Graphene Coating for Enhancing the Atom Oxygen Erosion Resistance of Kapton
Abstract
1. Introduction
2. Experimental
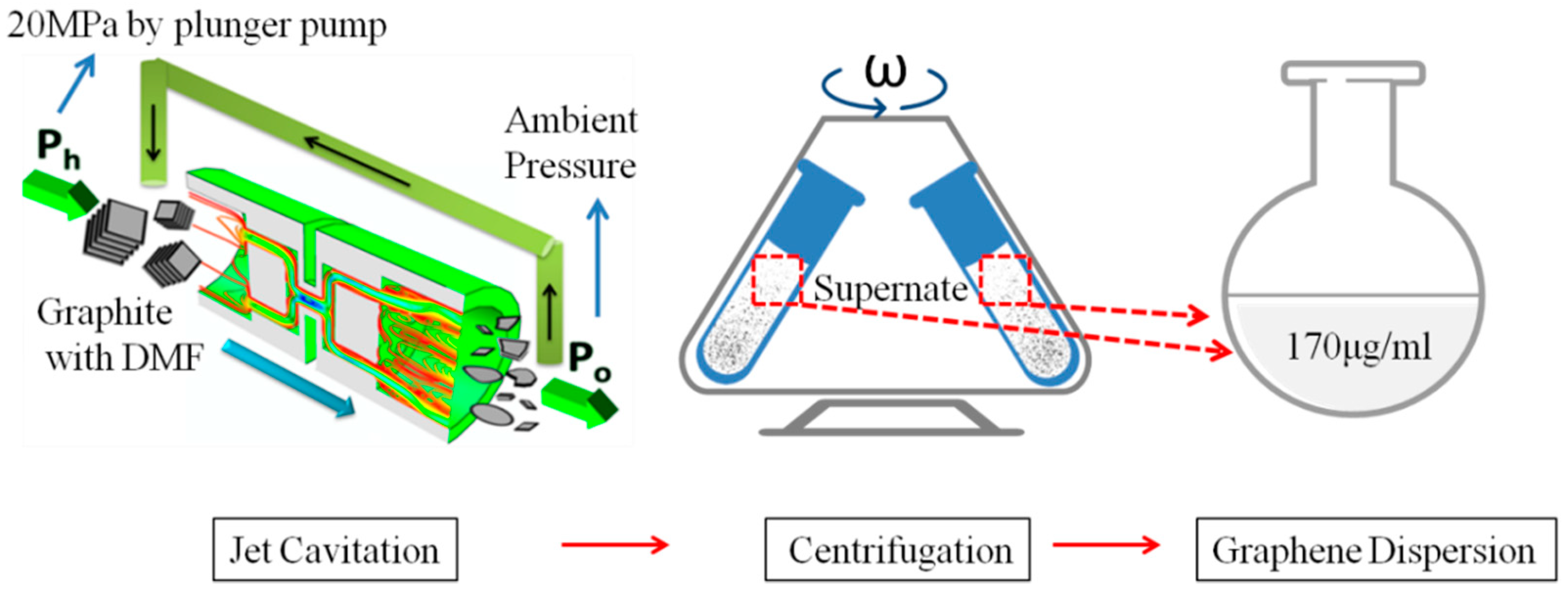
2.1. Preparation of Graphene Dispersion
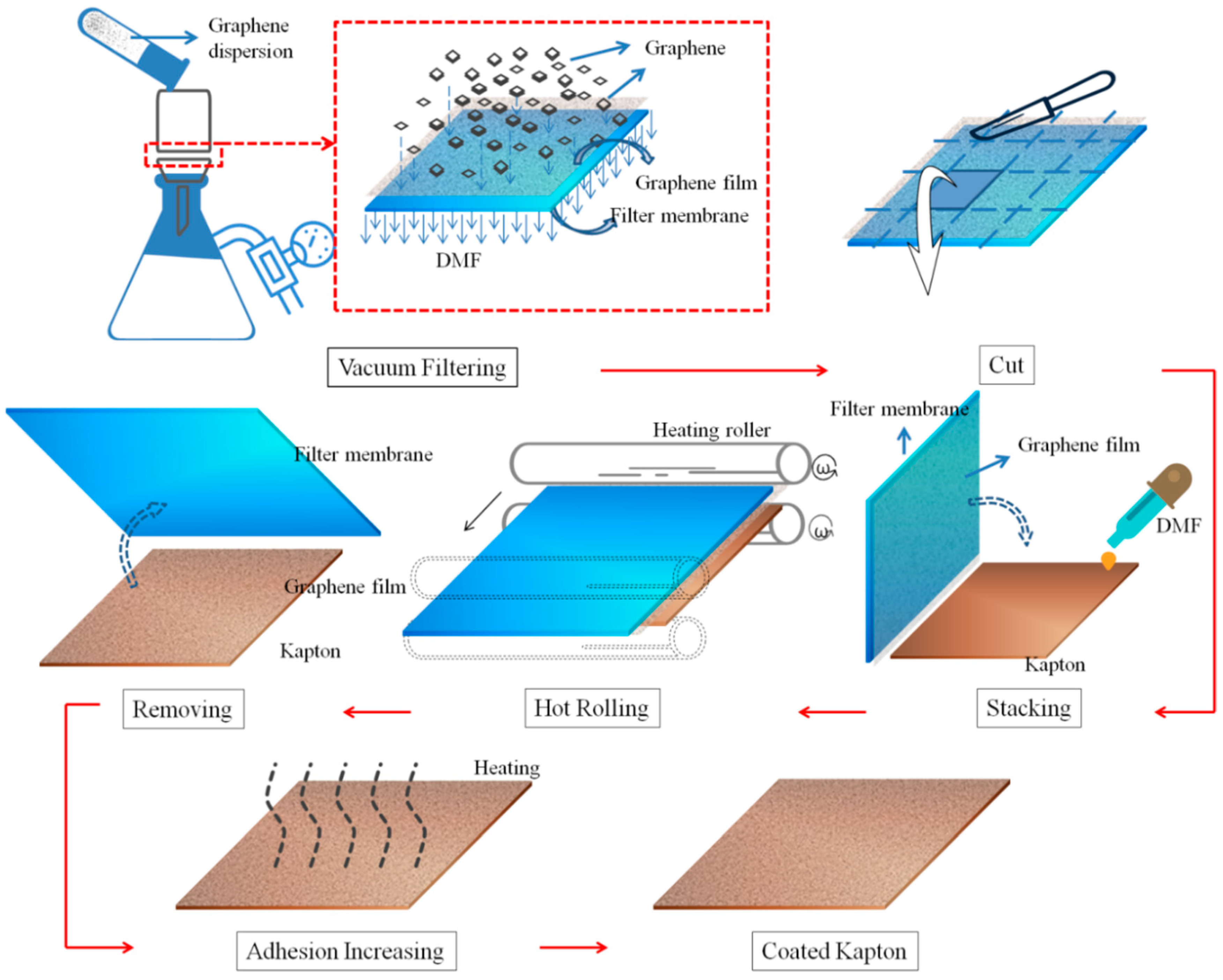
2.2. Preparation of Graphene Coatingon Kapton
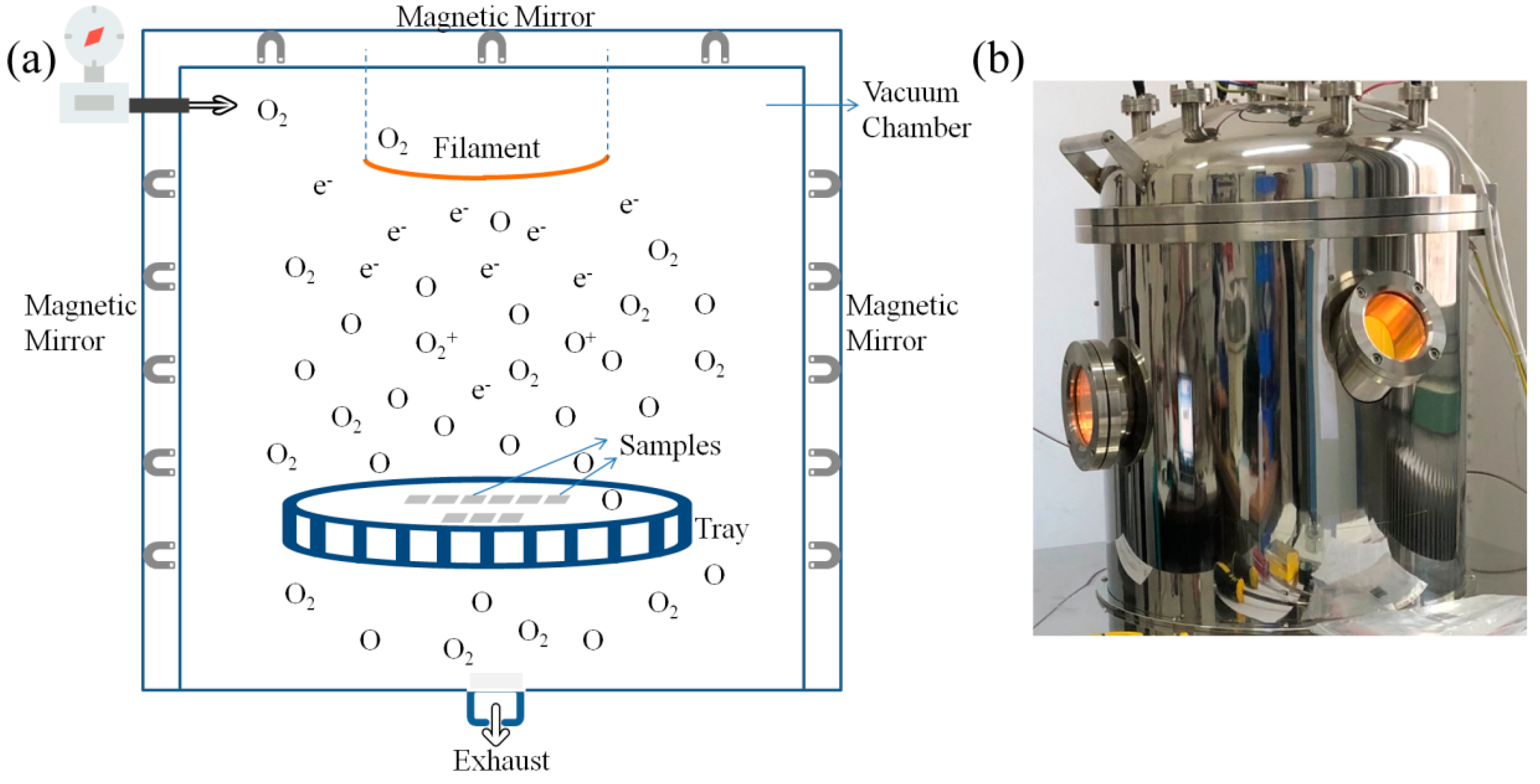
2.3. AO Exposure Experiment
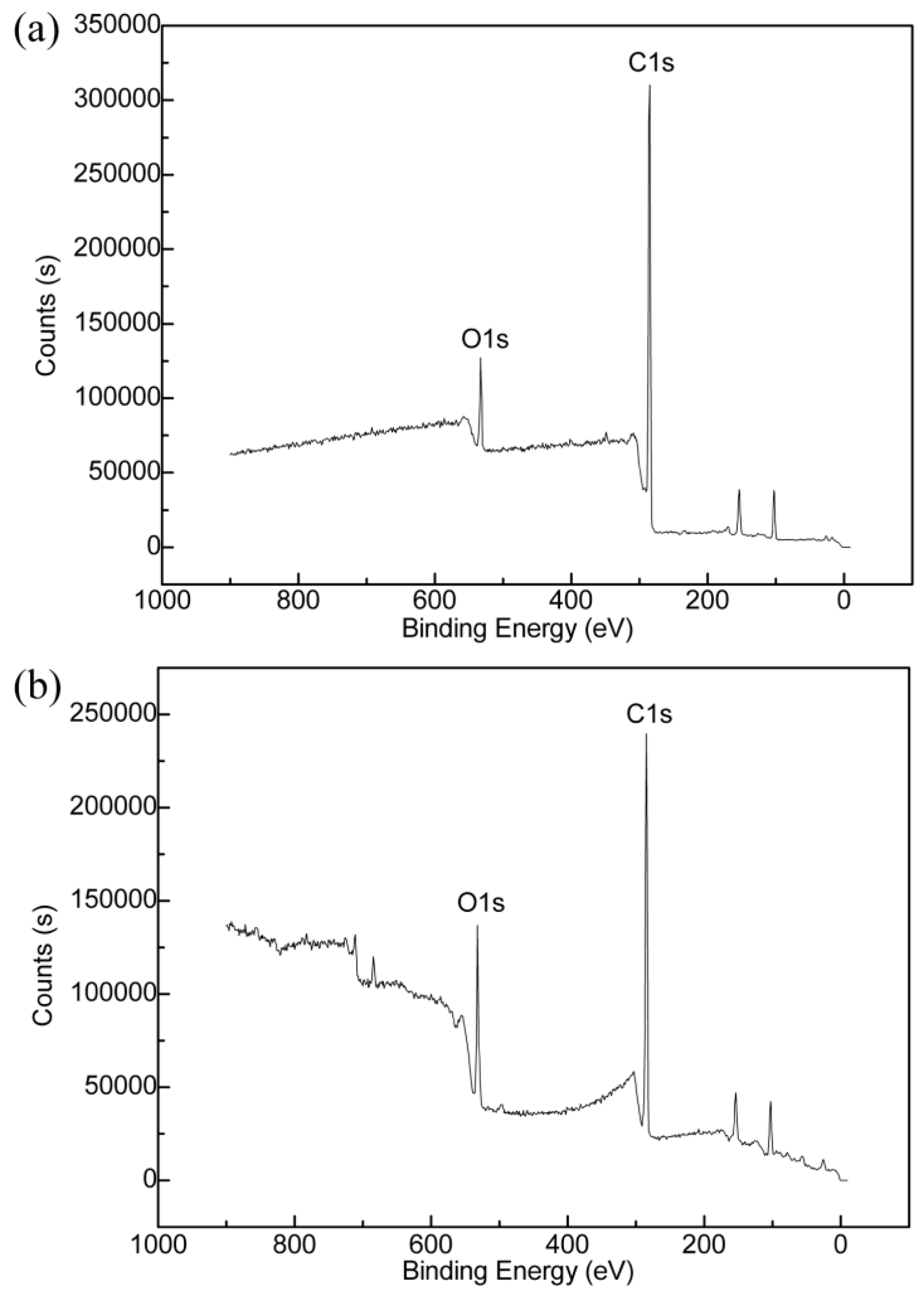
2.4. Characterization
3. Results and Discussion
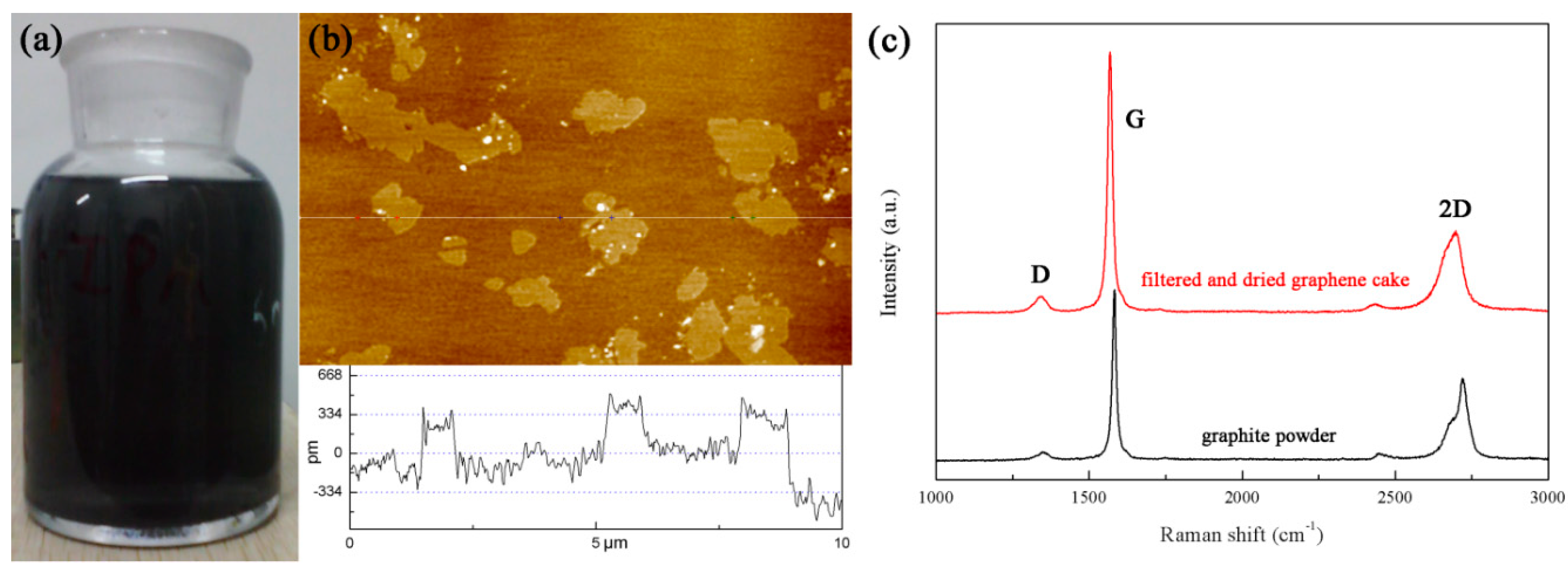
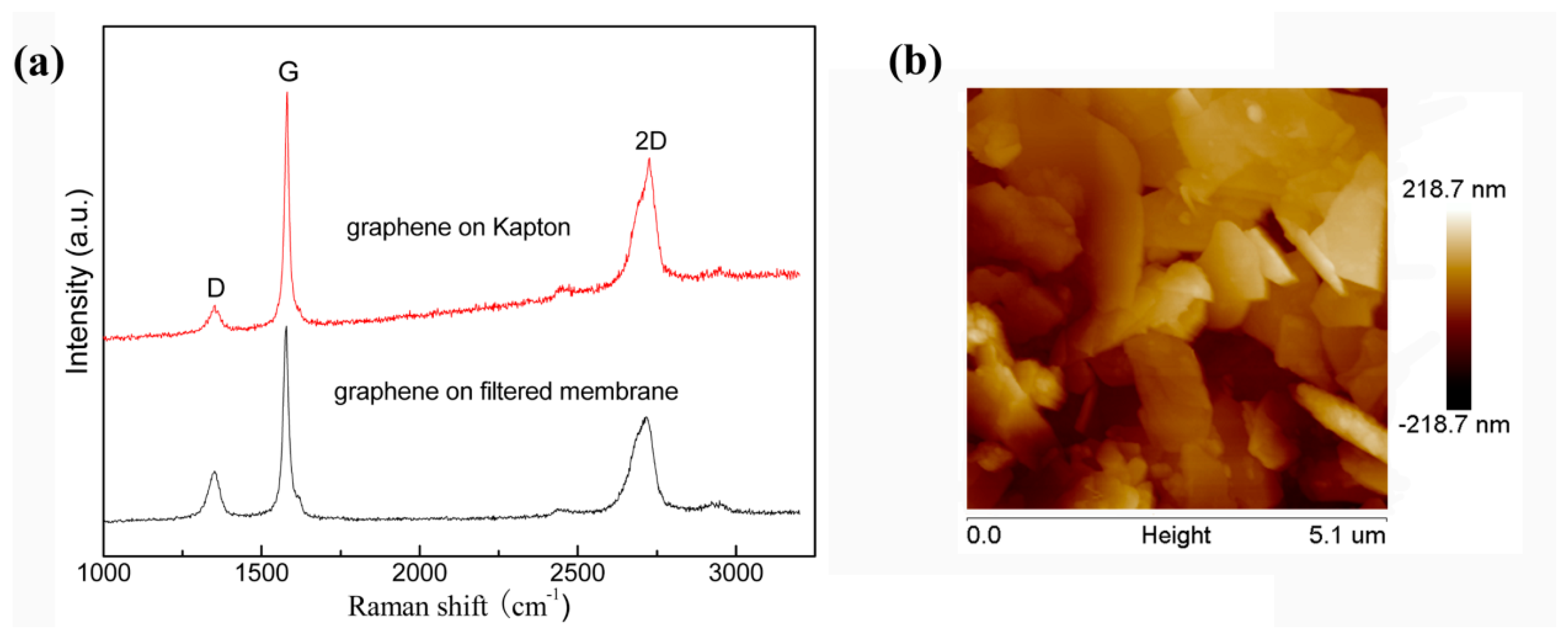
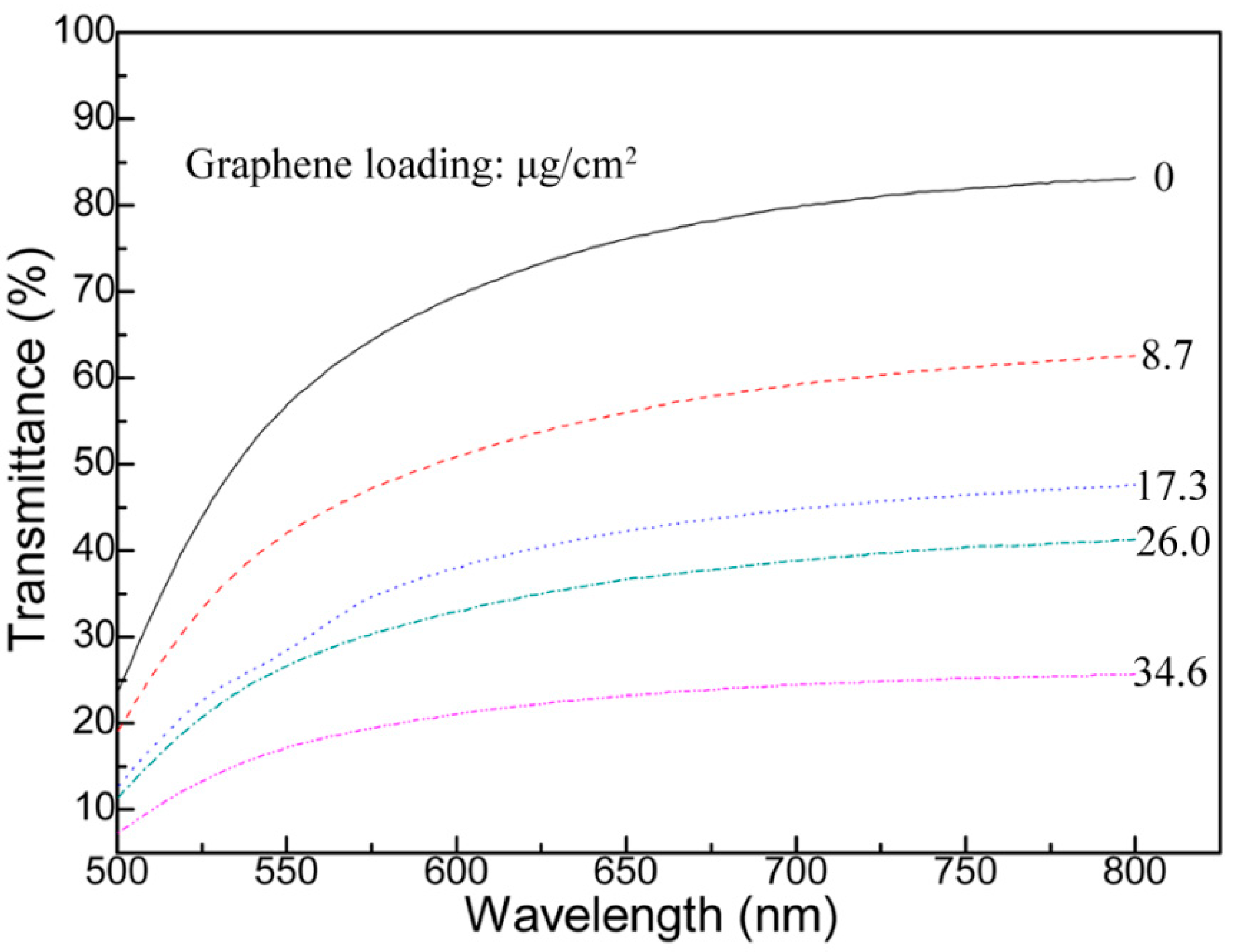
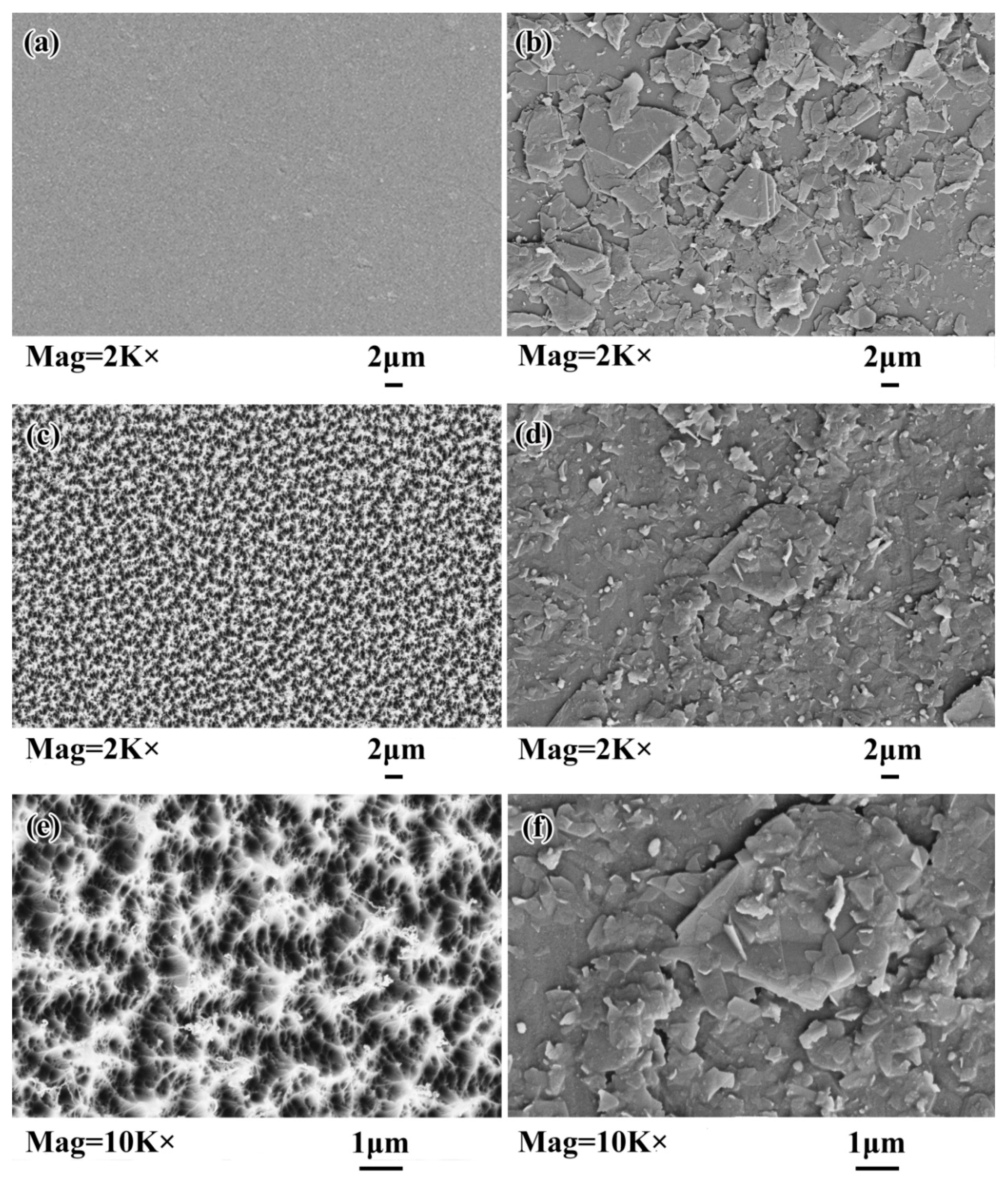
3.1. Graphene and Graphene Film Prepared
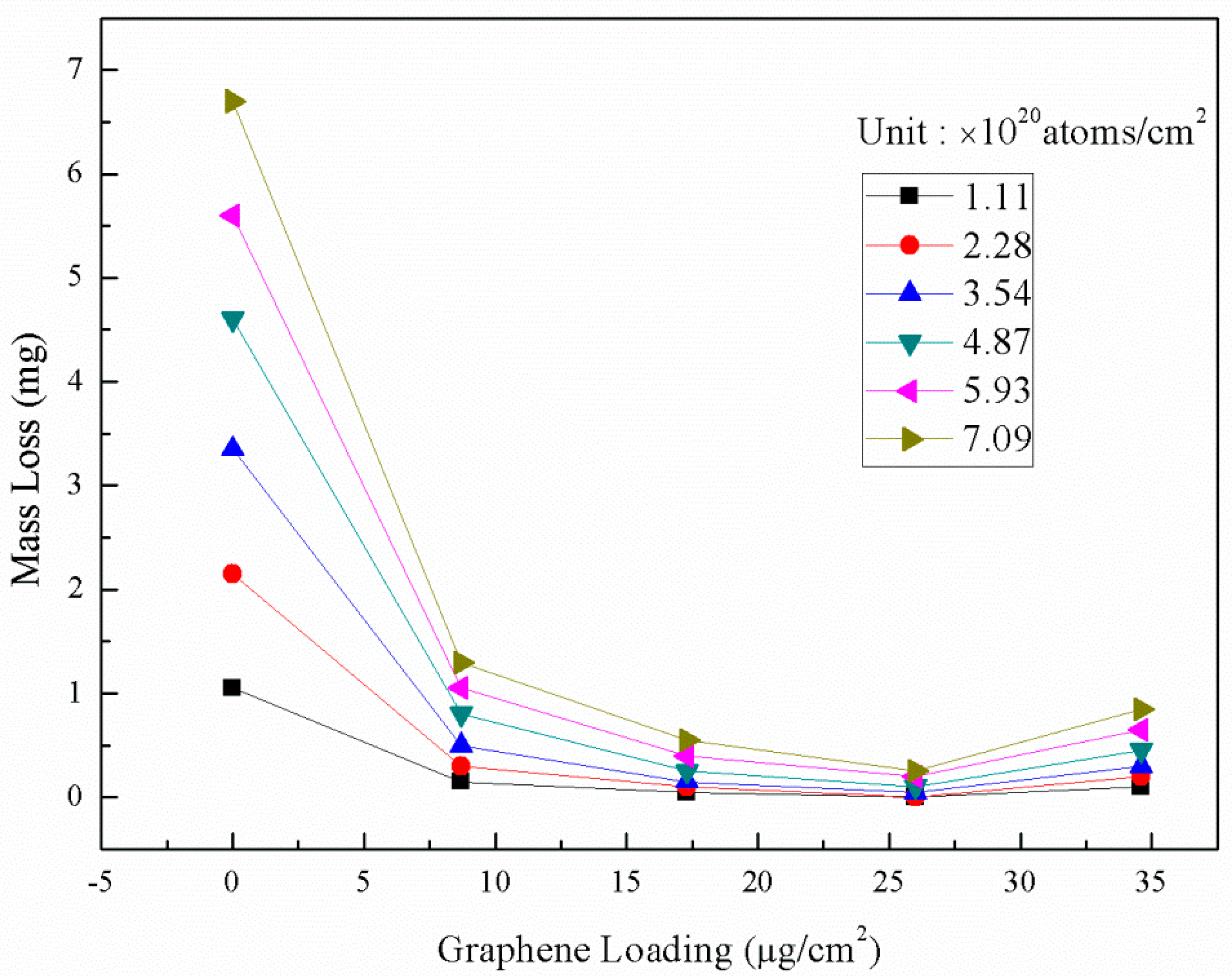
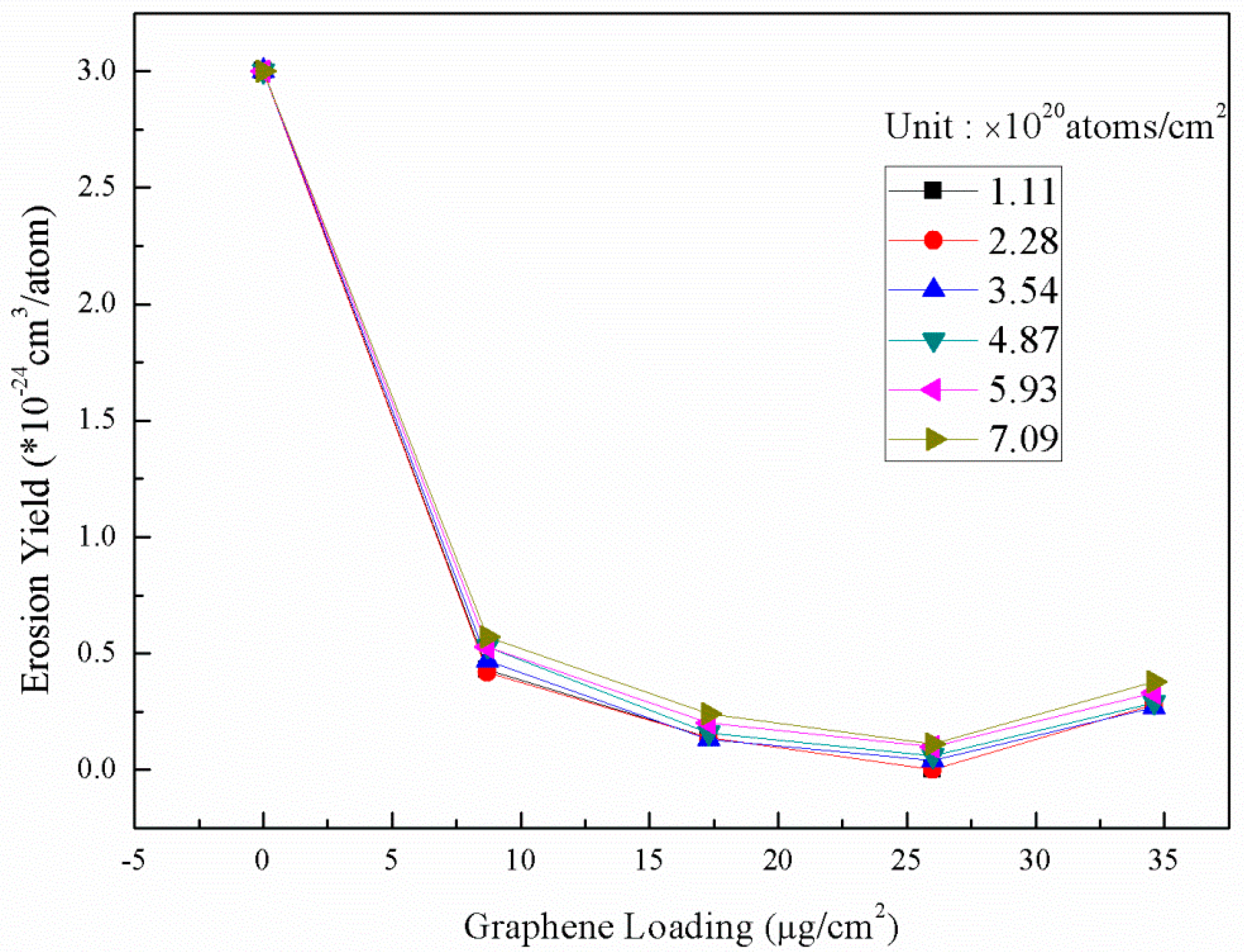
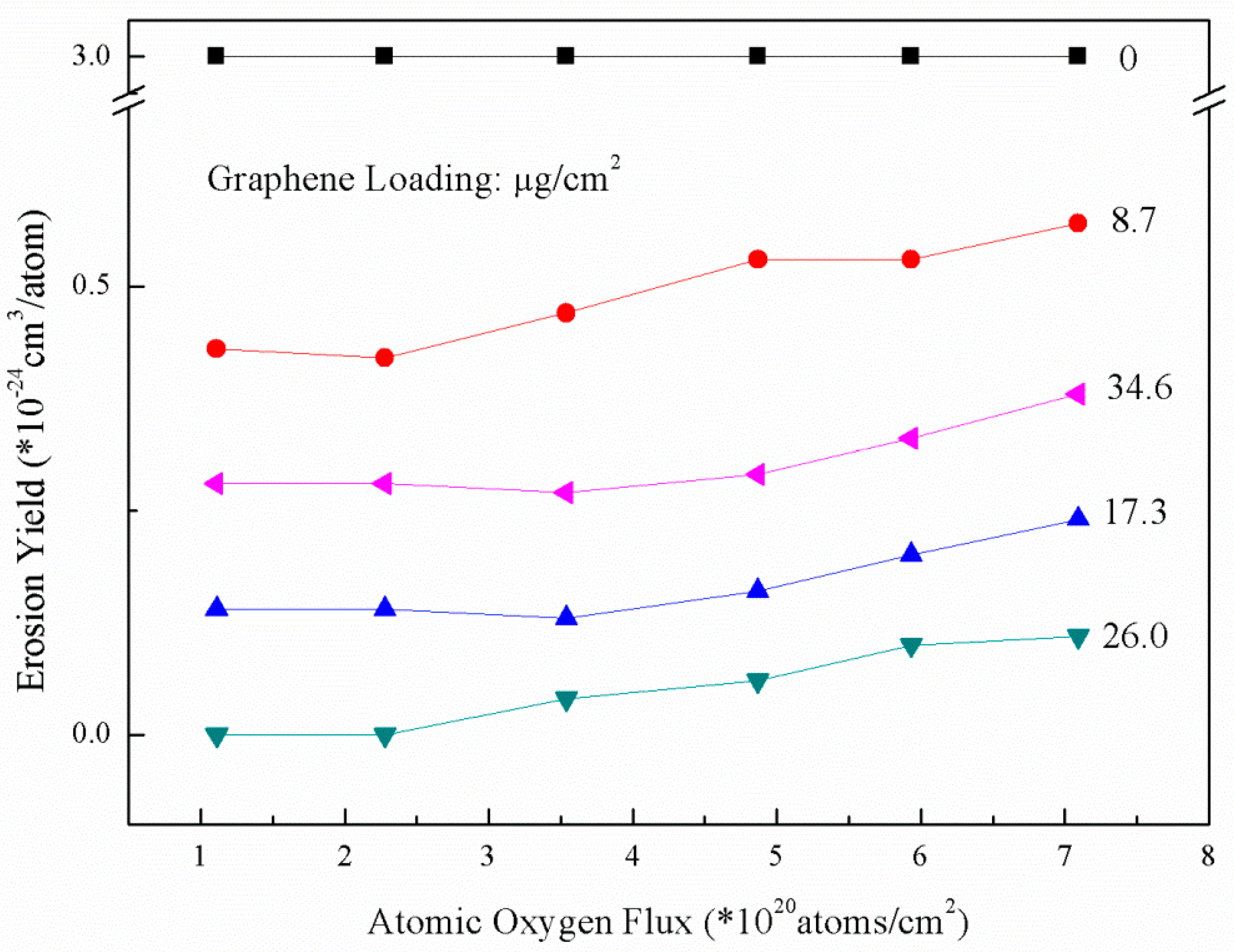
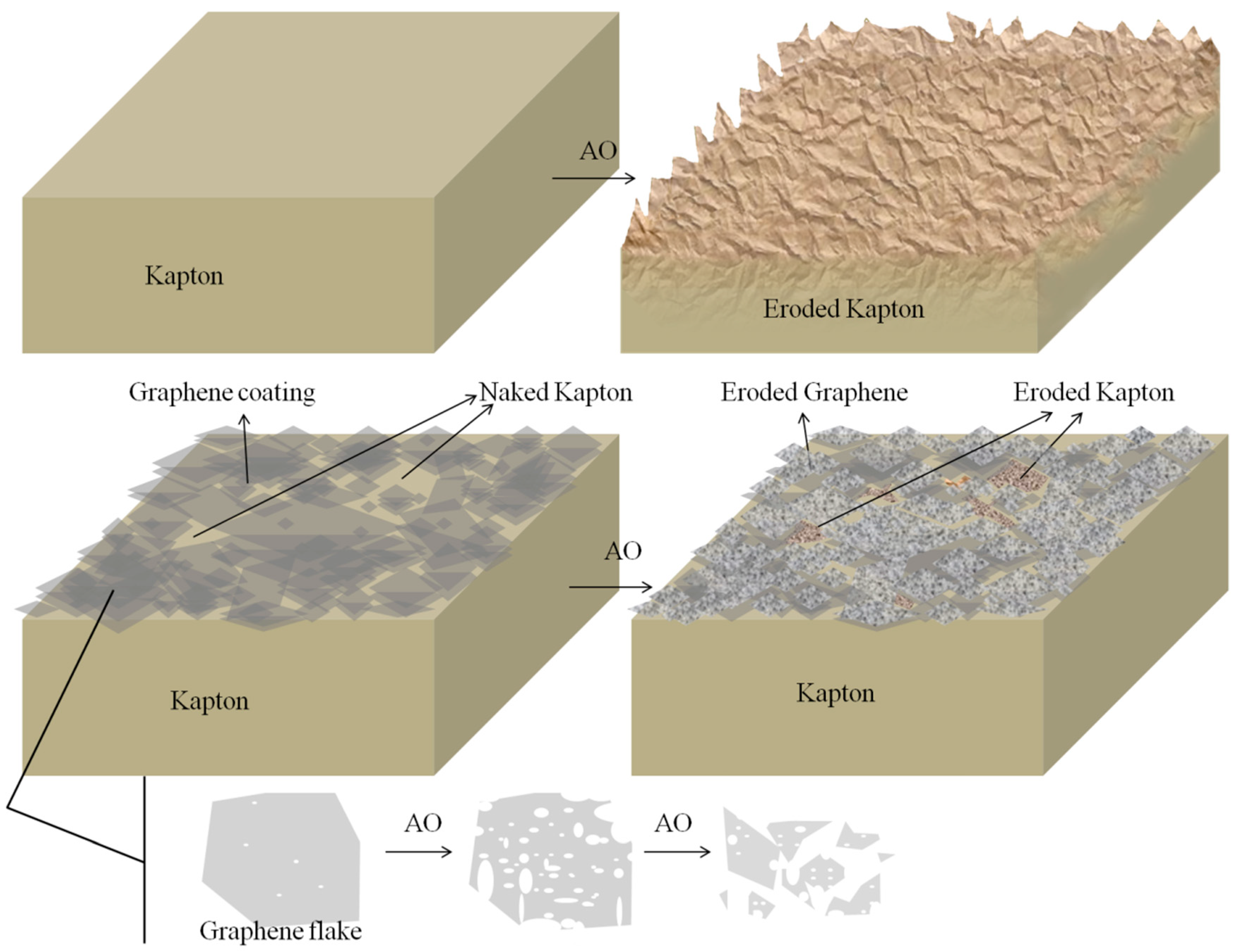
3.2. AO Resistance of Coated Kapton
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dever, J.A.; Miller, S.K.; Sechkar, E.A.; Wittberg, T.N. Space Environment Exposure of Polymer Films on the Materials International Space Station Experiment: Results from MISSE 1 and MISSE 2. High Perform. Polym. 2008, 20, 371–387. [Google Scholar] [CrossRef]
- Shimamura, H.; Nakamura, T. Mechanical properties degradation of polyimide films irradiated by atomic oxygen. Polym. Degrad. Stab. 2009, 94, 1389–1396. [Google Scholar] [CrossRef]
- Devapal, D.; Packirisamy, S.; Nair, C.P.R.; Ninan, K.N. Phosphazene-based polymers as atomic oxygen resistant materials. J. Mater. Sci. 2006, 41, 5764–5766. [Google Scholar] [CrossRef]
- Su, L.; Tao, L.; Wang, T.; Wang, Q. Phenylphosphine oxide-containing aromatic polyamide films with high atomic oxygen erosion resistance. Polym. Degrad. Stab. 2012, 97, 981–986. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, K.; Zhan, M.S. Atomic oxygen resistant phosphorus-containing polyimides for LEO environment. J. Mater. Sci. 2012, 47, 4904–4913. [Google Scholar] [CrossRef]
- Verker, R.; Atar, N.; Quero, F.; Eichhorn, S.J.; Grossman, E. Tensile stress effect on the macromolecular orientation and erosion mechanism of an atomic oxygen irradiated polyimide. Polym. Degrad. Stab. 2013, 98, 997–1005. [Google Scholar] [CrossRef]
- Samwel, S. Low Earth Orbital Atomic Oxygen Erosion Effect on Spacecraft Materials. Space Res. J. 2014, 7, 1–13. [Google Scholar] [CrossRef]
- Packirisamy, S.; Schwam, D.; Litt, M.H. Atomic oxygen resistant coatings for low earth orbit space structures. J. Mater. Sci. 1995, 30, 308–320. [Google Scholar] [CrossRef]
- Duo, S.; Chang, Y.; Liu, T.; Zhang, H. Atomic Oxygen Erosion Resistance of Polysiloxane/POSS Hybrid Coatings on Kapton. Phys. Procedia 2013, 50, 337–342. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Xu, C.; Luo, Y. Perhydropolysilazane derived silica coating protecting Kapton from atomic oxygen attack. Thin Solid Films 2011, 520, 1063–1068. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; He, S.; Yang, D.; Li, F. An investigation on the atomic oxygen erosion resistance of surface sol–gel silica films. Surf. Coat. Technol. 2008, 202, 3464–3469. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, Y.; Qin, X.; Liu, H.; Yin, J. Preparation and properties of atomic oxygen protective films deposited on Kapton by solvothermal and sol–gel methods. Surf. Coat. Technol. 2012, 206, 4384–4388. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Liu, G.; He, S.; Yang, D. Investigation on sol–gel boehmite-AlOOH films on Kapton and their erosion resistance to atomic oxygen. Thin Solid Films 2008, 516, 5020–5026. [Google Scholar] [CrossRef]
- Duo, S.; Li, M.; Zhu, M.; Zhou, Y. Polydimethylsiloxane/silica hybrid coatings protecting Kapton from atomic oxygen attack. Mater. Chem. Phys. 2008, 112, 1093–1098. [Google Scholar] [CrossRef]
- Liu, K.; Mu, H.; Shu, M.; Li, Z.; Gao, Y. Improved adhesion between SnO2/SiO2 coating and polyimide film and its applications to atomic oxygen protection. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 356–362. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Xu, C.; Luo, Y.; Zhou, Y. A polysilazane coating protecting polyimide from atomic oxygen and vacuum ultraviolet radiation erosion. Surf. Coat. Technol. 2009, 203, 3338–3343. [Google Scholar] [CrossRef]
- Gouzman, I.; Girshevitz, O.; Grossman, E.; Eliaz, N.; Sukenik, C.N. Thin Film Oxide Barrier Layers: Protection of Kapton from Space Environment by Liquid Phase Deposition of Titanium Oxide. ACS Appl. Mater. Interfaces 2010, 2, 1835–1843. [Google Scholar] [CrossRef]
- Gotlib-Vainstein, K.; Gouzman, I.; Girshevitz, O.; Bolker, A.; Atar, N.; Grossman, E.; Sukenik, C.N. Liquid Phase Deposition of a Space-Durable, Antistatic SnO2 Coating on Kapton. ACS Appl. Mater. Interfaces 2015, 7, 3539–3546. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, X.; Lv, S.; Yang, S.; Fu, R.; Chu, P.K.; Leng, J.; Li, Y. An undercutting model of atomic oxygen for multilayer silica/alumina films fabricated by plasma immersion implantation and deposition on polyimide. Appl. Surf. Sci. 2011, 257, 9158–9163. [Google Scholar] [CrossRef]
- Mu, H.; Wang, X.; Li, Z.; Xie, Y.; Gao, Y.; Liu, H. Preparation and atomic oxygen erosion resistance of SiOx coating formed on polyimide film by plasma polymer deposition. Vacuum 2019, 165, 7–11. [Google Scholar] [CrossRef]
- Qi, H.; Qian, Y.; Xu, J.; Li, M. Studies on atomic oxygen erosion resistance of deposited Mg-alloy coating on Kapton. Corros. Sci. 2017, 124, 56–62. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Kamel, S.; El-Sakhawy, M.; Anis, B.; Tohamy, H.-A.S. Graphene: Structure, Synthesis, and Characterization; a brief review. Egypt. J. Chem. 2019, 63, 9–10. [Google Scholar] [CrossRef]
- Fang, B.; Chang, D.; Xu, Z.; Gao, C. A Review on Graphene Fibers: Expectations, Advances, and Prospects. Adv. Mater. 2019, 32, e1902664. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F. Graphene: A perfect nanoballoon. Appl. Phys. Lett. 2008, 93, 193107. [Google Scholar] [CrossRef]
- Topsakal, M.; Sahin, H.; Ciraci, S. Graphene coatings: An efficient protection from oxidation. Phys. Rev. B 2012, 85, 85. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, S.; Pu, J.; Xue, Q. Barrier mechanism of multilayers graphene coated copper against atomic oxygen irradiation. Appl. Surf. Sci. 2018, 444, 28–35. [Google Scholar] [CrossRef]
- Ren, S.; Cui, M.; Li, Q.; Li, W.; Pu, J.; Xue, Q.; Wang, L. Barrier mechanism of nitrogen-doped graphene against atomic oxygen irradiation. Appl. Surf. Sci. 2019, 479, 669–678. [Google Scholar] [CrossRef]
- Chen, L.; Wei, F.; Liu, L.; Cheng, W.; Hu, Z.; Wu, G.; Du, Y.; Qu, M.; Huang, Y. Grafting of silane and graphene oxide onto PBO fibers: Multifunctional interphase for fiber/polymer matrix composites with simultaneously improved interfacial and atomic oxygen resistant properties. Compos. Sci. Technol. 2015, 106, 32–38. [Google Scholar] [CrossRef]
- Peng, D.; Qin, W.; Wu, X. Improvement of the atomic oxygen resistance of carbon fiber-reinforced cyanate ester composites modified by POSS-graphene-TiO2. Polym. Degrad. Stab. 2016, 133, 211–218. [Google Scholar] [CrossRef]
- Shen, Z.; Li, J.; Yi, M.; Zhang, X.; Ma, S. Preparation of graphene by jet cavitation. Nanotechnology 2011, 22, 365306. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, M.; Shen, Z.; Zhao, X.; Zhang, X.; Ma, S. Graphene-reinforced epoxy resin with enhanced atomic oxygen erosion resistance. J. Mater. Sci. 2012, 48, 2416–2423. [Google Scholar] [CrossRef]
- Liu, L.; Shen, Z.; Zheng, Y.; Yi, M.; Zhang, X.; Ma, S. Boron nitride nanosheets with controlled size and thickness for enhancing mechanical properties and atomic oxygen erosion resistance. RSC Adv. 2014, 4, 37726–37732. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z.; Zhao, X.; Liu, L.; Liang, S.; Zhang, X. Exploring few-layer graphene and graphene oxide as fillers to enhance the oxygen-atom corrosion resistance of composites. Phys. Chem. Chem. Phys. 2014, 16, 11162–11167. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Li, G.; Li, T. Numerical investigation on atomic oxygen undercutting of the protective polymer film using Monte Carlo approach. Appl. Surf. Sci. 2010, 256, 6096–6106. [Google Scholar] [CrossRef]
- Güneş, F.; Shin, H.-J.; Biswas, C.; Han, G.H.; Kim, E.S.; Chae, S.J.; Choi, J.-Y.; Lee, Y.H. Layer-by-Layer Doping of Few-Layer Graphene Film. ACS Nano 2010, 4, 4595–4600. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Tsoi, S.; Dev, P.; Friedman, A.L.; Stine, R.; Robinson, J.T.; Reinecke, T.L.; Sheehan, P.E. van der Waals Screening by Single-Layer Graphene and Molybdenum Disulfide. ACS Nano 2014, 8, 12410–12417. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Shen, Z.; Xing, Y.-S.; Ma, S.-L. An experimental study of low earth orbit atomic oxygen and ultraviolet radiation effects on a spacecraft material-polytetrafluoroethylene. Polym. Degrad. Stab. 2005, 88, 275–285. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Z.; Yi, M.; Liu, L.; Zhang, X.; Cai, C.; Ma, S. Effects of Processing Parameters on Massive Production of Graphene by Jet Cavitation. J. Nanosci. Nanotechnol. 2015, 15, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Shen, Z.; Zhu, J. A fluid dynamics route for producing graphene and its analogues. Chin. Sci. Bull. 2014, 59, 1794–1799. [Google Scholar] [CrossRef]
- Wang, S.; Geng, Y.; Zheng, Q.; Kim, J.-K. Fabrication of highly conducting and transparent graphene films. Carbon 2010, 48, 1815–1823. [Google Scholar] [CrossRef]
- Caldwell, J.D.; Anderson, T.J.; Culbertson, J.C.; Jernigan, G.G.; Hobart, K.D.; Kub, F.J.; Tadjer, M.J.; Tedesco, J.L.; Hite, J.K.; Mastro, M.A.; et al. Technique for the Dry Transfer of Epitaxial Graphene onto Arbitrary Substrates. ACS Nano 2010, 4, 1108–1114. [Google Scholar] [CrossRef]
- Wajid, A.S.; Das, S.; Irin, F.; Ahmed, H.T.; Shelburne, J.L.; Parviz, D.; Fullerton, R.J.; Jankowski, A.F.; Hedden, R.C.; Green, M.J. Polymer-stabilized graphene dispersions at high concentrations in organic solvents for composite production. Carbon 2012, 50, 526–534. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, S.; Kim, J.H.; Kim, M.H.; Ryu, J.; Seo, S.; Hong, B.H.; Kim, M.K.; Choi, J.-B. Efficient Transfer of Large-Area Graphene Films onto Rigid Substrates by Hot Pressing. ACS Nano 2012, 6, 5360–5365. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Suk, J.W.; Lee, J.; Hao, Y.; Park, J.; Yang, J.W.; Ha, H.-W.; Murali, S.; Chou, H.; Akinwande, D.; et al. Simultaneous Transfer and Doping of CVD-Grown Graphene by Fluoropolymer for Transparent Conductive Films on Plastic. ACS Nano 2012, 6, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.W.; Kitt, A.; Magnuson, C.W.; Hao, Y.; Ahmed, S.; An, J.; Swan, A.K.; Goldberg, B.B.; Ruoff, R.S. Transfer of CVD-Grown Monolayer Graphene onto Arbitrary Substrates. ACS Nano 2011, 5, 6916–6924. [Google Scholar] [CrossRef]
- Cazaubon, B.; Paillous, A.; Siffre, J.; Thomas, R. Mass Spectrometric Analysis of Reaction Products of Fast Oxygen Atoms-Material Interactions. J. Spacecr. Rockets 1998, 35, 797–804. [Google Scholar] [CrossRef]
- Lotya, M.; Hernández, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Shen, Z. Kitchen blender for producing high-quality few-layer graphene. Carbon 2014, 78, 622–626. [Google Scholar] [CrossRef]
- Yi, M.; Liang, S.; Liu, L.; Shen, Z.; Zheng, Y.; Zhang, X.; Ma, S. Investigating the nature of graphene-based films prepared by vacuum filtration of graphene dispersions. J. Nanosci. Nanotechnol. 2014, 14, 4969–4975. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Fujii, S.; Enoki, T. Cutting of Oxidized Graphene into Nanosized Pieces. J. Am. Chem. Soc. 2010, 132, 10034–10041. [Google Scholar] [CrossRef]
- Li, J.-L.; Kudin, K.N.; McAllister, M.J.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Oxygen-Driven Unzipping of Graphitic Materials. Phys. Rev. Lett. 2006, 96, 176101. [Google Scholar] [CrossRef]
- Sun, T.; Fabris, S. Mechanisms for Oxidative Unzipping and Cutting of Graphene. Nano Lett. 2011, 12, 17–21. [Google Scholar] [CrossRef]
- Spady, B.R.; Synowicki, R.A.; Hale, J.S.; De Vries, M.J.; Woollam, J.A.; Moore, A.W.; Lake, M. Low-Earth-orbit exposure of carbon-based materials aboard Shuttle flight STS-46. J. Spacecr. Rockets 1995, 32, 1015–1017. [Google Scholar] [CrossRef]
- Novoselov, K.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shen, Z.; Liang, S.; Yi, M.; Zhang, X.; Ma, S. Enhanced atomic oxygen erosion resistance and mechanical properties of graphene/cellulose acetate composite films. J. Appl. Polym. Sci. 2013, 131, 40292. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, J.H.; Ding, F. The edges of graphene. Nanoscale 2013, 5, 2556. [Google Scholar] [CrossRef] [PubMed]
- Barinov, A.; Malcioǧlu, O.B.; Fabris, S.; Sun, T.; Gregoratti, L.; Dalmiglio, M.M.; Kiskinova, M. Initial Stages of Oxidation on Graphitic Surfaces: Photoemission Study and Density Functional Theory Calculations. J. Phys. Chem. C 2009, 113, 9009–9013. [Google Scholar] [CrossRef]
| Material | Kapton | Coated Kapton with Graphene | |||
|---|---|---|---|---|---|
| Element | Before | After | Before | After | |
| C | 78.88 | 68.25 | 94.34 | 86.96 | |
| O | 14.04 | 26.49 | 4.96 | 12.50 | |
| N | 7.08 | 5.26 | 0.70 | 0.54 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Shen, Z.; Zhang, W.; Yi, M.; Ma, H.; Liu, L.; Liu, L.; Zhao, Y. Graphene Coating for Enhancing the Atom Oxygen Erosion Resistance of Kapton. Coatings 2020, 10, 644. https://doi.org/10.3390/coatings10070644
Zhang X, Shen Z, Zhang W, Yi M, Ma H, Liu L, Liu L, Zhao Y. Graphene Coating for Enhancing the Atom Oxygen Erosion Resistance of Kapton. Coatings. 2020; 10(7):644. https://doi.org/10.3390/coatings10070644
Chicago/Turabian StyleZhang, Xiaojing, Zhigang Shen, Wen Zhang, Min Yi, Han Ma, Lei Liu, Lixin Liu, and Yizhi Zhao. 2020. "Graphene Coating for Enhancing the Atom Oxygen Erosion Resistance of Kapton" Coatings 10, no. 7: 644. https://doi.org/10.3390/coatings10070644
APA StyleZhang, X., Shen, Z., Zhang, W., Yi, M., Ma, H., Liu, L., Liu, L., & Zhao, Y. (2020). Graphene Coating for Enhancing the Atom Oxygen Erosion Resistance of Kapton. Coatings, 10(7), 644. https://doi.org/10.3390/coatings10070644





