Vertical Guided Bone Augmentation Using Titanium Mesh Domes Coated with Natural Latex Extracted from Hevea brasiliensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Latex–Titanium Domes
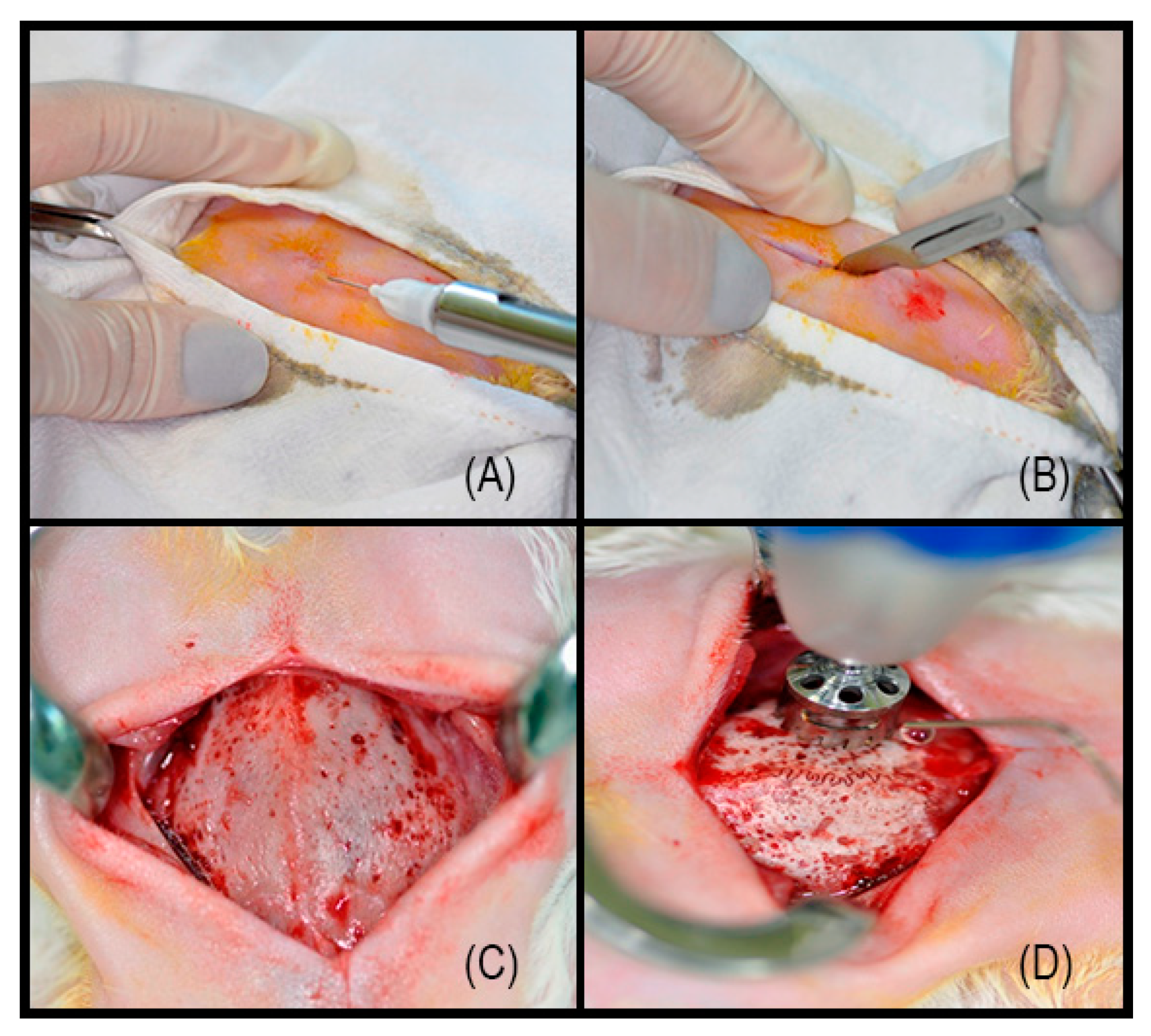
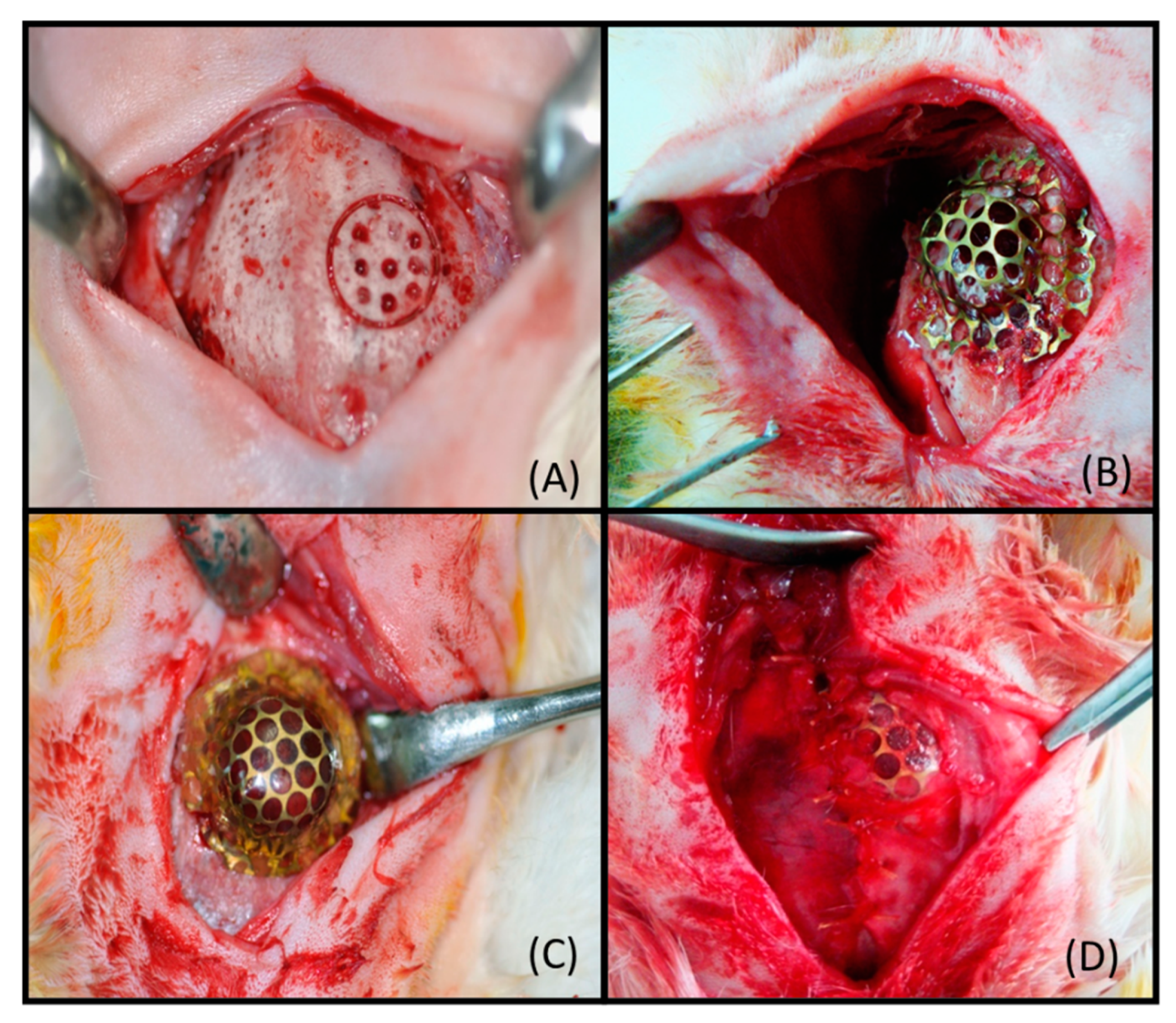
2.2. Surgical Procedure
2.3. Computed Tomography Images
2.4. Microscopic Analysis
3. Results
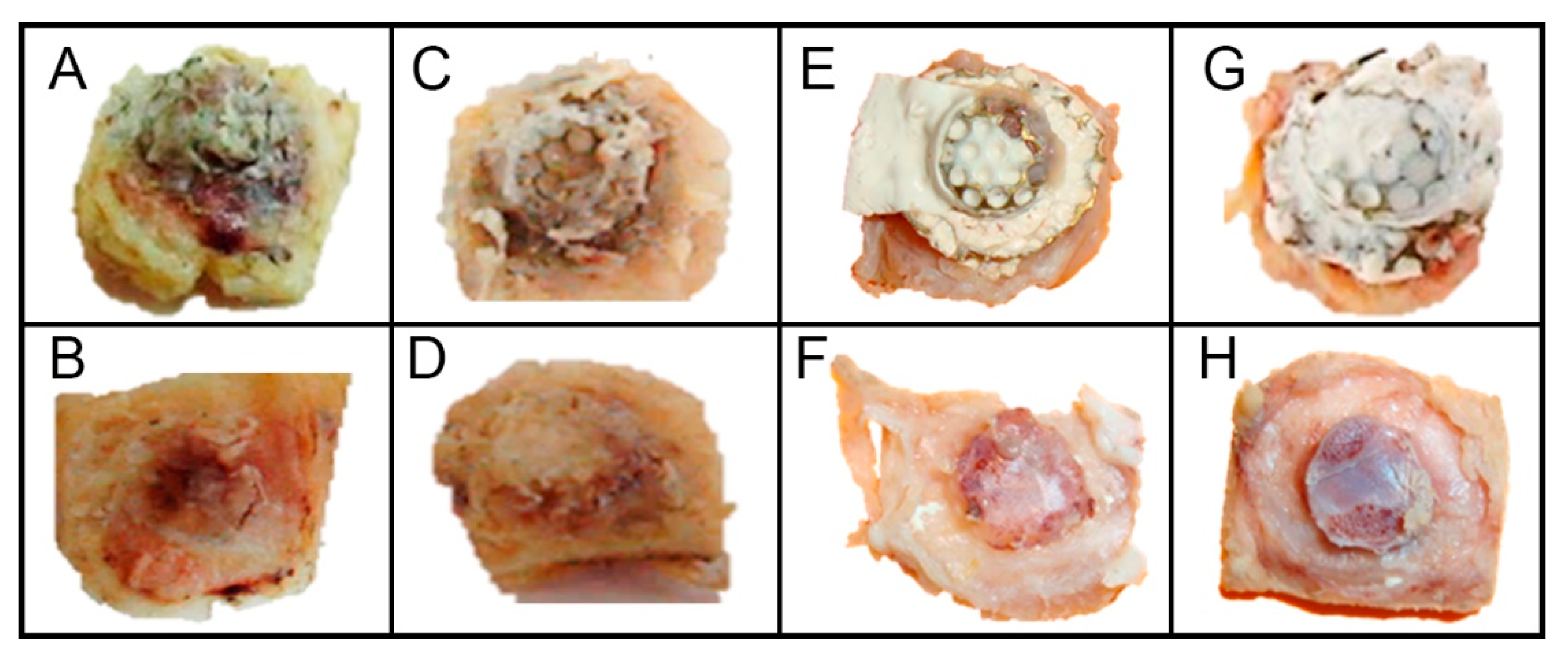
3.1. Macroscopic Analysis
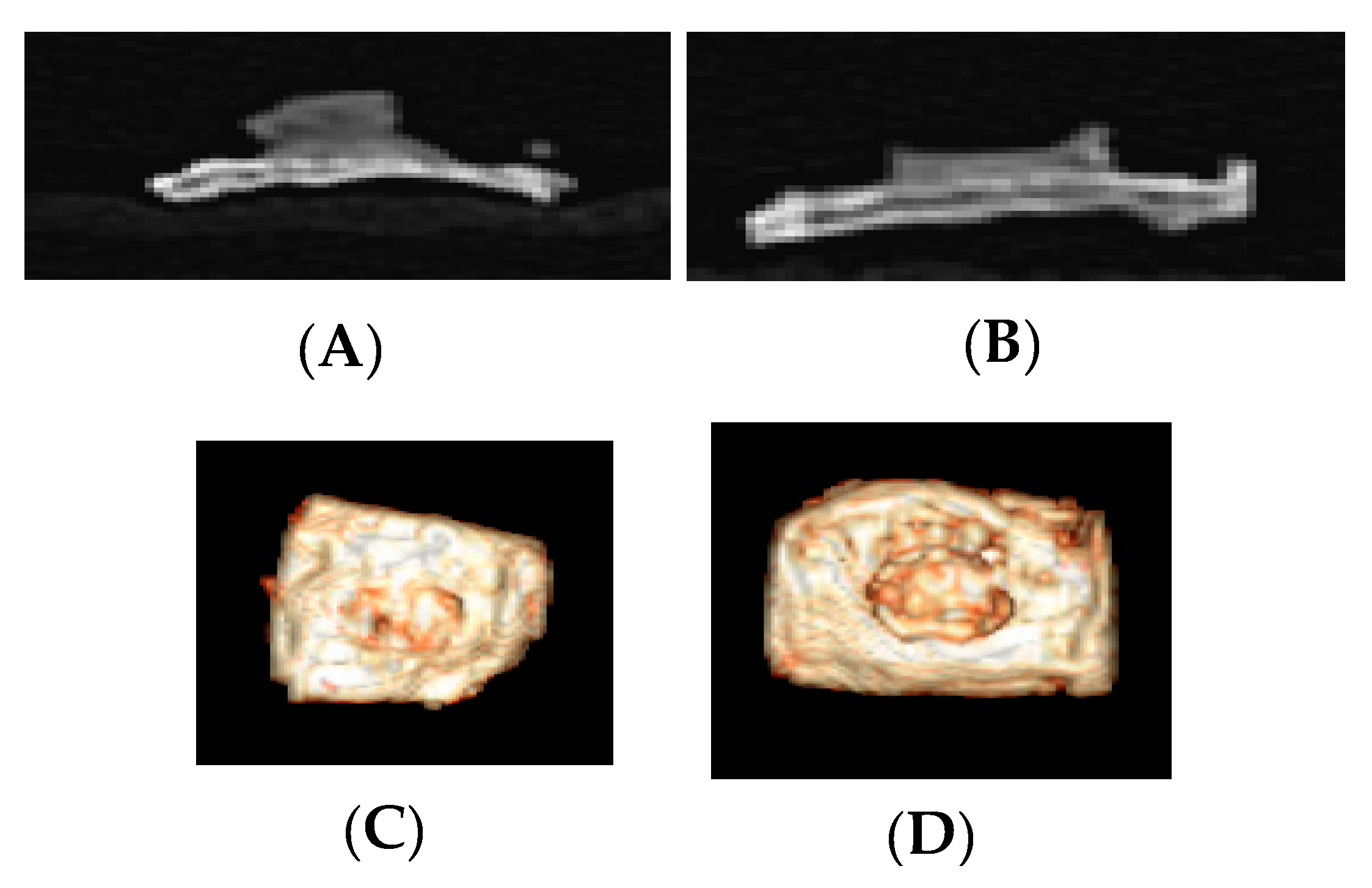
3.2. Computed Tomography Images
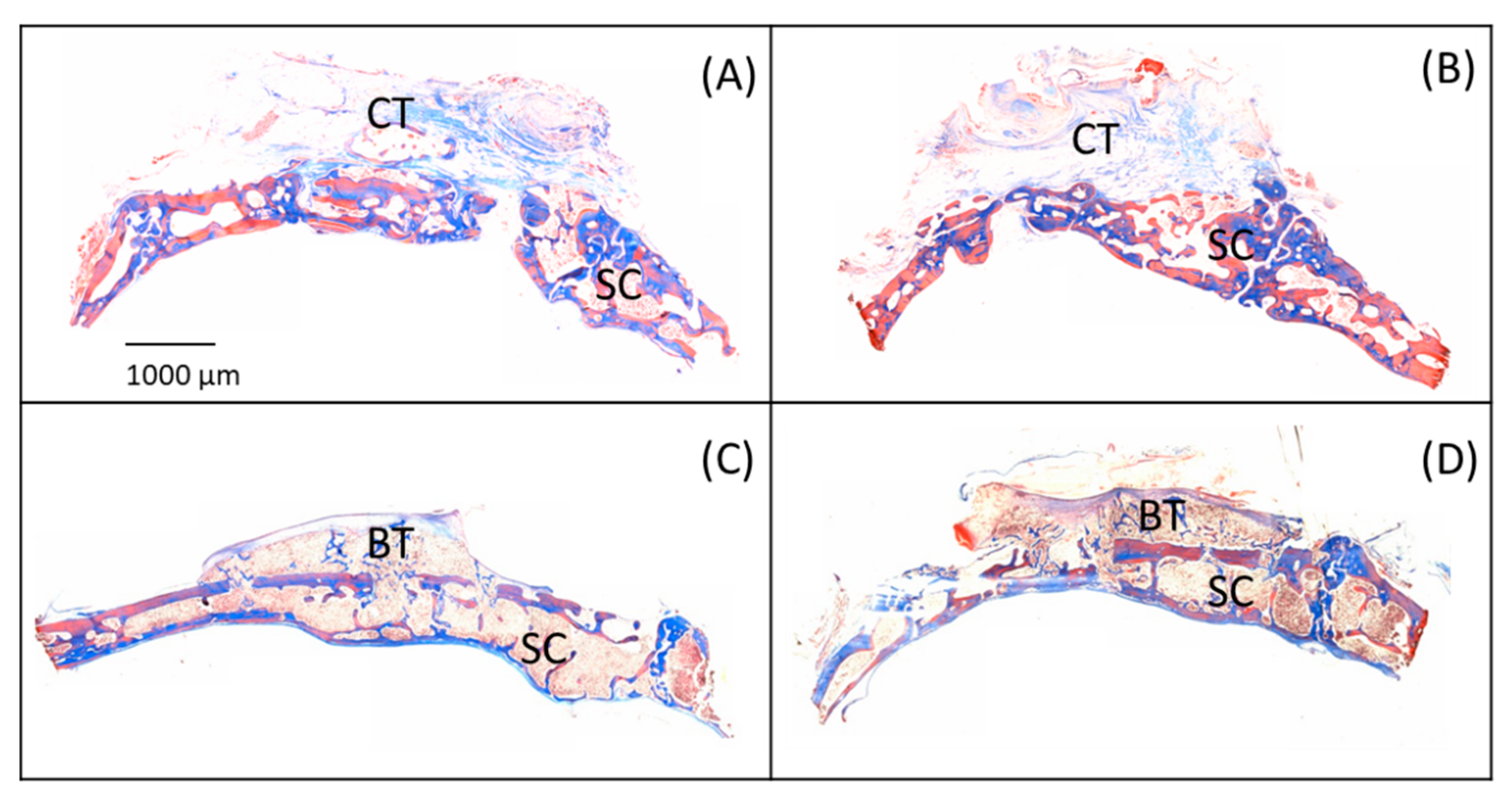
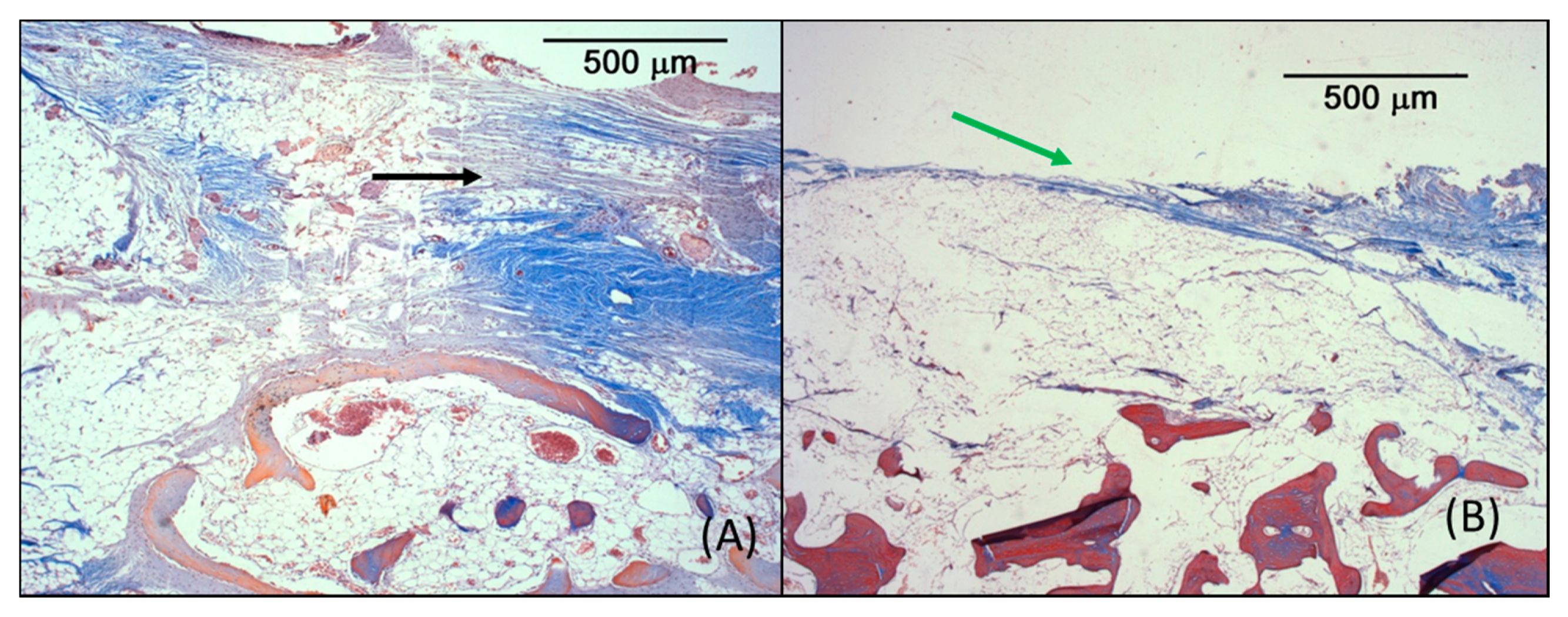
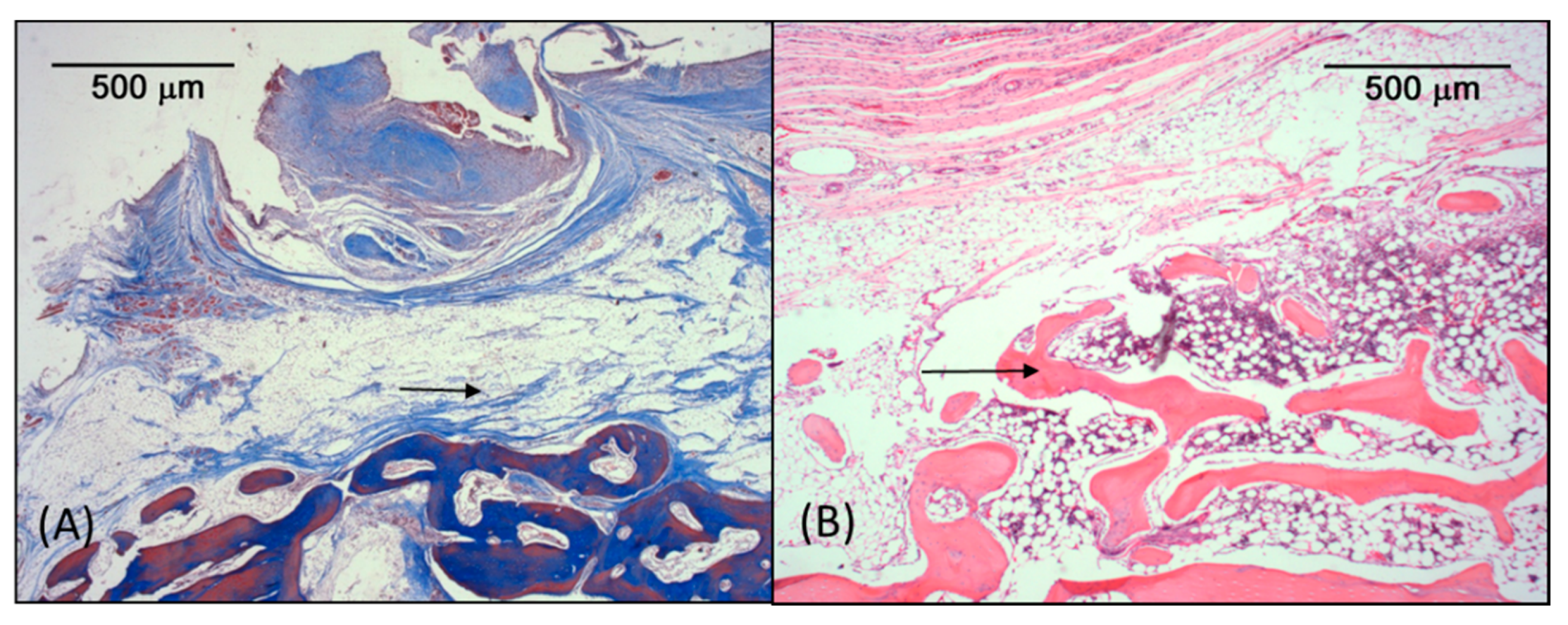
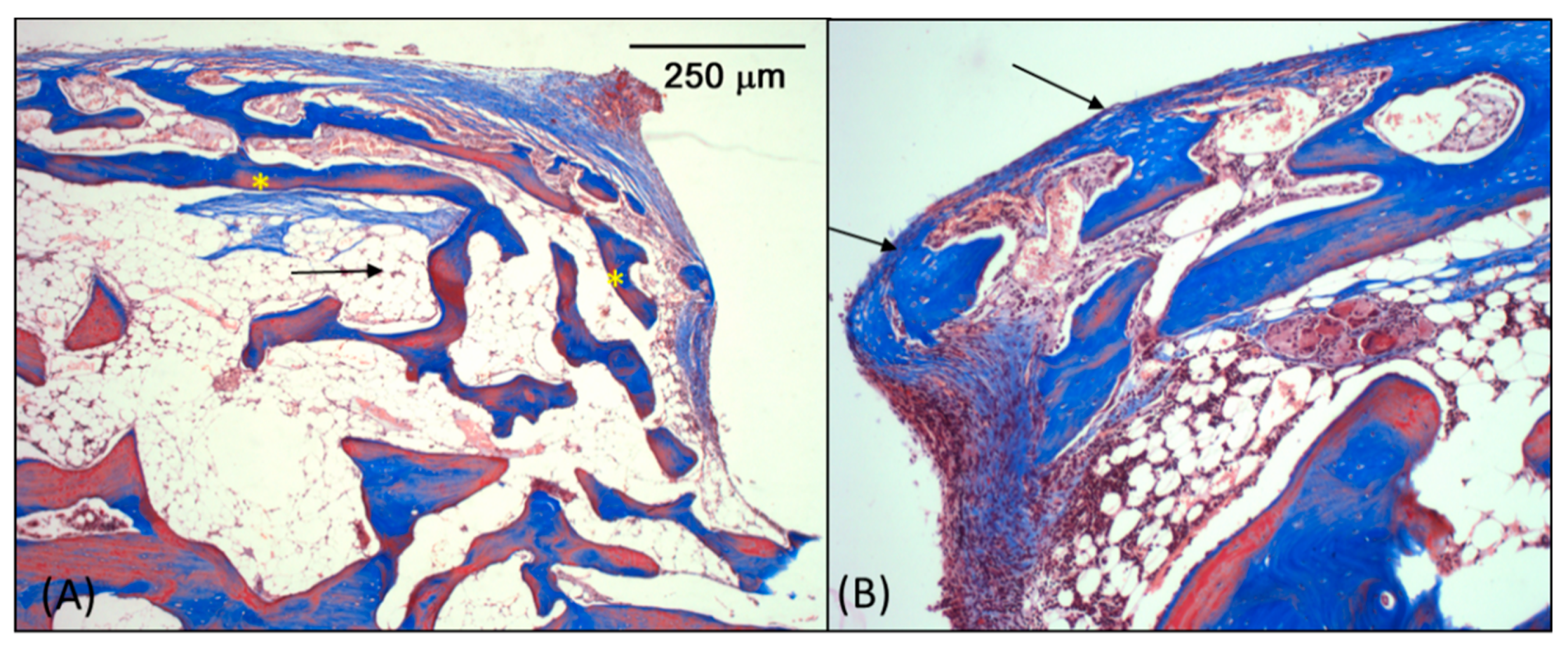
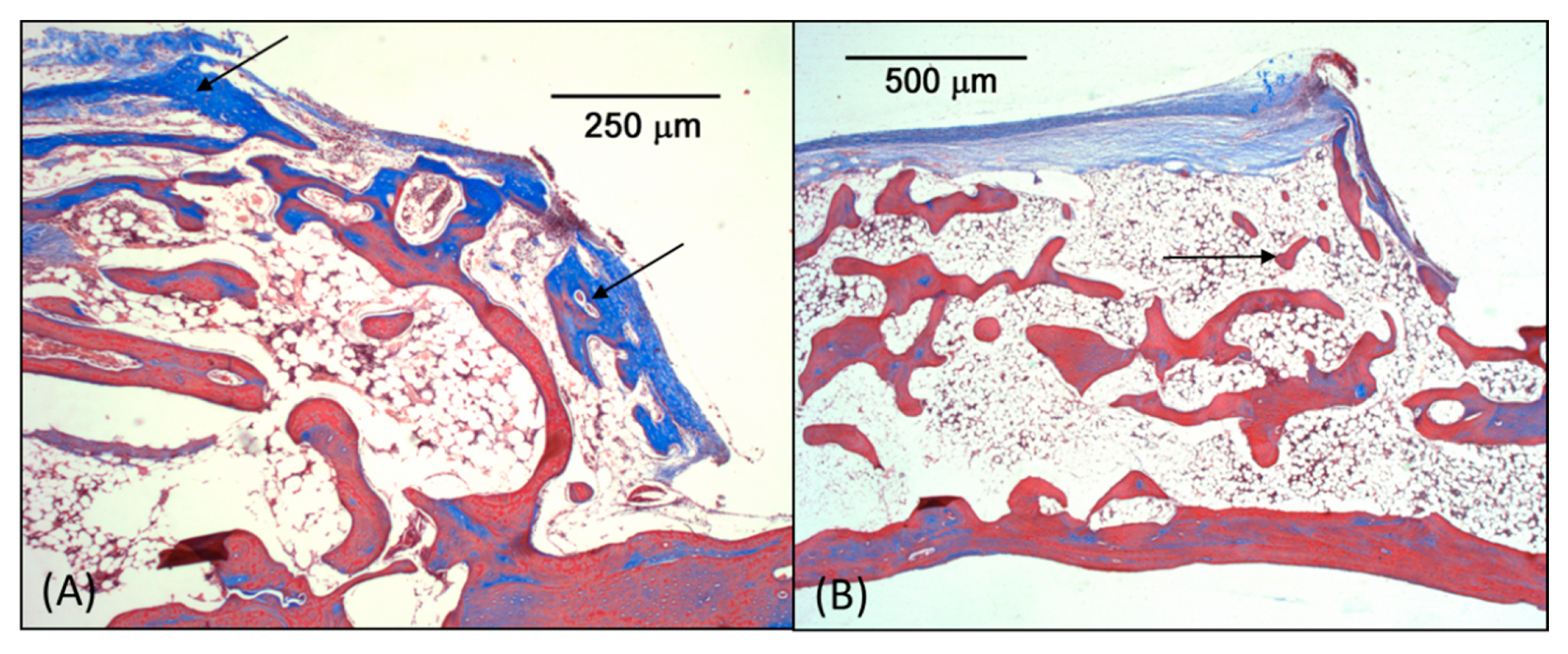
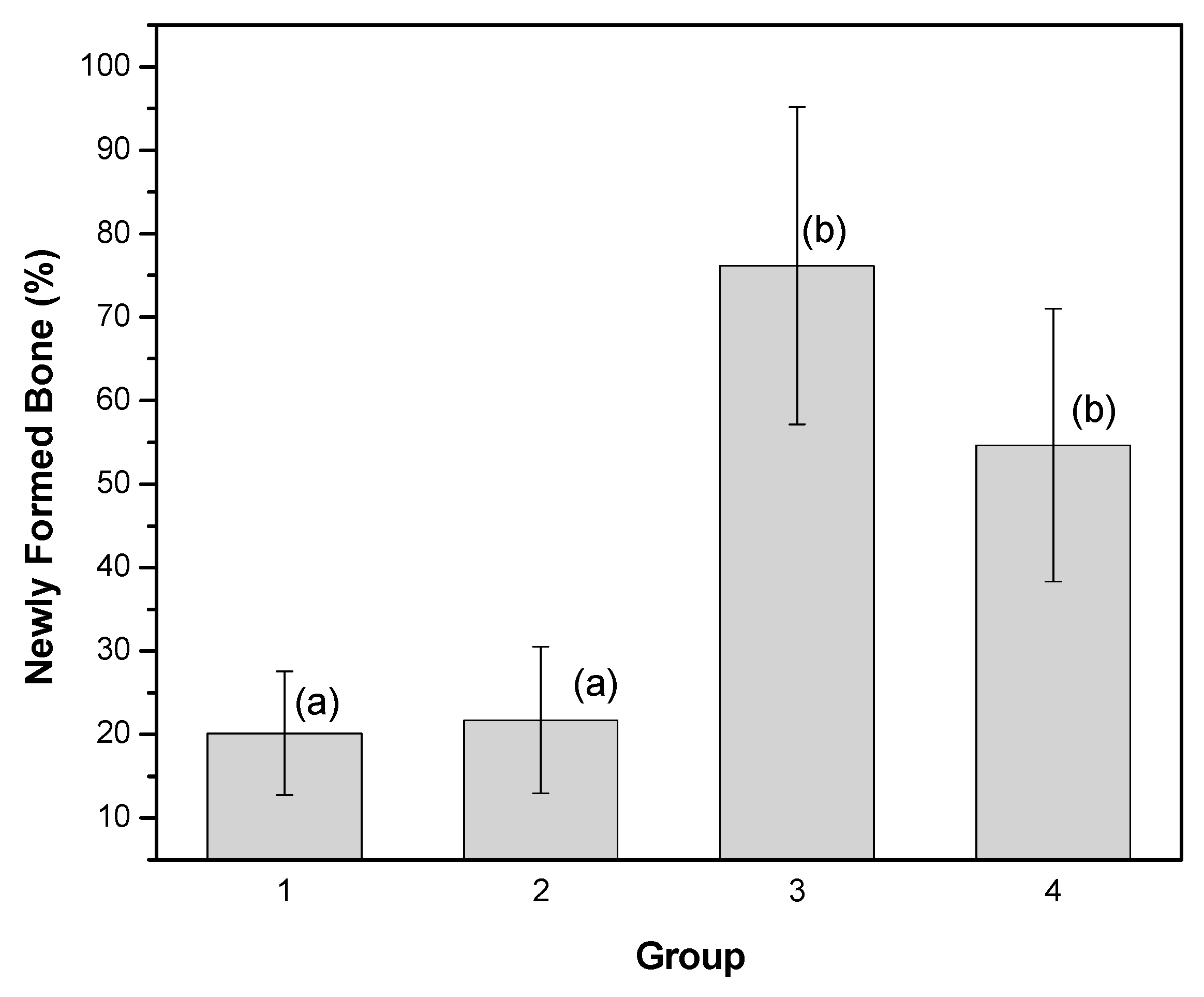
3.3. Microscopic Analysis
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Avila-Ortiz, G.; Bartold, M.; Giannobile, W.; Katagiri, W.; Nares, S.; Rios, H.; Spagnoli, D.; Wikesjö, U.M.E. Biologics and cell therapy tissue engineering approaches for the management of the edentulous maxilla: A systematic review. Int. J. Oral Maxillofac. Implant. 2016, 31, s121–s164. [Google Scholar] [CrossRef]
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buser, D. Guided Bone Regeneration in Implant Dentistry, 2nd ed.; Quintessence: Chicago, IL, USA, 2009; ISBN 0867152494. [Google Scholar]
- Roccuzzo, M.; Savoini, M.; Dalmasso, P.; Ramieri, G. Long-term outcomes of implants placed after vertical alveolar ridge augmentation in partially edentulous patients: A 10-year prospective clinical study. Clin. Oral Implant. Res. 2017, 28, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Ding, L.; Kasugai, S. Effect of doxycycline doped bone substitute on vertical bone augmentation on rat calvaria. Dent. Mater. J. 2019, 38, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misch, C.M.; Jensen, O.T.; Pikos, M.A.; Malmquist, J.P. Vertical bone augmentation using recombinant bone morphogenetic protein, mineralized bone allograft, and titanium mesh: A retrospective cone beam computed tomography study. Int. J. Oral Maxillofac. Implant. 2015, 30, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Carrera-Arrabal, T.; Calvo-Guirado, J.L.; Passador-Santos, F.; Sorgi da Costa, C.E.; Teles Costa, F.R.; Aloise, A.C.; Napimoga, M.H.; Aragoneses, J.M.; Pelegrine, A.A. Vertical bone construction with bone marrow-derived and adipose tissue-derived stem cells. Symmetry 2019, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Dupoirieux, L.; Pourquier, D.; Picot, M.; Neves, M. Comparative study of three different membranes for guided bone regeneration of rat cranial defects. Int. J. Oral Maxillofac. Surg. 2001, 30, 58–62. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.G.; Kowolik, M.J.; Janowski, G.M.; Lafayette, W. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Wöltje, M.; Brünler, R.; Böbel, M.; Ernst, S.; Neuss, S.; Aibibu, D.; Cherif, C. Functionalization of silk fibers by PDGF and bioceramics for bone tissue regeneration. Coatings 2020, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, R.J.; Maurício, V.B.; Teixeira, L.d.B.; Lachat, J.J.; Coutinho-Netto, J. Increased vascular permeability, angiogenesis and wound healing induced by the serum of natural latex of the rubber tree Hevea brasiliensis. Phytother. Res. 2010, 24, 8–764. [Google Scholar] [CrossRef]
- Kerche-silva, L.E.; Gomes, D.; Morais, S.; Danna, C.S.; Gomes, A.S.; Moratto, I.; Cecchini, A.L.; Yoshihara, E.; Job, A.E. Free-radical Scavenging properties and cytotoxic activity evaluation of latex C-serum from hevea brasiliensis RRIM 600. Free Radic. Antioxid. 2017, 7, 107–114. [Google Scholar] [CrossRef]
- Ereno, C.; Guimarães, S.A.C.; Pasetto, S.; Herculano, R.D.; Silva, C.P.; Graeff, C.F.O.; Tavano, O.; Baffa, O.; Kinoshita, A. Latex use as an occlusive membrane for guided bone regeneration. J. Biomed. Mater. Res. Part A 2010, 95A, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.M.L.; Ferreira, J.F.; Marques, L.; Holgado, L.; Graeff, C.F.O.; Kinoshita, A. Comparison of the performance of natural latex membranes prepared with different procedures and PTFE membrane in guided bone regeneration (GBR) in rabbits. J. Mater. Sci. Mater. Med. 2014, 25, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Herculano, R.D.; Silva, C.P.; Ereno, C.; Guimaraes, S.A.C.; Kinoshita, A.; Graeff, C.F.d.O. Natural rubber latex used as drug delivery system in guided bone regeneration (GBR). Mater. Res. 2009, 12, 253–256. [Google Scholar] [CrossRef] [Green Version]
- Herculano, R.D.; Alencar de Queiroz, A.A.; Kinoshita, A.; Oliveira, O.N.; Graeff, C.F.O. On the release of metronidazole from natural rubber latex membranes. Mater. Sci. Eng. C 2011, 31, 272–275. [Google Scholar] [CrossRef]
- Herculano, R.D.; Guimarães, S.A.C.; Belmonte, G.C.; Duarte, M.A.H.; de Oliveira, O.N.; Kinoshita, A.; Graeff, C.F.d.O. Metronidazole release using natural rubber latex as matrix. Mater. Res. 2010, 13, 57–61. [Google Scholar] [CrossRef]
- de Barros, N.R.; Miranda, M.C.R.; Borges, F.A.; Gemeinder, J.L.P.; de Mendonça, R.J.; Cilli, E.M.; Herculano, R.D. Natural rubber latex: Development and in vitro characterization of a future transdermal patch for enuresis treatment. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Almeida, G.F.B.; Cardoso, M.R.; Zancanela, D.C.; Bernardes, L.L.; Norberto, A.M.Q.; Barros, N.R.; Paulino, C.G.; Chagas, A.L.D.; Herculano, R.D.; Mendonça, C.R. Controlled drug delivery system by fs-laser micromachined biocompatible rubber latex membranes. Appl. Surf. Sci. 2020, 506, 144762. [Google Scholar] [CrossRef]
- Guidelli, É.J.; Kinoshita, A.; Ramos, A.P.; Baffa, O.; Guidelli, E.J.; Kinoshita, A.; Ramos, A.P.; Baffa, O. Silver nanoparticles delivery system based on natural rubber latex membranes. J. Nanoparticle Res. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Marques, L.; Martinez, G.; Guidelli, É.; Tamashiro, J.; Segato, R.; Payão, S.L.M.; Baffa, O.; Kinoshita, A. Performance on bone regeneration of a silver nanoparticle delivery system based on natural rubber membrane NRL-AgNP. Coatings 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Fukase, Y.; Goke, E.; Yamada, Y.; Sato, S.; Nishiyama, M.; Ito, K. Three-dimensional evaluation for augmented bone using guided bone regeneration. J. Periodontal Res. 2005, 40, 269–276. [Google Scholar] [CrossRef]
- Min, S.; Sato, S.; Murai, M.; Okuno, K.; Fujisaki, Y.; Yamada, Y.; Ito, K. Effects of marrow penetration on bone augmentation within a titanium cap in rabbit calvarium. J. Periodontol. 2007, 78, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Sato, S.; Saito, M.; Ebihara, H.; Arai, Y.; Ito, K. Micro–computerized tomography analysis: Dynamics of bone augmentation within a titanium cap in rabbit calvarium. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Vignoletti, F. Key aspects on the use of bone substitutes for bone regeneration of edentulous ridges. Dent. Mater. 2015, 31, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Arrington, E.D.; Smith, W.J.; Chambers, H.G.; Bucknell, A.L.; Davino, N.A. Complications of iliac crest bone graft harvesting. Clin. Orthop. Relat. Res. 1996, 329, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 1985; Volume 23–86. [Google Scholar]
- Khojasteh, A.; Kheiri, L.; Motamedian, S.R.; Khoshkam, V. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann. Maxillofac. Surg. 2017, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Moest, T.; Frabschka, J.; Kesting, M.R.; Schmitt, C.M.; Frohwitter, G.; Lutz, R.; Schlegel, K.A. Osseous ingrowth in allogeneic bone blocks applied for vertical bone augmentation: A preclinical randomised controlled study. Clin. Oral Investig. 2019, 1–13. [Google Scholar] [CrossRef]
- Balabanian, C.A.C.A.; Coutinho-Netto, J.; Lamano-Carvalho, T.L.; Lacerda, S.A.; Brentegani, L.G. Biocompatibility of natural latex implanted into dental alveolus of rats. J. Oral Sci. 2006, 48, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Floriano, J.; Mota, L.; Furtado, E.; Rossetto, V.; Graeff, C.O. Biocompatibility studies of natural rubber latex from different tree clones and collection methods. J. Mater. Sci. Mater. Med. 2014, 25, 461–470. [Google Scholar] [CrossRef]
- Louis, P.J. Vertical ridge augmentation using titanium mesh. Oral Maxillofac. Surg. Clin. 2010, 22, 353–368. [Google Scholar] [CrossRef]
- Yamada, Y.; Nanba, K.; Ito, K. Effects of occlusiveness of a titanium cap on bone generation beyond the skeletal envelope in the rabbit calvarium. Clin. Oral Implant. Res. 2003, 14, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, A.K.; Lundgren, D.; Hämmerle, C.H.; Nyman, S.; Sennerby, L. Influence of decortication of the donor bone on guided bone augmentation. An experimental study in the rabbit skull bone. Clin. Oral Implant. Res. 2000, 11, 99–106. [Google Scholar] [CrossRef]
- Lundgren, D.; Slotte, C.; Lundgren, D.; Sennerby, L. Bone morphology and vascularization of untreated and guided bone augmentation-treated rabbit calvaria: Evaluation of an augmentation model. Clin. Oral Implant. Res. 2005, 16, 228–235. [Google Scholar] [CrossRef]
- Miyamoto, I.; Tsuboi, Y.; Takahashi, K.; Hyon, S.-H.; Iizuka, T. Enhancement of bone volume in guided bone augmentation by cell transplants derived from periosteum: An experimental study in rabbit calvarium bone. Clin. Oral Implant. Res. 2004, 15, 308–314. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pons, D.K.; Eleuterio, R.G.; Paiva, F.F.G.; Holgado, L.A.; Marques, L.; Consolaro, A.; Graeff, C.F.O.; Baffa, O.; Kinoshita, A. Vertical Guided Bone Augmentation Using Titanium Mesh Domes Coated with Natural Latex Extracted from Hevea brasiliensis. Coatings 2020, 10, 595. https://doi.org/10.3390/coatings10060595
Pons DK, Eleuterio RG, Paiva FFG, Holgado LA, Marques L, Consolaro A, Graeff CFO, Baffa O, Kinoshita A. Vertical Guided Bone Augmentation Using Titanium Mesh Domes Coated with Natural Latex Extracted from Hevea brasiliensis. Coatings. 2020; 10(6):595. https://doi.org/10.3390/coatings10060595
Chicago/Turabian StylePons, Diego K., Rachel G. Eleuterio, Fábio F. G. Paiva, Leandro A. Holgado, Leonardo Marques, Alberto Consolaro, Carlos F. O. Graeff, Oswaldo Baffa, and Angela Kinoshita. 2020. "Vertical Guided Bone Augmentation Using Titanium Mesh Domes Coated with Natural Latex Extracted from Hevea brasiliensis" Coatings 10, no. 6: 595. https://doi.org/10.3390/coatings10060595
APA StylePons, D. K., Eleuterio, R. G., Paiva, F. F. G., Holgado, L. A., Marques, L., Consolaro, A., Graeff, C. F. O., Baffa, O., & Kinoshita, A. (2020). Vertical Guided Bone Augmentation Using Titanium Mesh Domes Coated with Natural Latex Extracted from Hevea brasiliensis. Coatings, 10(6), 595. https://doi.org/10.3390/coatings10060595




