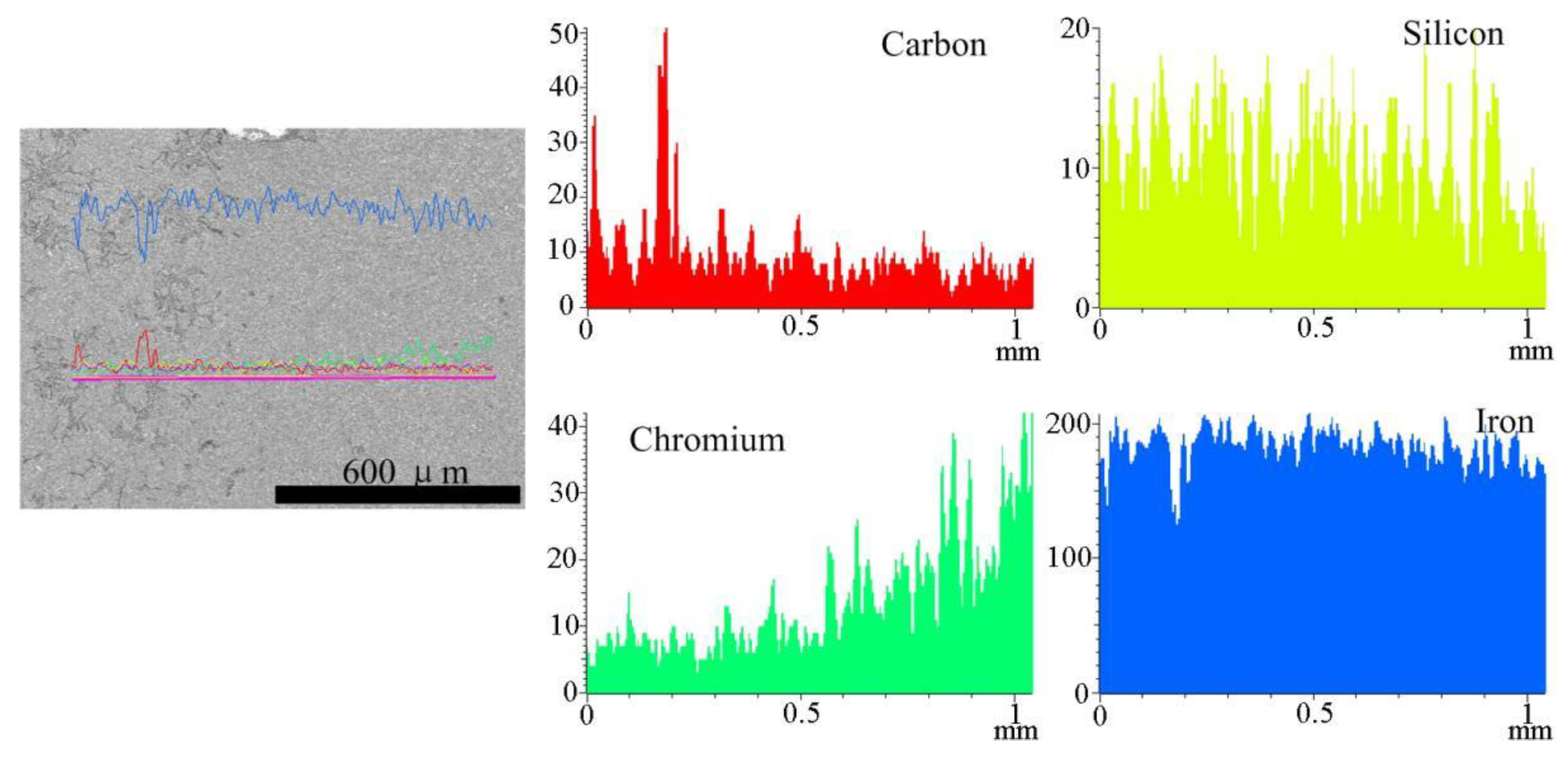
3.2. Microstructure and Composition of the Coating
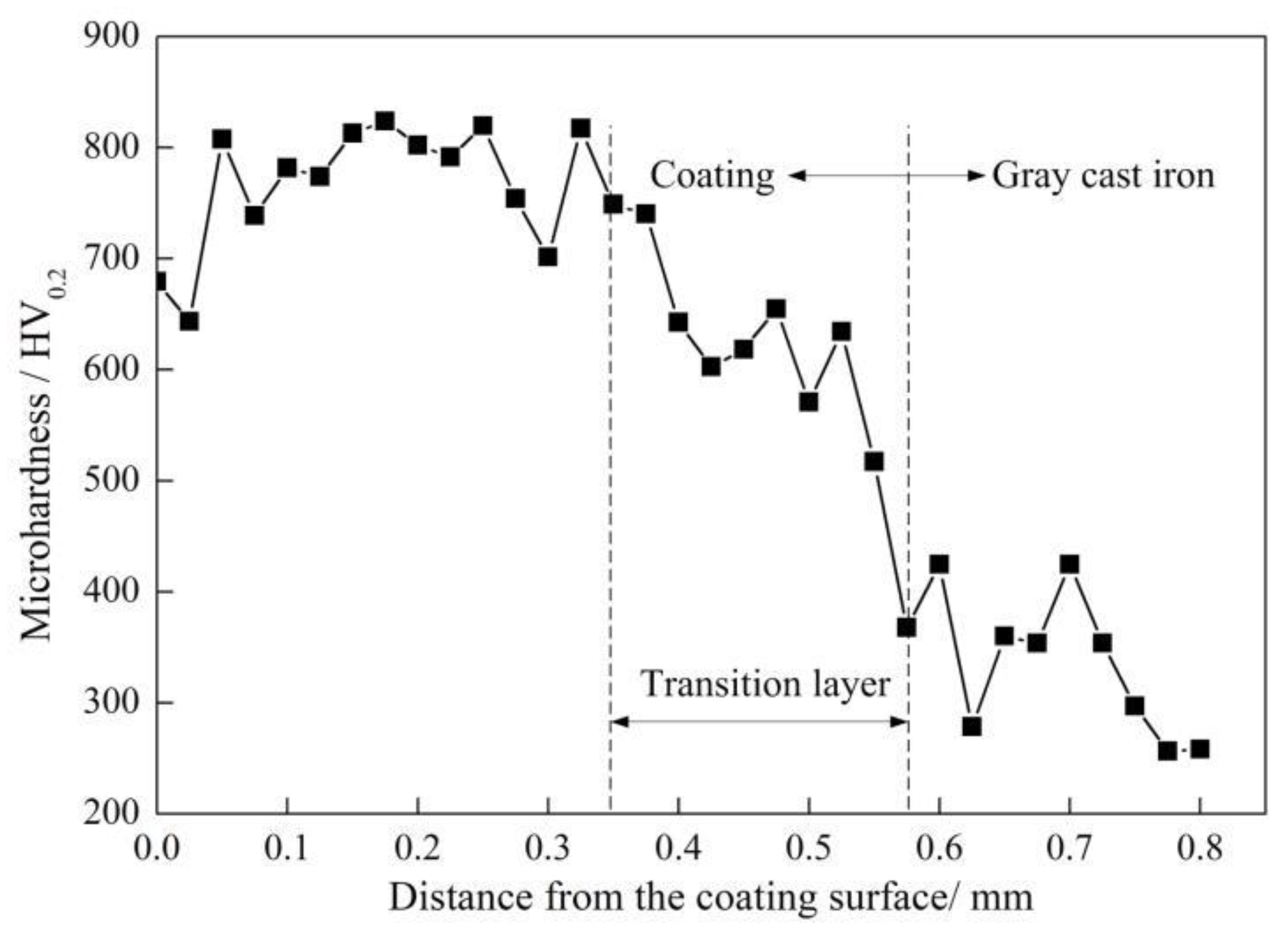
3.3. Microhardness
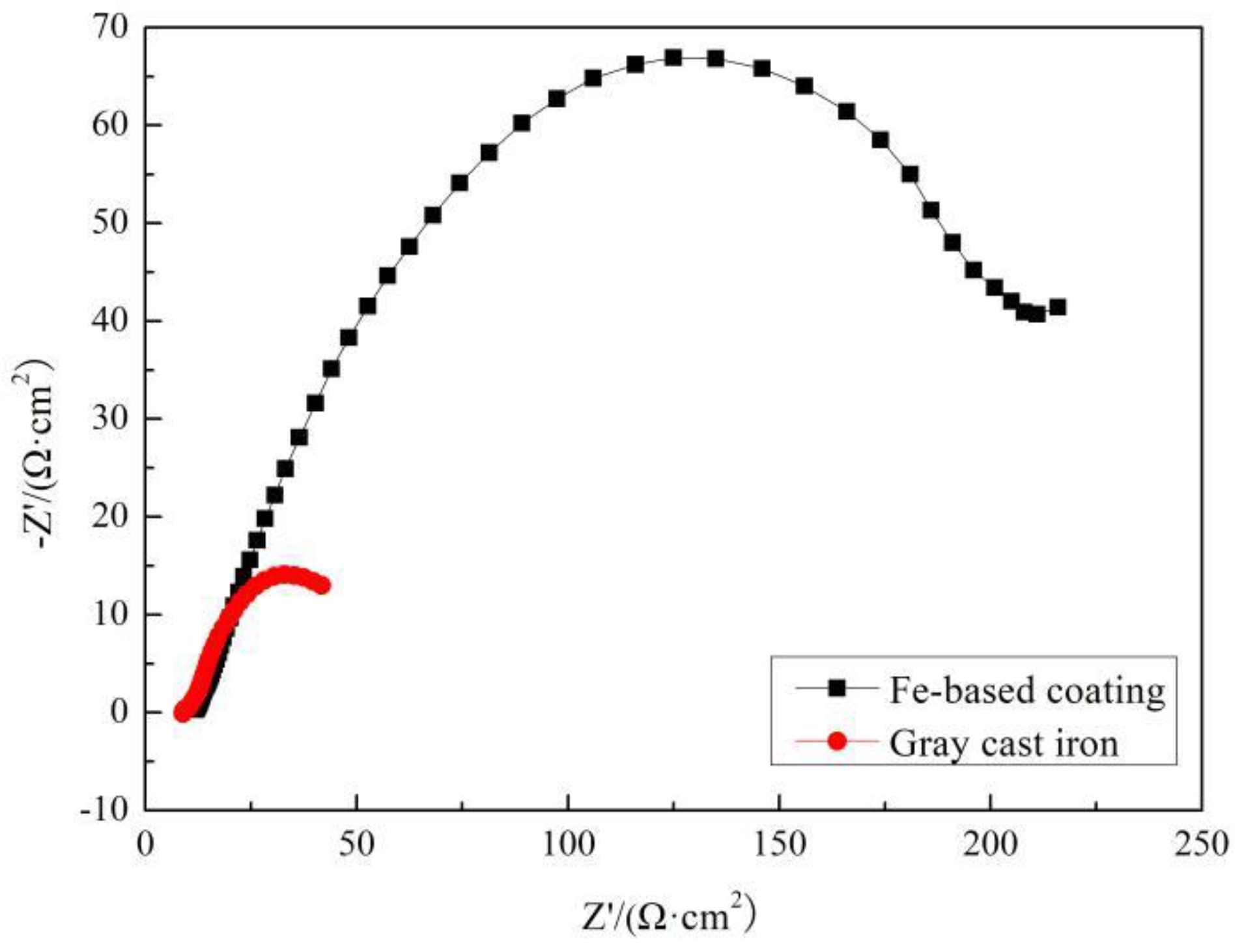
3.4. Electrochemical Corrosion Behavior in 3.5% NaCl
3.5. Friction and Wear Properties
4. Conclusions
- a.
- Because the Fe-based alloy powder is easily oxidized during the induction cladding, the iron oxides cover outside the Fe-based alloy powder, consequently increasing the melting temperature and further hindering the heating process. The additive of welding flux can react with the iron oxides and the reaction products can fill the gaps between the alloy particles, therefore increasing the heat transfer. In addition, prolonging the heating time promotes the complete reaction of welding flux so as to reduce the gas pore in the coating. Therefore, the additive of welding flux and prolonged heating time is a feasible approach to fabricate good-quality Fe-based alloy coating onto gray cast iron.
- b.
- The elemental distribution at the bonding interface shows that the coating is metallurgically bonded with the substrate. The XRD diffraction pattern and SEM images reveal that the phase composition of the coating consists of α-(Fe, Cr), (Fe, Cr)7C3, and Fe3C. Due to the uneven distribution of the hard phase in the coating, the hardness curve presents fluctuation and its mean value is approximately four times higher than that of substrate.
- c.
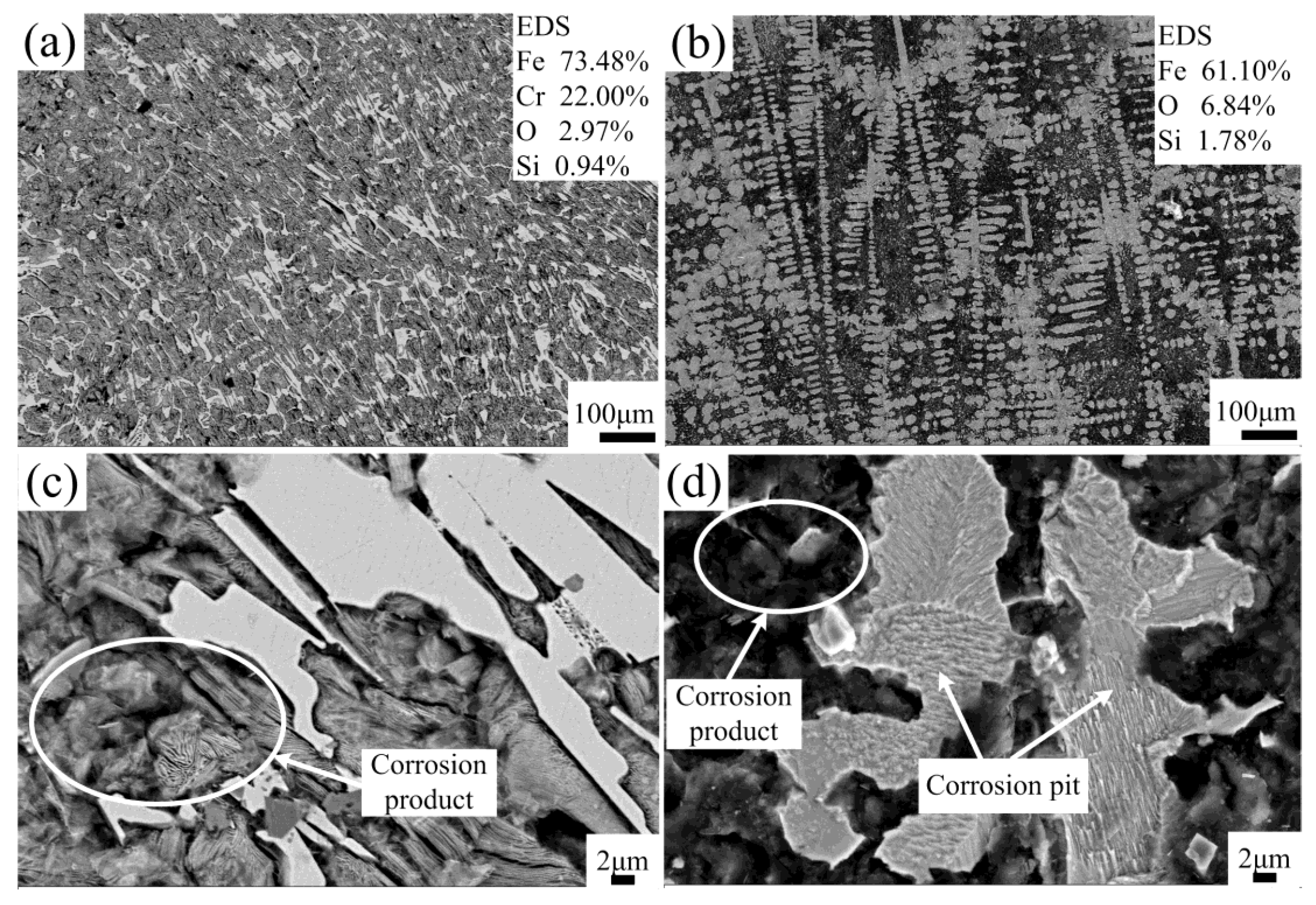
- The results of potentiodynamic polarization and ac impedance testings in 3.5% NaCl solution indicate that the Fe-based coating possesses better anti-corrosion properties than gray cast iron, and the corrosion products continuously and firmly attach on the coating surface, which is beneficial for preventing the electrochemical corrosion.
- d.
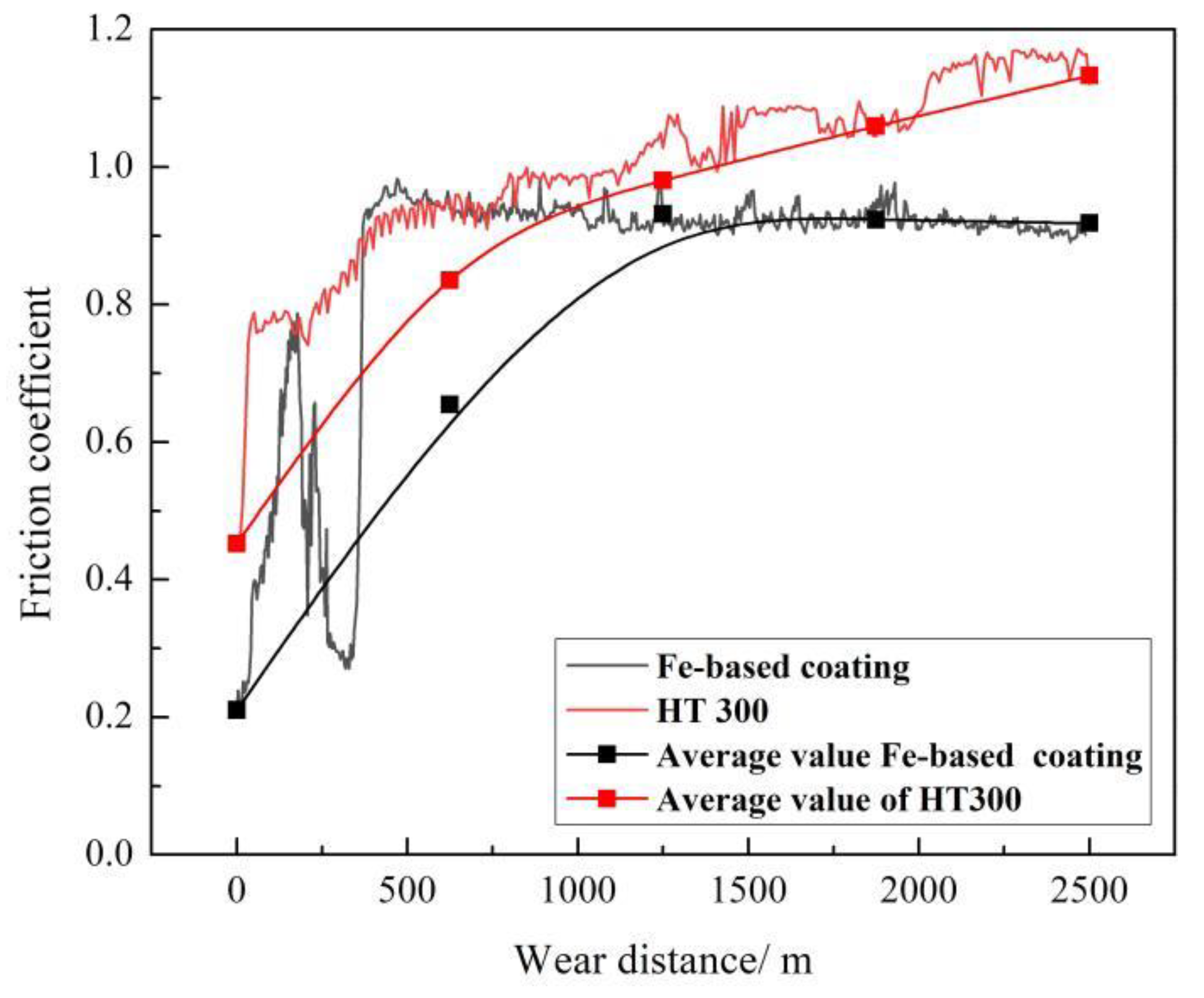
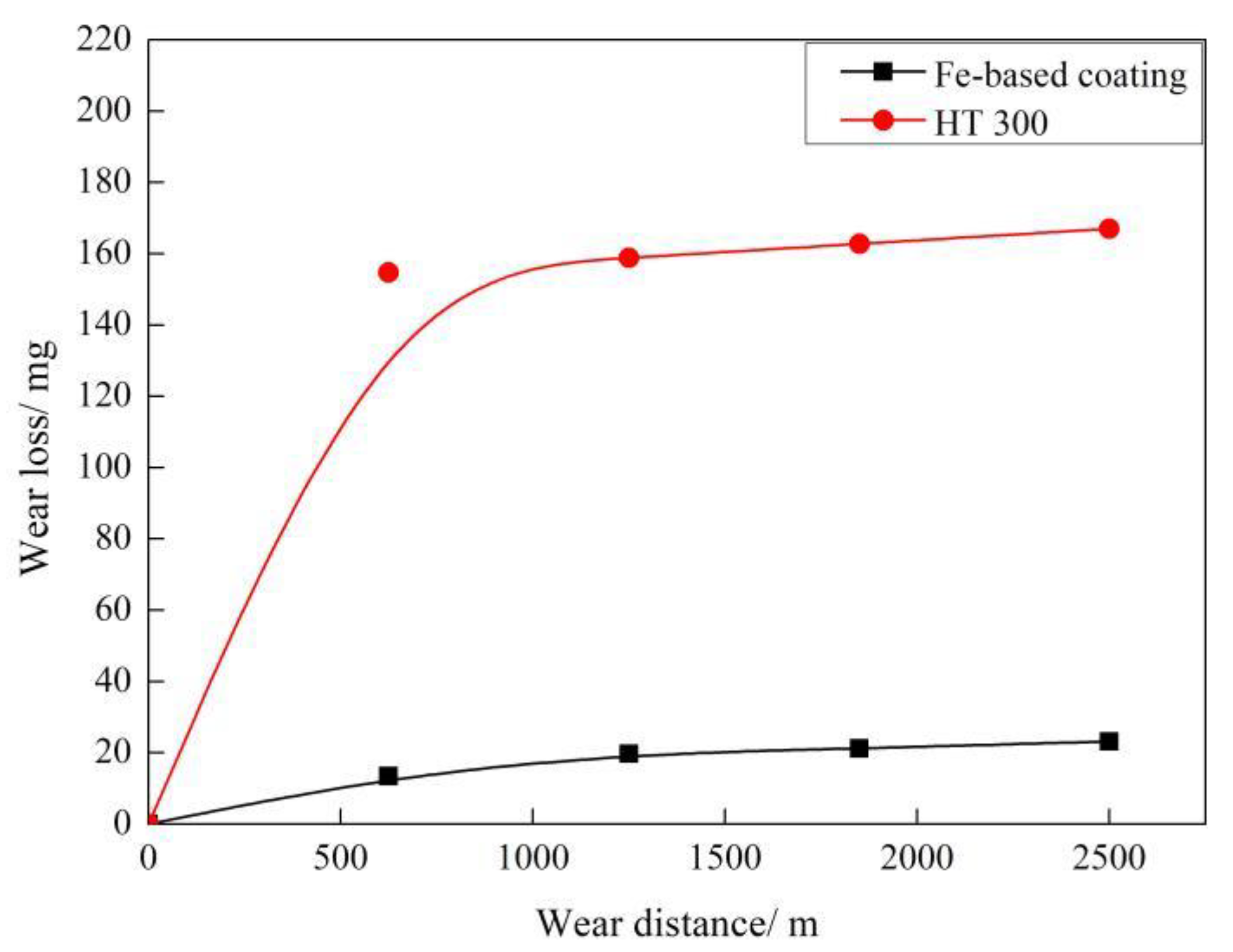
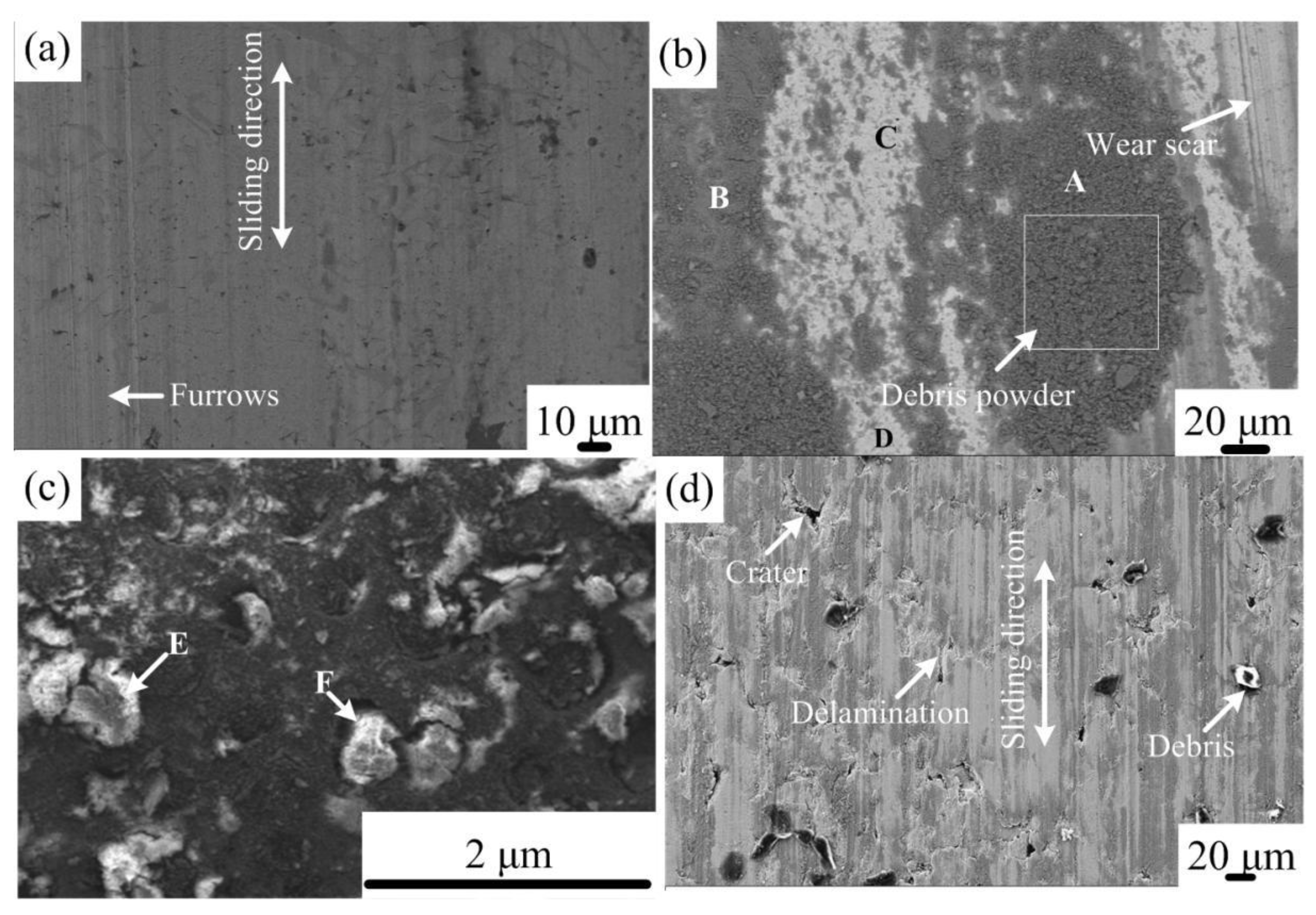
- Friction coefficient of the coating violently fluctuates before the sliding distance of 625 m and then gradually tends to be stable at roughly 0.9, always lower than that of the gray cast iron. Wear loss of the coating increases with the sliding distance and is approximately seven times lower than that of the substrate. The wear mechanism of the coating is changed mainly from light abrasive wear to moderate abrasive and oxidation wear.
Author Contributions
Funding
Conflicts of Interest
References
- Guo, Z.W.; Yuan, C.Q.; Liu, P.; Yan, Y.P. Effect of cylinder surface treatment on diesel engine performance. Adv. Mater. Res. 2011, 199–200, 689–695. [Google Scholar] [CrossRef]
- Fu, P.; Zhao, C.; Tian, H. Experimental research and performance testing of Fe-based composite plating. Adv. Mater. Res. 2011, 221, 364–368. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, S.K.; Agarwal, A.K. Wear evaluation of engine piston rings coated with dual layer hard and soft coatings. J. Tribol. 2018, 141, 031301. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, C.; Zhang, G.; Liao, H. Wear and corrosion resistant performance of thermal-sprayed Fe-based amorphous coatings: A review. Surf. Coat. Technol. 2019, 377, 124896. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, P.; Yan, H.; Yu, Z.; Wu, D.; Shi, H.; Li, S.; Tian, Y. Magnetic-field-assisted laser cladding in the preparation of a crack-free Fe-Cr-Mo-C-Y-B amorphous coating on steel. Philos. Mag. Lett. 2020. [Google Scholar] [CrossRef]
- Bing, Z.; Shi, Y.; Sun, R.; Guo, Y. Study on microstructure and properties of 304/316L coating prepared by ultra-high frequency induction cladding with wire feeding. Surf. Coat. Technol. 2020, 387, 1–13. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, W.; Wang, H.; Ji, X.; Song, Z.; Li, X.; Xu, B. In-situ synthesis of TiC/Ti composite coating by high frequency induction cladding. J. Alloys Compd. 2017, 701, 244–255. [Google Scholar] [CrossRef]
- Sun, G.; Zhou, R.; Li, P.; Feng, A.; Zhang, Y. Laser surface alloying of C-B-W-Cr powders on nodular cast iron rolls. Surf. Coat. Technol. 2011, 205, 2747–2754. [Google Scholar] [CrossRef]
- dos Santos Filho, D.; Tschipstschin, A.P.; Goldenstein, H. Effects of ethanol content on cast iron cylinder wear in a flex-fuel internal combustion engine-A cast study. Wear 2018, 406–407, 105–117. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Tian, X.; Yang, H.; Hao, J. Microstructural evolution and corrosion properties of Ni-based alloy coatings fabricated by multi-layer laser cladding on cast iron. J. Alloys Compd. 2020, 822, 153708. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; He, P.; Yan, S.; Li, E.; Liu, X.; Xu, B. Microstructure characteristics and mechanical properties of new-type FeNiCr laser cladding alloy coating on nodular cast iron. J. Mater. Process. Technol. 2019, 269, 163–171. [Google Scholar] [CrossRef]
- Jing, Y.; Song, B. Friction and wear behavior of a Ni-based alloy coating fabricated using a multistep induction cladding technique. Results Phys. 2018, 11, 105–111. [Google Scholar]
- Jing, Y.; Song, B. Effects of heating time on the microstructure and properties of an induction cladding coating. Results Phys. 2018, 11, 212–218. [Google Scholar]
- An, Y.-J.; Zhu, L.; Li, W.-Q.; Lu, J.-J.; Liu, X.-Y. Study of fly ash-based flux effects on microstructure and wear of TIG-cladded Ni-based composite coating. Results Phys. 2019, 12, 970–974. [Google Scholar] [CrossRef]
- Lu, X.-L.; Liu, X.-B.; Yu, P.-C.; Zhai, Y.-J.; Qiao, S.-J.; Wang, M.-D.; Wang, Y.-G.; Chen, Y. Effects of heat treatment on micostructure and mechanical properties of Ni60/h-BN self-lubricating anti-wear composite coatings on 304 stainless steel by laser. Appl. Surf. Sci. 2015, 355, 350–358. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Z. Microstructure and wear performance of high volume fraction carbide M7C3 reinforced Fe-based composite coating fabricated by plasma transferred arc welding. J. Wuhan Univ. Technol. Sci. Ed. 2014, 29, 1028–1035. [Google Scholar] [CrossRef]
- Luo, K.; Xu, X.; Zhao, Z.; Zhao, S.; Cheng, Z.; Lu, J. Microstructural evolution and characteristics of bonding zone in multilayer laser cladding of Fe-based coating. J. Mater. Process. Technol. 2019, 263, 50–58. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Yan, S.; Liu, X.; He, P.; Xu, B. Surface remanufacturing of ductile cast iron by laser cladding Ni-Cu alloy coatings. Surf. Coat. Technol. 2018, 347, 20–28. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Yan, S.; Liu, X.; He, P.; Xu, B. Microstructure evolution during laser cladding Fe-Cr alloy coatings on ductile cast iron. Opt. Laser Technol. 2018, 108, 255–264. [Google Scholar] [CrossRef]
- Hu, G.; Meng, H.; Liu, J. Microstructure and elevated temperature wear behavior of induction melted Fe-based composite coating. Appl. Surf. Sci. 2014, 317, 378–384. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Jiang, C.-P.; Zhang, F.-Y.; Xing, Y.-Z. The evaluation of microstructure characteristic and corrosion performance of laser-re-melted Fe-based amorphous coating deposited via plasma spraying. Mater. Express 2019, 9, 1100–1105. [Google Scholar] [CrossRef]
- Hu, G.; Meng, H.; Liu, J. Microstructure and corrosion resistance of induction melted Fe-based alloy coating. Surf. Coat. Technol. 2014, 251, 300–306. [Google Scholar] [CrossRef]
- Hu, G.; Meng, H.; Liu, J. Friction and sliding wear behavior of induction melted FeCrB metamorphic alloy coating. Appl. Surf. Sci. 2014, 308, 363–371. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Zhang, Y.; Liu, H.; Ren, H.; Zhong, Y.; Huang, X.; Huang, W. Influence of Sn and Nb additions on the microstructure and wear characteristics of a gray cast iron. Appl. Phys. A 2020, 126, 1–8. [Google Scholar] [CrossRef]




| Item | C | Si | Mn | S | P | Cr | Fe |
|---|---|---|---|---|---|---|---|
| HT 300 | 2.9–3.2 | 1.0–2.5 | 0.5–1.4 | ≤0.5–1.0 | ≤0.5–1.0 | – | Bal. |
| Fe 30 | 0.9–1.1 | 3.0–4.0 | – | – | – | 18–20 | Bal. |
| Sample | Preheating | Heating | Heat Preservation | |||
|---|---|---|---|---|---|---|
| Current/A | Time/s | Current/A | Time/s | Current/A | Time/s | |
| Fe-1 | 10 | 90 | 20 | 11 | – | – |
| Fe-2 | 10 | 90 | 20 | 11 | – | – |
| Fe-3 | 10 | 90 | 20 | 11 | 10 | 30 |
| Sample | Ecorr (V) | Icorr (µA/cm−2) | Rp (Ω·cm2) | Corr Rate (mm/a) |
|---|---|---|---|---|
| Coating | −1.120 | 110.94 | 376.2 | 0.836 |
| Substrate | −1.037 | 269.00 | 106.1 | 2.251 |
| Sample | Rsol (Ω·cm2) | Qdl (F·cm−2·sn) | n1 | Rt (Ω·cm2) | Qa (F·cm−2·sn) | n2 | Ra (Ω·cm2) |
|---|---|---|---|---|---|---|---|
| Coating | 11.84 | 0.004 | 0.57 | 15.03 | 0.0008344 | 0.819 | 223.8 |
| Substrate | 9.016 | 0.022 | 0.55 | 3.22 | 0.00804 | 0.752 | 46.17 |
| Testing Point | O | Si | Cr | Fe |
|---|---|---|---|---|
| A | 23.71 | 2.29 | 3.66 | 69.72 |
| B | 12.14 | 3.22 | 1.23 | 74.34 |
| C | – | 3.39 | 1.33 | 95.29 |
| D | – | 3.15 | 1.02 | 95.83 |
| E (wear debris) | 30.68 | 1.37 | 8.33 | 59.62 |
| F (wear debris) | 34.93 | 1.65 | 8.43 | 53.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Liu, Y.; Song, B.; Wang, J. Microstructure and Properties of Fe-Based Alloy Coating on Gray Cast Iron Fabricated Using Induction Cladding. Coatings 2020, 10, 801. https://doi.org/10.3390/coatings10090801
Yu J, Liu Y, Song B, Wang J. Microstructure and Properties of Fe-Based Alloy Coating on Gray Cast Iron Fabricated Using Induction Cladding. Coatings. 2020; 10(9):801. https://doi.org/10.3390/coatings10090801
Chicago/Turabian StyleYu, Jing, Yanchuan Liu, Bo Song, and Jinlong Wang. 2020. "Microstructure and Properties of Fe-Based Alloy Coating on Gray Cast Iron Fabricated Using Induction Cladding" Coatings 10, no. 9: 801. https://doi.org/10.3390/coatings10090801
APA StyleYu, J., Liu, Y., Song, B., & Wang, J. (2020). Microstructure and Properties of Fe-Based Alloy Coating on Gray Cast Iron Fabricated Using Induction Cladding. Coatings, 10(9), 801. https://doi.org/10.3390/coatings10090801





