Effect on Silt Capillary Water Absorption upon Addition of Sodium Methyl Silicate (SMS) and Microscopic Mechanism Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Capillary Water Rise Testing
2.3. Contact Angle Measurement
2.4. X-ray Diffraction (XRD) and X-ray Fluorescence (XRF) Testing
2.5. Scanning Electron Microscopy (SEM) Testing
2.6. Mercury Intrusion Porosimetry (MIP) Testing
3. Results and Discussion
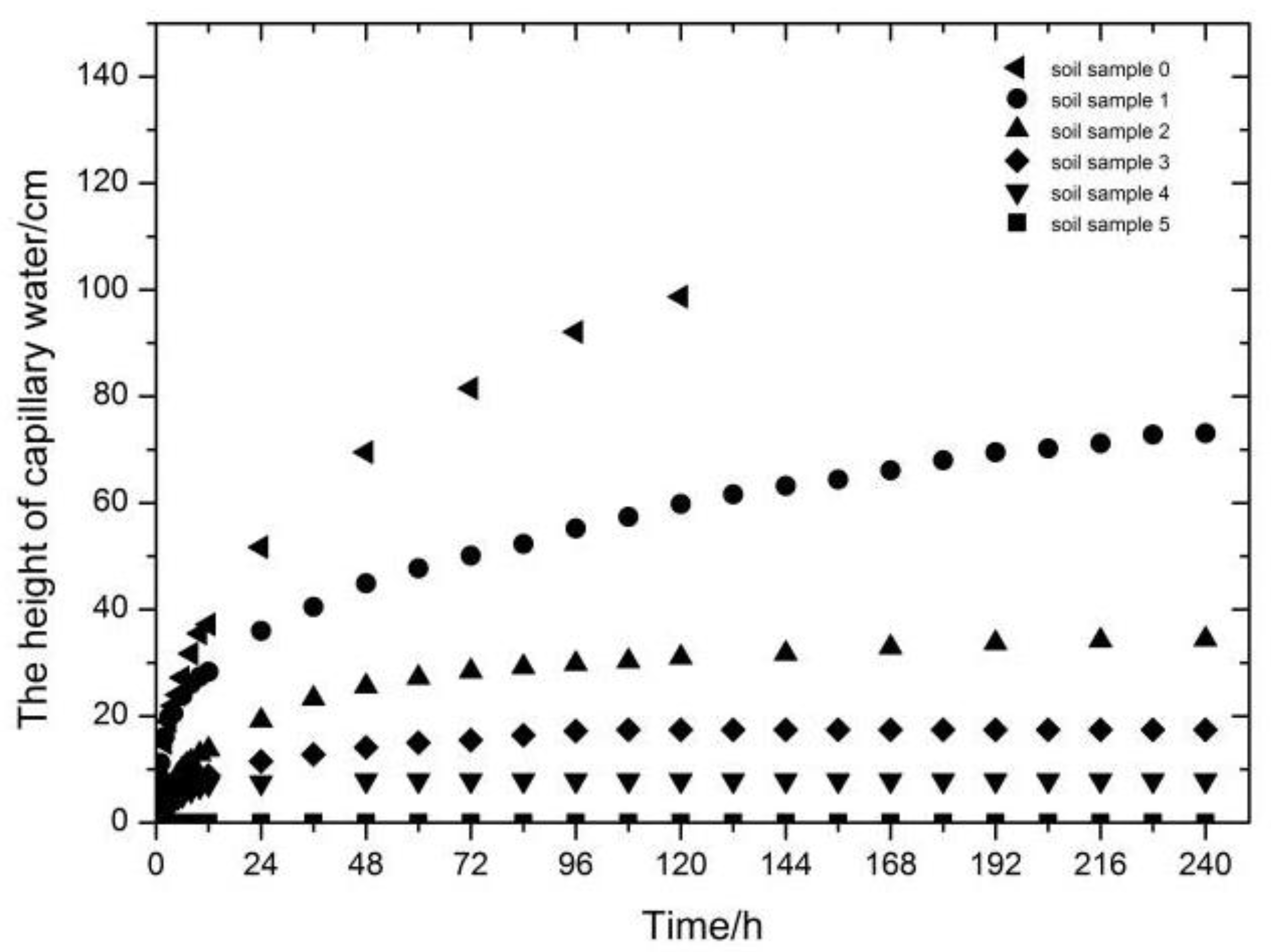
3.1. Observations on the Speed of Soil Capillary Water Absorption
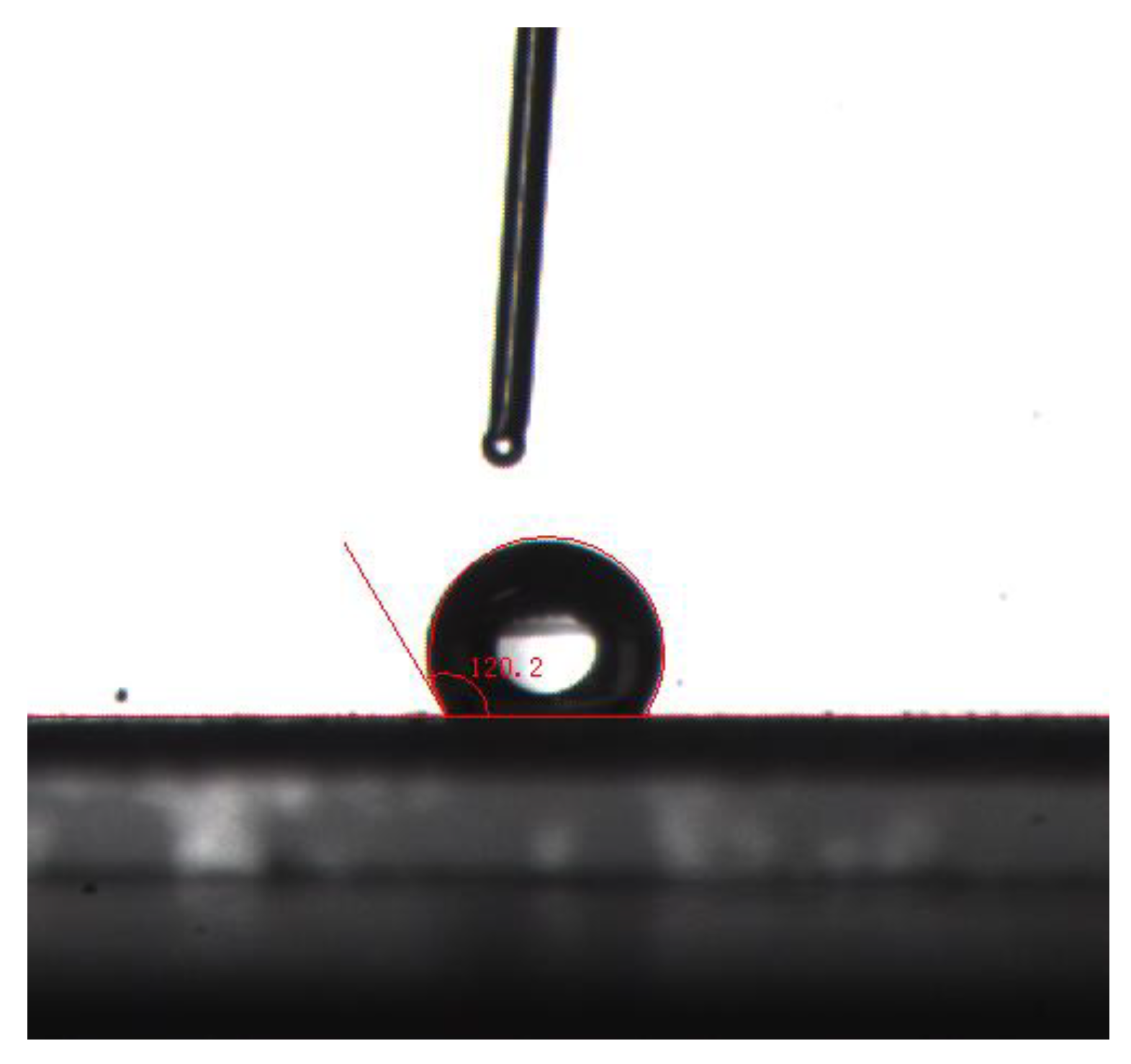
3.2. Contact Angle Measurement and Analysis
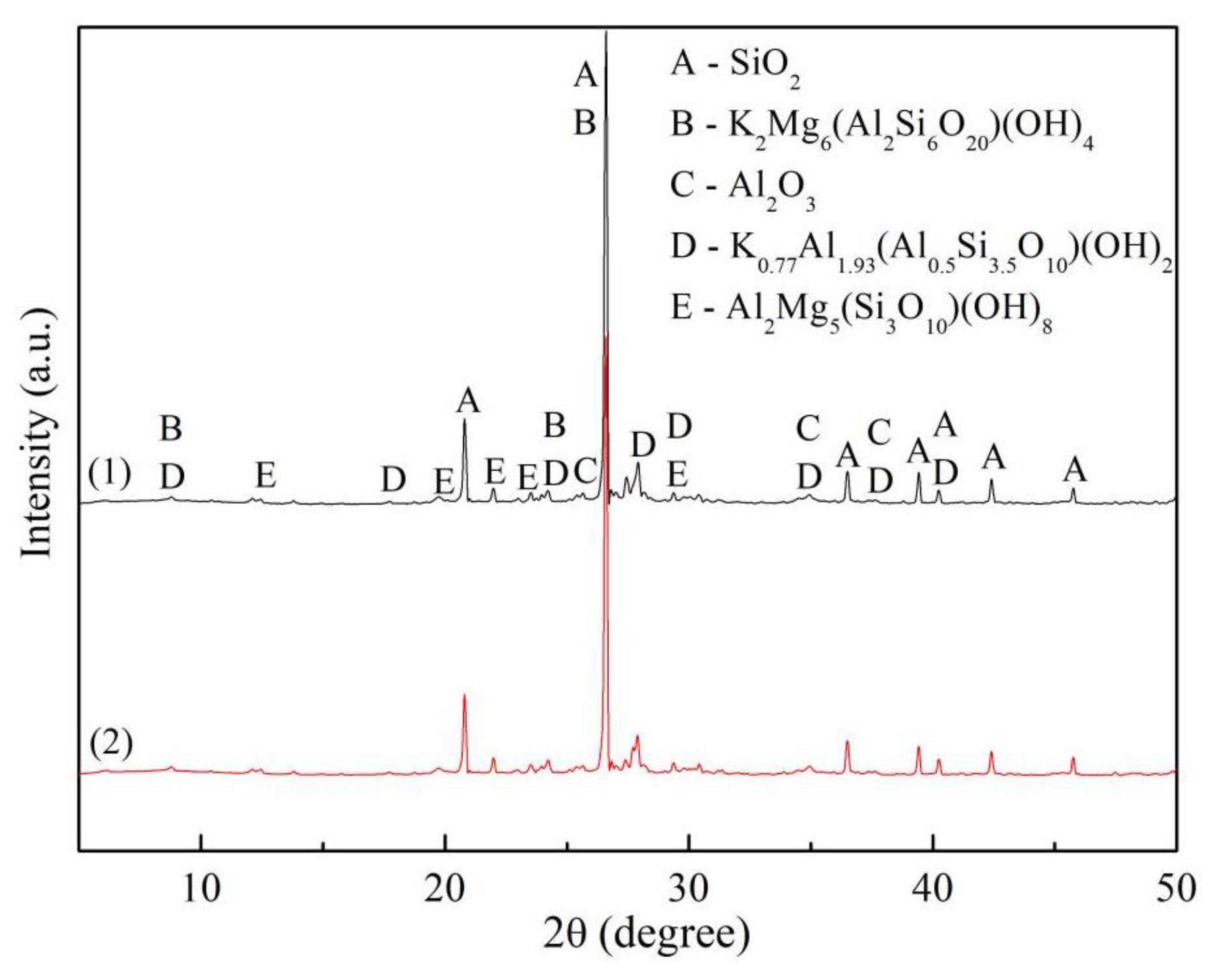
3.3. XRD/XRF Test Results and Analysis
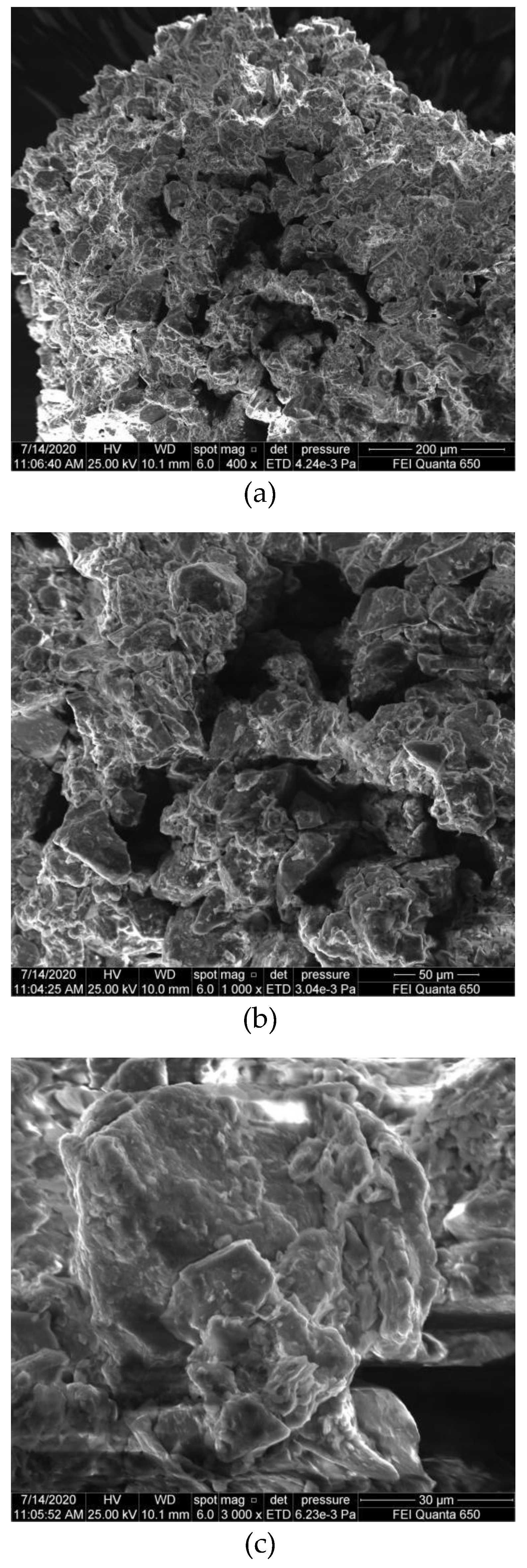
3.4. SEM Testing Results and Analysis
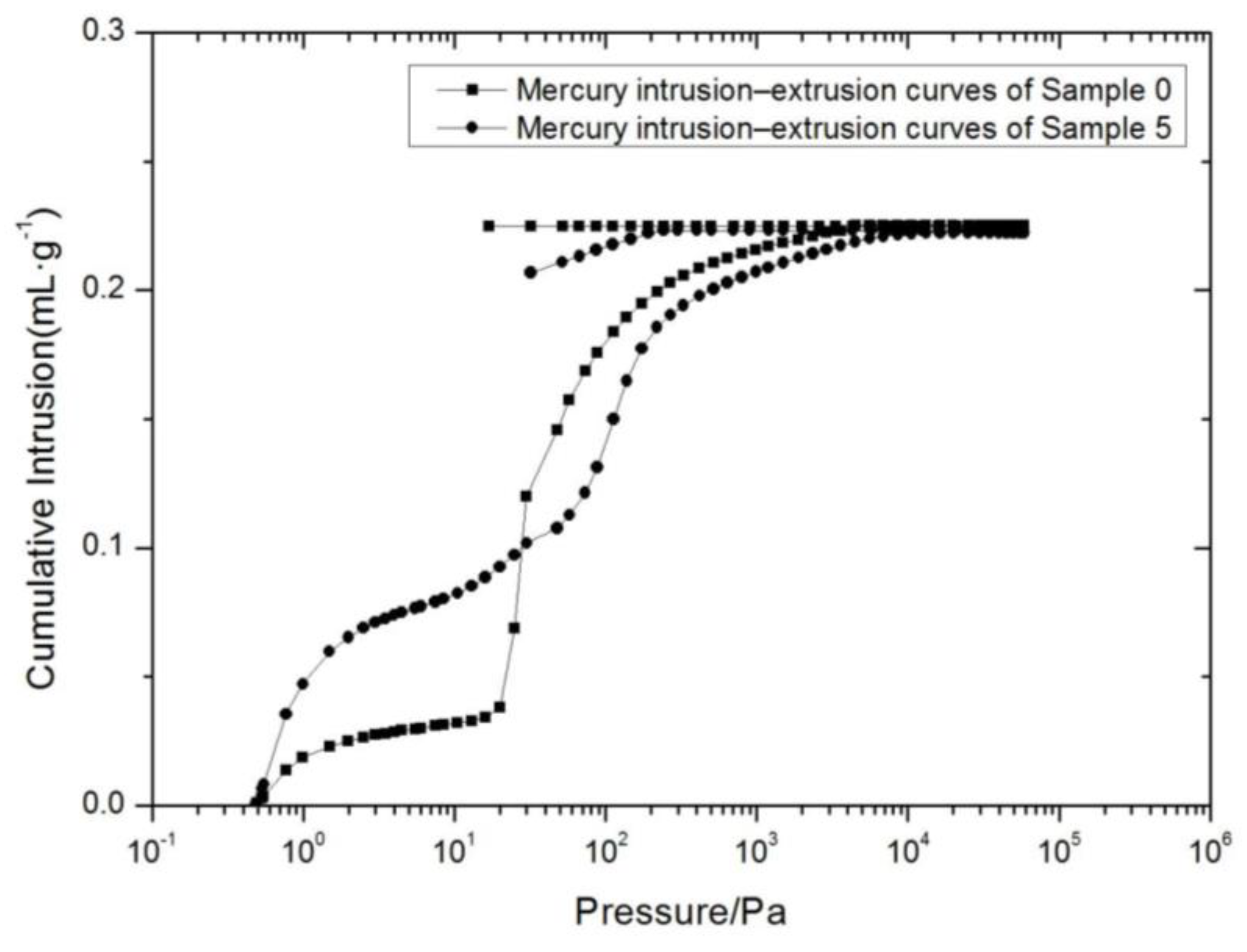
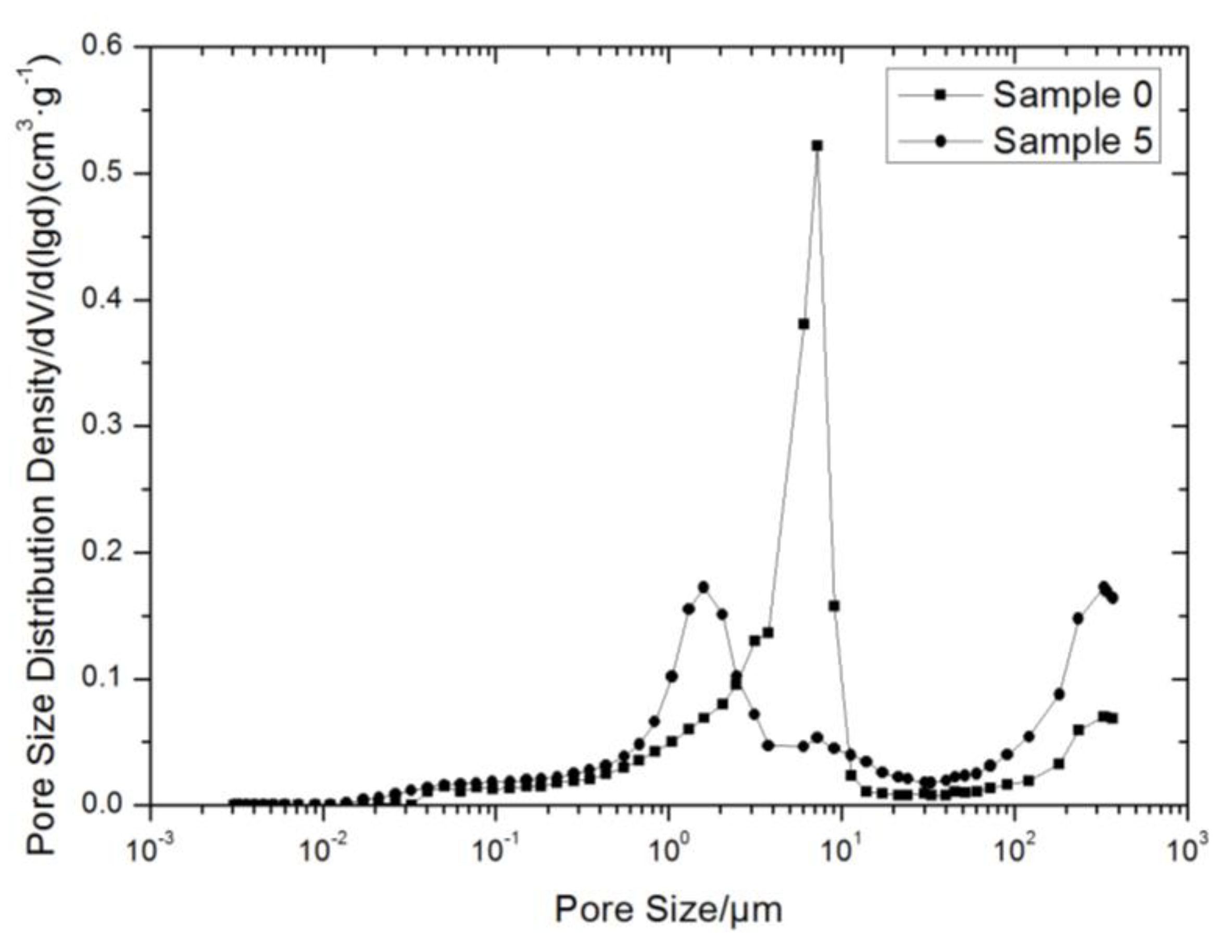
3.5. MIP Testing Results and Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.H. Study on the Water Movement of Silt and the Seepage Characteristics of the City Wall Considering Clay content. Master’s Thesis, Zhongyuan Institute of Technology, Henan, China, April 2019. [Google Scholar]
- Li, L. Study on the Freeze-Thaw Cycle Effect and Micromechanism of the Strength and Disintegration of the Silt at the Site. Master’s Thesis, Zhongyuan Institute of Technology, Henan, China, April 2019. [Google Scholar]
- Yan, C.G.; Wan, Q.; Xu, Y.; Xie, Y.; Yin, P. Experimental study of barrier effect on moisture movement and mechanical behaviors of loess soil. Eng. Geol. 2018, 240, 1–9. [Google Scholar] [CrossRef]
- He, F.; Pan, Y.; Tan, L.; Zhang, Z.; Li, P.; Liu, J.; Song, X. Study of the water transportation characteristics of marsh saline soil in the Yellow River Delta. Sci. Total Environ. 2017, 574, 716–723. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhang, X.F.; Leng, Y.F. The research on stable rising height and harmful rising height of capillary water. In Proceedings of the 2011 International Conference on Transportation and Mechanical & Electrical Engineering (TMEE), Changchun, China, 16 December 2011; pp. 2190–2197. [Google Scholar]
- Dong, B.; Zhang, X.F.; Li, X.; Zhang, D.Q. Comprehensive test research of capillary water rising height. Chin. J. Geotech. Eng. 2008, 10, 1569–1574. [Google Scholar]
- Spennemann, D.H.R. The creeping disaster: Dryland and urban salinity and its impact on heritage. Cult. Resour. Prot. Emerg. Prep. 2001, 24, 22–26. [Google Scholar]
- Ren, K.B.; Wang, B.; Li, X.M.; Yin, S. Strength characteristics and pore distribution characteristics of soil ruins under the action of capillary water dry-wet cycle. Rock Soil Mech. 2019, 40, 962–970. [Google Scholar]
- Zhu, D.Y.; Guan, Y.H. The effect of capillary water on the stability of silt subgrade. J. Shandong Univ. 2012, 42, 9398. [Google Scholar]
- Walker, P. Terra 2003. In Proceedings of the 9th International Conference on the Study and Conservation of Earthen Architecture, Terra, Iran, 29 November 2003; GCI: Los Angeles, CA, USA, 2003. [Google Scholar]
- Rainer, L.H. Water, wind, salt, biological, environmental, dete rioration/pathology. In Terra Literature Review; GCI: Los Angeles, CA, USA, 2003. [Google Scholar]
- Cui, C. Disease Analysis of a City Wall Soil Site and the Stability of Modified Loess after Restoration. Master’s Thesis, Xi’an University of Technology, Shanxi, China, June 2019. [Google Scholar]
- Zhang, H.Y.; Zhu, S.B.; Li, M.; Zhang, X.C. Water repellency of monument soil treated by tung oil. Geotech. Geol. Eng. 2016, 34, 205–216. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, L.; Li, W.; Cao, R. Research on silty soil capillary water rising in yellow river flooded area of eastern henan. J. Highw. Transp. Res. Dev. 2016, 10, 40–46. [Google Scholar] [CrossRef]
- Ahmed, A. Recycled bassanite for enhancing the stability of poor subgrades clay soil in road construction projects. Constr. Build. Mater. 2013, 48, 151–159. [Google Scholar] [CrossRef]
- Ahmed, A.; Ugai, K.; Kamei, T. Investigation of recycled gypsum in conjunction with waste plastic trays for ground improvement. Constr. Build. Mater. 2011, 25, 208–217. [Google Scholar] [CrossRef]
- Shafran, A.W.; Gross, A.; Ronen, Z.; Weisbrod, N.; Adar, E. Effects of surfactants originating from reuse of greywater on capillary rise in the soil. Water Sci. Technol. 2005, 52, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.M.; Xu, H.Z.; Zhu, D.H.; Zhu, H. Experimental study on polymer-modified silt under different water environments. Chin. J. Geotech. Eng. 2013, 35, 1316–1322. [Google Scholar]
- Mobbs, T.L.; Peters, R.T.; Davenport, J.R.; Evans, M.A.; Wu, J.Q. Effects of four soil surfactants on four soil-water properties in sand and silt loam. J. Soil Water Conserv. 2012, 67, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Abu-Zreig, M.; Rudra, R.P.; Dickinson, W.T. Effect of application of surfactants on hydraulic properties of soils. Biosyst. Eng. 2003, 84, 363–372. [Google Scholar] [CrossRef]
- Kong, D.; Wan, R.; Chen, J.; Jing, Y.; Huang, W.; Wang, Y. The study on engineering characteristics and compression mechanisms of typical historical earthen site soil. Constr. Build. Mater. 2019, 213, 386–403. [Google Scholar] [CrossRef]
- Han, W.C.; Li, Y.; Tan, X.F.; Guo, M.Y.; Xu, H.W. Methyl silicate inhibits clay hydration and its mechanism. Prospect. Eng. (Rock & Soil Drill. Eng.) 2018, 45, 19–23. [Google Scholar]
- Jiang, G.C.; Wang, J.S.; Xuan, Y. Performance evaluation and action mechanism of potassium methyl silicate shale inhibitor. Sci. Technol. Eng. 2014, 14, 6–10. [Google Scholar]
- Yang, H.T.; Yu, J.J. Research on the influence of active components on the properties of concrete. New Build. Mater. 2019, 46, 131–133. [Google Scholar]
- Guo, Z.; Zhu, Q.; Liu, C.; Xing, Z. Preparation of Ca-Al-Fe deicing salt and modified with sodium methyl silicate for reducing the influence of concrete structure. Constr. Build. Mater. 2018, 172, 263–271. [Google Scholar] [CrossRef]
- Liu, G. Experimental Study on Adding Chloride Ion Transmission Blocking Materials to Cement Concrete. Master’s Thesis, Chang’an University, Shanxi, China, May 2017. [Google Scholar]
- Li, X.J. Microwave Hardened Water Glass Sand Composite Hardening Process and Anti-Hygroscopicity. Master’s Thesis, Huazhong University of Science and Technology, Hubei, China, January 2013. [Google Scholar]
- Wang, H.F. Key Technical Foundations of Microwave Hardened Water Glass Sand Application. Ph.D. Thesis, Huazhong University of Science and Technology, Hubei, China, January 2012. [Google Scholar]
- Yuan, Y.Q.; Jia, M.; Li, W. Experimental study of yellow silty soil stabilized by sodium methyl silicate. China Sci. Technol. Pap. 2017, 12, 831–844. [Google Scholar]
- JTGE40-2007. Highway Geotechnical Test Regulations [S]; People’s Communications Press: Beijing, China, 2007. [Google Scholar]
- Zhang, W.P.; Sun, Y.F.; Tong, W.W.; Song, Y.P.; Dong, L.F.; Liu, X.Y. An analytical method for studying the distribution characteristics of soil particles and pores based on sem images. Adv. Mar. Sci. 2018, 36, 605–613. [Google Scholar]
- Liu, Y.J.; Wu, J.S.H.; Xie, Z.H. Experimental study on microstructure characteristics of soft soil based on NMR and SEM. J. Guangdong Univ. Technol. 2018, 35, 49–56. [Google Scholar]
- Wu, S.; Yang, J.; Yang, R.; Zhu, J.; Liu, S.; Wang, C. Investigation of microscopic air void structure of anti-freezing asphalt pavement with X-ray CT and MIP. Constr. Build. Mater. 2018, 178, 473–483. [Google Scholar] [CrossRef]
- Lu, F.; Nie, J.T. Application research of sodium methyl silicate (organic silicone water repellent). Jiangxi Chem. Ind. 1995, 2, 31–34. [Google Scholar]
- Shear, D.L.; Olsen, H.W.; Nelson, K.R. Effects of desiccation on the hydraulic conductivity versus void ratio relationship for a natural clay. In Transportation Research Record; National Academy Press: Washington, DC, USA, 1993; pp. 1365–1370. [Google Scholar]

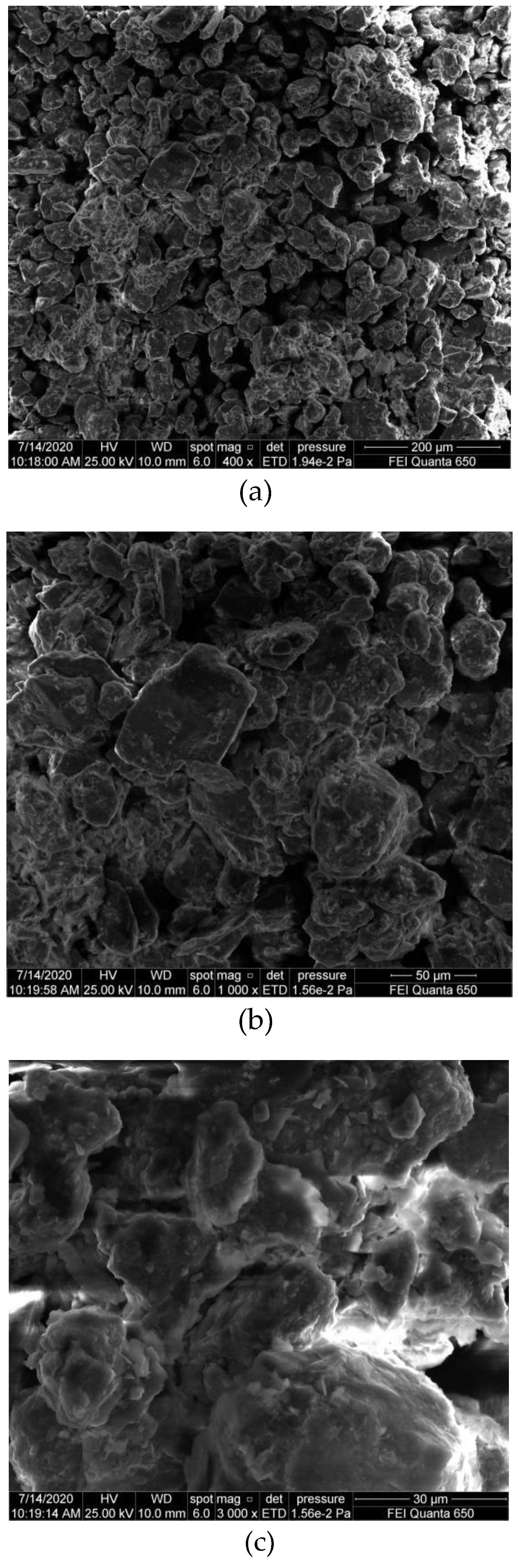
| Soil Type | Density/g·cm−3 | Initial Moisture Content/% | Porosity/% | Liquid Limit/% | Plastic Limit/% | Plasticity Index |
|---|---|---|---|---|---|---|
| Silt | 1.7 | 6.8 | 33.8 | 23.4 | 18.1 | 8.9 |
| Na2O | MgO | Al2O3 | SiO2 | Cl | K2O | CaO | TiO2 | MnO | Fe2O3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample 0 | 1.01 | 3.44 | 15.3 | 62.6 | - | 3.00 | 5.04 | 0.832 | 0.103 | 5.25 |
| Sample 5 | 2.37 | 3.40 | 15.0 | 65.7 | 0.123 | 2.81 | 4.65 | 0.769 | - | 4.72 |
| Total Intrusion Volume (mL/g) | Total Pore Area (m2/g) | Porosity (%) | |
|---|---|---|---|
| Soil sample 0 | 0.2253 | 0.878 | 33.8093 |
| Soil sample 5 | 0.2224 | 1.544 | 36.3578 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Liu, S. Effect on Silt Capillary Water Absorption upon Addition of Sodium Methyl Silicate (SMS) and Microscopic Mechanism Analysis. Coatings 2020, 10, 724. https://doi.org/10.3390/coatings10080724
Ma Q, Liu S. Effect on Silt Capillary Water Absorption upon Addition of Sodium Methyl Silicate (SMS) and Microscopic Mechanism Analysis. Coatings. 2020; 10(8):724. https://doi.org/10.3390/coatings10080724
Chicago/Turabian StyleMa, Qingwen, and Sihan Liu. 2020. "Effect on Silt Capillary Water Absorption upon Addition of Sodium Methyl Silicate (SMS) and Microscopic Mechanism Analysis" Coatings 10, no. 8: 724. https://doi.org/10.3390/coatings10080724
APA StyleMa, Q., & Liu, S. (2020). Effect on Silt Capillary Water Absorption upon Addition of Sodium Methyl Silicate (SMS) and Microscopic Mechanism Analysis. Coatings, 10(8), 724. https://doi.org/10.3390/coatings10080724




