Zn-Al Layered Double Hydroxide Thin Film Fabricated by the Sputtering Method and Aqueous Solution Treatment
Abstract
1. Introduction
2. Materials and Methods
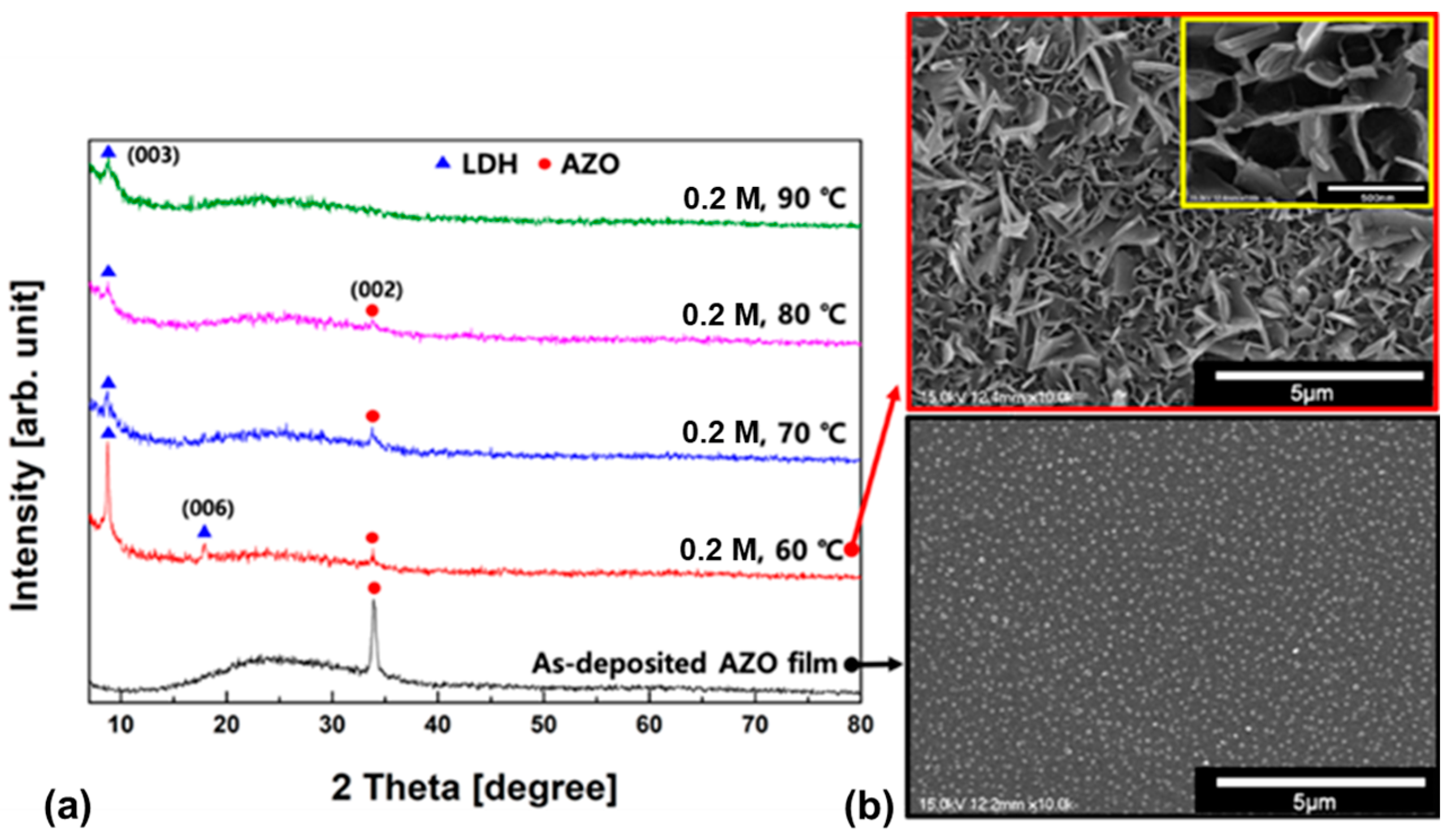
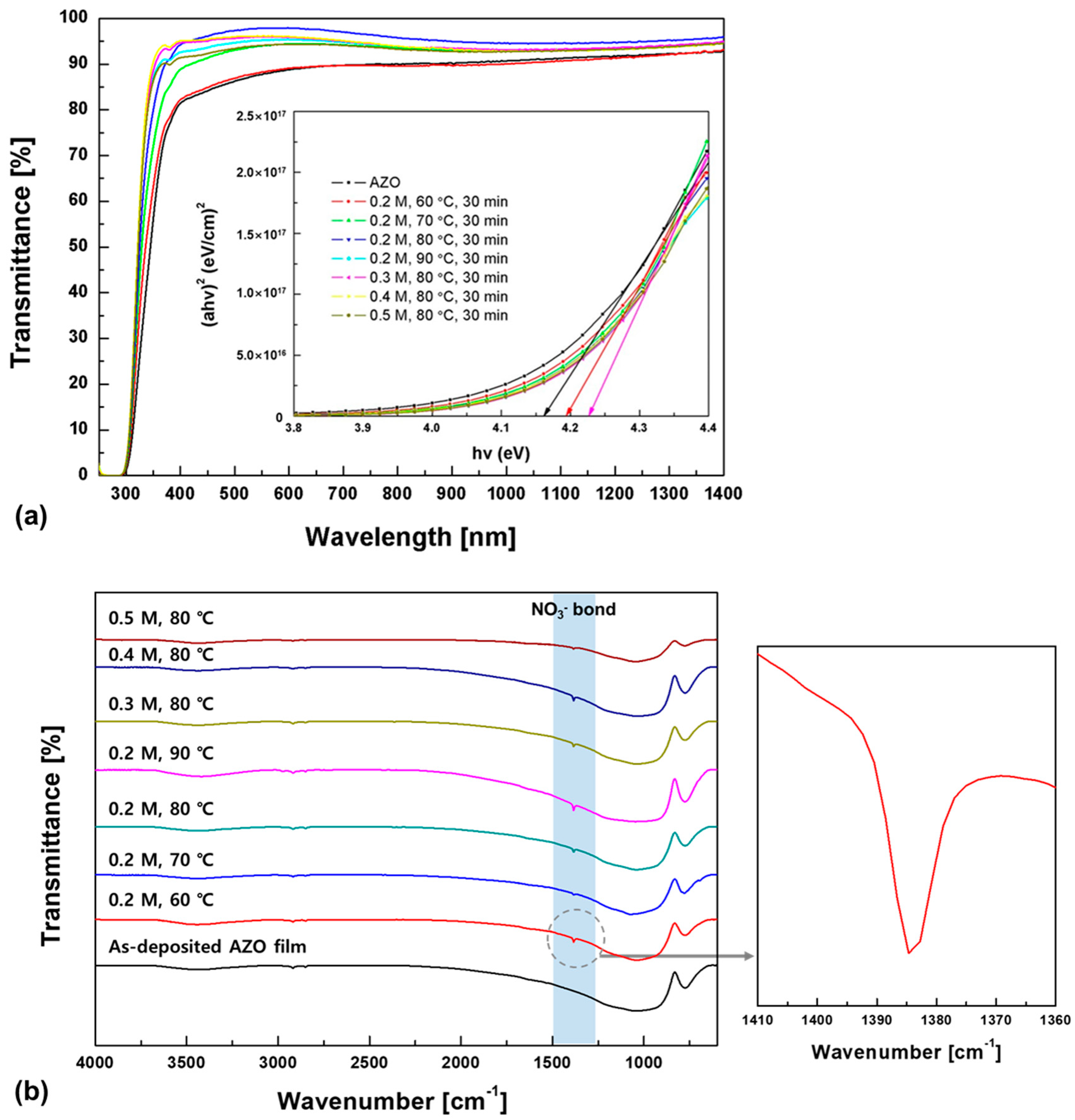
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Al Ali, S.H.; Al-Qubaisi, M.; Hussein, M.Z.; Ismail, M.; Zainal, Z.; Hakim, M.N. Controlled release and angiotensin-converting enzyme inhibition properties of an antihypertensive drug based on a perindopril erbumine-layered double hydroxide nanocomposite. Int. J. Nanomed. 2012, 7, 2129–2141. [Google Scholar] [CrossRef]
- Elkhattabi, E.H.; Badreddine, M.; Berraho, M.; Legrouri, A. Incorporation of fluorophosphate into zinc–aluminium–nitrate layered double hydroxide by ion exchange. Bull. Mater. Sci. 2012, 35, 693–700. [Google Scholar] [CrossRef]
- Prasanna, S.V.; Kamath, P.V. Anion-Exchange Reactions of Layered Double Hydroxides: Interplay between Coulombic and H-Bonding Interactions. Ind. Eng. Chem. Res. 2009, 48, 6315–6320. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Abderrazek, K.; Najoua, F.S.; Srasra, E. Synthesis and characterization of [Zn–Al] LDH: Study of the effect of calcination on the photocatalytic activity. Appl. Clay Sci. 2016, 119, 229–235. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Z.; Guo, Z.; Jiang, C.; Chu, W. Layered double hydroxide and related catalysts for hydrogen production and a biorefinery. Chin. J. Catal. 2015, 36, 139–147. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Diniz da Costa, J.C. Layered Double Hydroxides for CO2 Capture: Structure Evolution and Regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [Google Scholar] [CrossRef]
- Yang, F.; Kadhim, M.S.; Babiker, M.; Elshekh, H.; Hou, W.; Huang, G.; Zhang, Y.; Zhao, Y.; Sun, B. Ion reaction tunable ON/OFF ratio of vertically oriented Zn-Al layered-double-hydroxide nanosheets based memristor. Mater. Today Commun. 2019, 20, 100573. [Google Scholar] [CrossRef]
- Hobbs, C.; Jaskaniec, S.; McCarthy, E.K.; Downing, C.; Opelt, K.; Güth, K.; Shmeliov, A.; Mourad MC, D.; Mandel, K.; Nicolosi, V. Structural transformation of layered double hydroxides: An in situ TEM analysis. npj 2D Mater. Appl. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Chang, Z.; Evans, D.G.; Duan, X.; Vial, C.; Ghanbaja, J.; Prevot, V.; De Roy, M.; Forano, C. Synthesis of [Zn–Al–CO3] layered double hydroxides by a coprecipitation method under steady-state conditions. J. Solid State Chem. 2005, 178, 2766–2777. [Google Scholar] [CrossRef]
- Xu, Z.P.; Lu, G.Q. Hydrothermal synthesis of layered double hydroxides (LDHs) from mixed MgO and Al2O3: LDH formation mechanism. Chem. Mater. 2005, 17, 1055–1062. [Google Scholar] [CrossRef]
- Mikhailau, A.; Maltanava, H.; Poznyak, S.K.; Salak, A.N.; Zheludkevich, M.L.; Yasakau, K.A.; Ferreira, M.G. One-step synthesis and growth mechanism of nitrate intercalated ZnAl LDH conversion coatings on zinc. Chem. Commun. 2019, 55, 6878–6881. [Google Scholar] [CrossRef] [PubMed]
- Neves, C.S.; Bastos, A.C.; Salak, A.N.; Starykevich, M.; Rocha, D.; Zheludkevich, M.L.; Cunha, A.; Almeida, A.; Tedim, J.; Ferreira, M.G. Layered double hydroxide clusters as precursors of novel multifunctional layers: A bottom-up approach. Coatings 2019, 9, 328. [Google Scholar] [CrossRef]
- Valeikiene, L.; Roshchina, M.; Grigoraviciute-Puroniene, I.; Prozorovich, V.; Zarkov, A.; Ivanets, A.; Kareiva, A. On the Reconstruction Peculiarities of Sol–Gel Derived Mg2− xMx/Al1 (M= Ca, Sr, Ba) Layered Double Hydroxides. Crystals 2020, 10, 470. [Google Scholar] [CrossRef]
- Hong, J.; Katsumata, K.; Matsushita, N. Fabrication of Al-Doped ZnO Film with High Conductivity Induced by Photocatalytic Activity. J. Electron. Mater. 2016, 45, 4875–4880. [Google Scholar] [CrossRef]
- Rim, Y.S.; Kim, S.M.; Kim, K.H. Properties of Indium-Zinc-Oxide Thin Films Prepared by Facing Targets Sputtering at Room Temperature. J. Kor. Phy. Soc. 2009, 54, 1267–1272. [Google Scholar] [CrossRef]
- Lei, H.; Ichikawa, K.; Hoshi, Y.; Wang, M.; Uchida, T.; Sawada, Y. A study of deposition of ITO films on organic layer using facing target sputtering in Ar and Kr gases. Thin Solid Films 2010, 518, 2926–2929. [Google Scholar] [CrossRef]
- Nose, M.; Nagae, T.; Yokota, M.; Saji, S.; Zhou, M.; Nakada, M. Electrical and colorimetric properties of TiN thin films prepared by DC reactive sputtering in a facing targets sputtering (FTS) system. Surface Coat. Technol. 1999, 116, 296–301. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Xiao, H.; Zhang, L.; Qin, Y.; Liu, D.; Hou, W.; Du, N. Synthesis and thermal properties of ZnAl layered double hydroxide by urea hydrolysis. Powder Technol. 2014, 253, 41–45. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; Hussein, M.Z. Influence of metallic molar ratio on the electron spin resonance and thermal diffusivity of Zn–Al layered double hydroxide. J. Nanomater. 2013, 2013, 639354. [Google Scholar] [CrossRef]
- Fang, G.; Li, D.; Yao, B.L. Fabrication and vacuum annealing of transparent conductive AZO thin films prepared by DC magnetron sputtering. Vacuum 2002, 68, 363–372. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Pavasaryte, L.; Yang, T.C.; Kareiva, A. Undoped and Eu3+ Doped Magnesium-Aluminium Layered Double Hydroxides: Peculiarities of Intercalation of Organic Anions and Investigation of Luminescence Properties. Materials 2019, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Zaid, M.; Hafiz, M.; Matori, K.A.; Abdul Aziz, S.H.; Zakaria, A.; Ghazali, M.; Sabri, M. Effect of ZnO on the Physical Properties and Optical Band Gap of Soda Lime Silicate Glass. Int. J. Mol. Sci. 2012, 13, 7550–7558. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.G.; Fujita, S.; Kawaharamura, T.; Nishinaka, H.; Kamada, Y.; Ohshima, T.; Ye, Z.Z.; Zeng, Y.J.; Zhang, Y.Z.; Zhu, L.P.; et al. Carrier concentration dependence of band gap shift in n-type ZnO:Al films. J. Appl. Phys. 2007, 101, 083705. [Google Scholar] [CrossRef]
- Pérez, M.R.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Hermosín, M.C.; Ulibarri, M.A. Uptake of Cu2+, Cd2+ and Pb2+ on Zn–Al layered double hydroxide intercalated with edta. Appl. Clay Sci. 2006, 32, 245–251. [Google Scholar] [CrossRef]



| Parameters | Conditions |
|---|---|
| Target | Al doped ZnO, 4 inch (Zn: 98 wt.%, Al2O3−: 2 wt.%) |
| Substrate | Glass |
| Working pressure | 2 mTorr |
| Gas flow | Ar, 10 sccm, O2, 0.8 sccm |
| Input power | 500 W (DC) |
| Thickness | 100 nm |
| Sample | 2 Theta(degree) | d-Value(Å) |
|---|---|---|
| AZO (002) | 34.5 | 2.598 |
| 0.2 M, 80 °C | 9.692 | 9.121 |
| 0.3 M, 80 °C | 9.703 | 9.110 |
| 0.4 M, 80 °C | 9.685 | 9.127 |
| 0.5 M, 80 °C | 9.734 | 9.081 |
| Sample | 2 Theta(degree) | d-Value(Å) |
|---|---|---|
| 0.2 M, 60 °C | 9.636 | 9.174 |
| 0.2 M, 70 °C | 9.632 | 9.177 |
| 0.2 M, 80 °C | 9.692 | 9.121 |
| 0.2 M, 90 °C | 9.727 | 9.088 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.; Kim, K.; Hong, J. Zn-Al Layered Double Hydroxide Thin Film Fabricated by the Sputtering Method and Aqueous Solution Treatment. Coatings 2020, 10, 669. https://doi.org/10.3390/coatings10070669
Shin J, Kim K, Hong J. Zn-Al Layered Double Hydroxide Thin Film Fabricated by the Sputtering Method and Aqueous Solution Treatment. Coatings. 2020; 10(7):669. https://doi.org/10.3390/coatings10070669
Chicago/Turabian StyleShin, Jaehwan, Kyunghwan Kim, and Jeongsoo Hong. 2020. "Zn-Al Layered Double Hydroxide Thin Film Fabricated by the Sputtering Method and Aqueous Solution Treatment" Coatings 10, no. 7: 669. https://doi.org/10.3390/coatings10070669
APA StyleShin, J., Kim, K., & Hong, J. (2020). Zn-Al Layered Double Hydroxide Thin Film Fabricated by the Sputtering Method and Aqueous Solution Treatment. Coatings, 10(7), 669. https://doi.org/10.3390/coatings10070669





