1. Introduction
The electroplating of zinc is an industrial process that is extensively used to protect steel surfaces and enhance their service life. This material is widely employed in the automotive industry to produce brake pipes, brake callipers and power steering components [
1]. Zinc electroplating is also used in the production of tanks, armoured personnel carriers and other heavy military vehicles [
2]. Steel articles coated by zinc exhibit better corrosion resistance as a result of sacrificial coating protection, known as white rust corrosion. The development of this white rust can be controlled by surface passivation [
3], where an additional barrier layer is created. The passivation is a process of dipping a material covered with zinc in a solution containing chromium compounds. The carcinogenic hexavalent chromium is still employed as a key compound in the passivation process. An alternative method of increasing the service life of zinc-coated materials is to include solid particles in a zinc matrix [
4,
5]. An application of inorganic or organic particles also creates opportunities for the multifunctional modification of zinc coatings. The addition of hard particles such as SiO
2, TiO
2 and CeO
2, improves coating hardness and wear resistance [
6]. Soft particles like PTFE, polystyrene or polymethylmethacrylate can equip zinc coatings with a low coefficient of friction or hydrophobic properties [
7]. However, the inclusion of microparticles not only provides various functional properties, but at the same time may cause porous structure and irregularities in the texture. In order to increase coating quality in terms of both anticorrosion and mechanical properties, the microparticles are replaced by nanoparticles. The nanoparticles fill voids in the zinc matrix, providing barrier properties by blocking active sites for corrosion initiation [
8]. In terms of enhancing mechanical properties, hard nanoparticles dispersed in the coating can hinder the easy movement of dislocations, thus increasing the coating’s hardness [
9]. To evaluate wear resistance, pin-on-disc and pin-on-flat tribological methods are the most widely used. Anticorrosion features are usually assessed by electrochemical techniques or a salt spray method.
In the present study, the tribological and corrosion behaviour of zinc and composite zinc coatings containing hard nanoparticles were evaluated. Besides traditional tribological methods, confocal laser scanning microscopy (CLSM) was used for the first time to characterise the metallic coatings. To our best knowledge, this was the first successful attempt to produce zinc nanocomposite coatings from commercially available products.
2. Materials and Methods
2.1. Sample Preparation
Austenitic steel 316L (
Table 1) was covered with plain zinc (Zn) and composite zinc coatings containing metal oxide hard nanoparticles (Zn/NP). Steel samples were prepared in the following steps: 1. sandpaper polishing (from 400 to 1200 grit), 2. rinsing, 3. degreasing with acetone, 4. alkaline cleaning, 5. rinsing, 6. acid pickling, and 7. rinsing. Coatings were prepared by electrodeposition from an acidic bath of 5 L, containing zinc sulphate as a source of zinc ions, provided by Gateros Plating Ltd. A bath temperature of 20 °C and current density of 2 A/dm
2 were applied. In the case of zinc nanocomposite coatings, the process parameters were kept the same and, to the 5 L of standard plating bath, 250 mL of nanoparticle-containing KA 201 additive provided by Coat-It Sp. z o. o. (Poland) was added. Applying the KA201 additive to prepare plating baths for nanocomposite coatings production allows for the uniform distribution of nanoparticles in a solution without intensive stirring and wetting agents. The coatings’ thickness of 20 ± 2 µm were analysed by a gravimetric method. The hardness, roughness, and corrosion and wear resistance of zinc and zinc nanocomposite coatings were tested.
2.2. Corrosion Tests
Corrosion resistance tests were performed using the PGP 201 potentiostat (Radiometer Analytical, Lyon, France) and the VoltaMaster 4 software. The measurements were carried out using a three-electrode system in a measuring vessel made of borosilicate glass filled with 70 cm3 of electrolyte (0.9% NaCl) at a temperature of 21 °C. A saturated calomel electrode of Hg/Hg2Cl2/Cl− REF421 (Radiometer Analytical, Loveland, CO, USA) was the reference electrode, the potential of which—relative to the normal hydrogen electrode—was +0.244 V. The auxiliary electrode was an XM 140 platinum electrode (Radiometer Analytical, USA) with an area of 8mm × 8 mm, and the working electrode was a sample. Cylinder-shaped samples with a diameter of 8 mm were placed in a specially constructed holder that enabled the contact of the sample with the electrolyte from the frontal surface only and simultaneously allowed the sample to connect with the potentiostat. Tests for pitting corrosion resistance began with the determination of the open circuit potential (OCP, EOCP). Then, the research was carried out using the potentiodynamic method, from the potential Einit = EOCP − 100 mV. The change in potential occurred in the anode direction at a rate of 3 mV/s. After the maximum value of the measuring range +2000 mV was reached or anode current density of 1 mA/cm2 was obtained, the polarization direction was changed. All tests were repeated three times.
2.3. Tribological Tests
Tribological studies were carried out using a UMT TriboLab tribometer (Bruker, Billerica, MA, USA), with a ball-on-disc system under dry friction conditions. The duration of each measurement was 300 s. A 6 mm immobile ball made of corundum (Al2O3) was a counterbody. Cylindrical samples, with a diameter of 8 mm, were placed in a special holder attached to the table performing reciprocating motion. The amplitude of the system displacements was 500 μm. Each measurement was repeated three times. Abrasion resistance of the coatings was tested with the following parameters:
Normal force (Fn) = 1 N, frequency (f) = 5 Hz;
Normal force (Fn) = 2 N, frequency (f) = 10 Hz.
2.4. Microscopy Analysis
The surfaces of the samples after tribological tests were subjected to microscopic observations using the LEXT OLS4000 confocal microscope (CLSM, Olympus, Tokyo, Japan) with 3D image feature and the scanning electron microscope (SEM) Phenom XL (Phenom-World, Eindhoven, The Netherlands) with the EDS X-ray microanalysis module.
Coating roughness (
Ra) measurements were made using a LEXT OLS400 confocal microscope. The 3D imaging feature of CLSM was used to measure the volume and depth of wear tracks. The details of the measurement method were described in our previous publication [
10]. Microhardness tests were carried out using the Vickers method with a load of 0.9807 N. For statistical purposes, each measurement was repeated ten times.
3. Results and Discussion
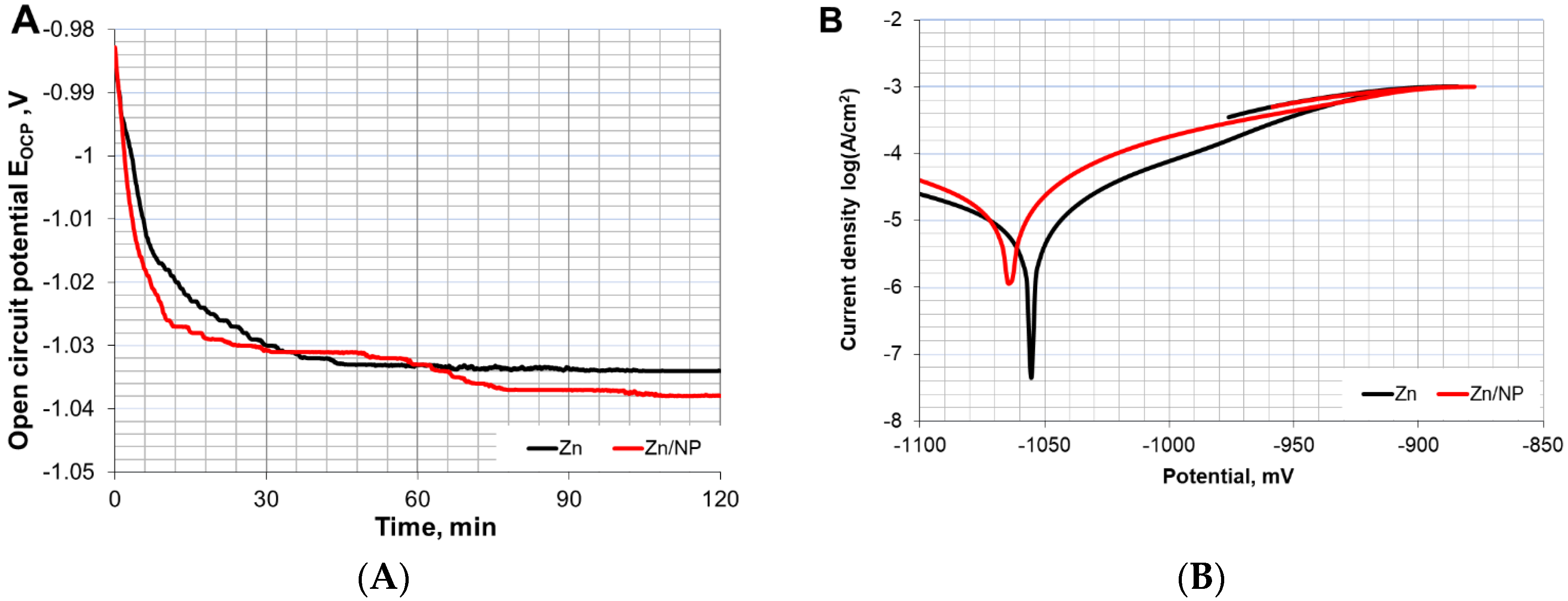
The coatings were successfully produced from a commercially available zinc bath and nanoparticle-containing additive. The results of the corrosion resistance tests of the pure zinc and Zn/NP coatings are presented in
Figure 1 and summarised in
Table 2.
Low open-circuit potential values suggest that sample surfaces are particularly susceptible to oxidation processes. This is usual for zinc layers, which act as sacrificial coatings. The corrosion resistance of both coatings is very similar, as samples with both Zn and Zn/NP coating were characterized by similar values of corrosion potential (E
corr) and corrosion current density (I
corr), as shown in
Table 2. This indicates that the addition of nanoparticles to the metal matrix does not compromise the anticorrosion properties as may happen in the case of microparticles [
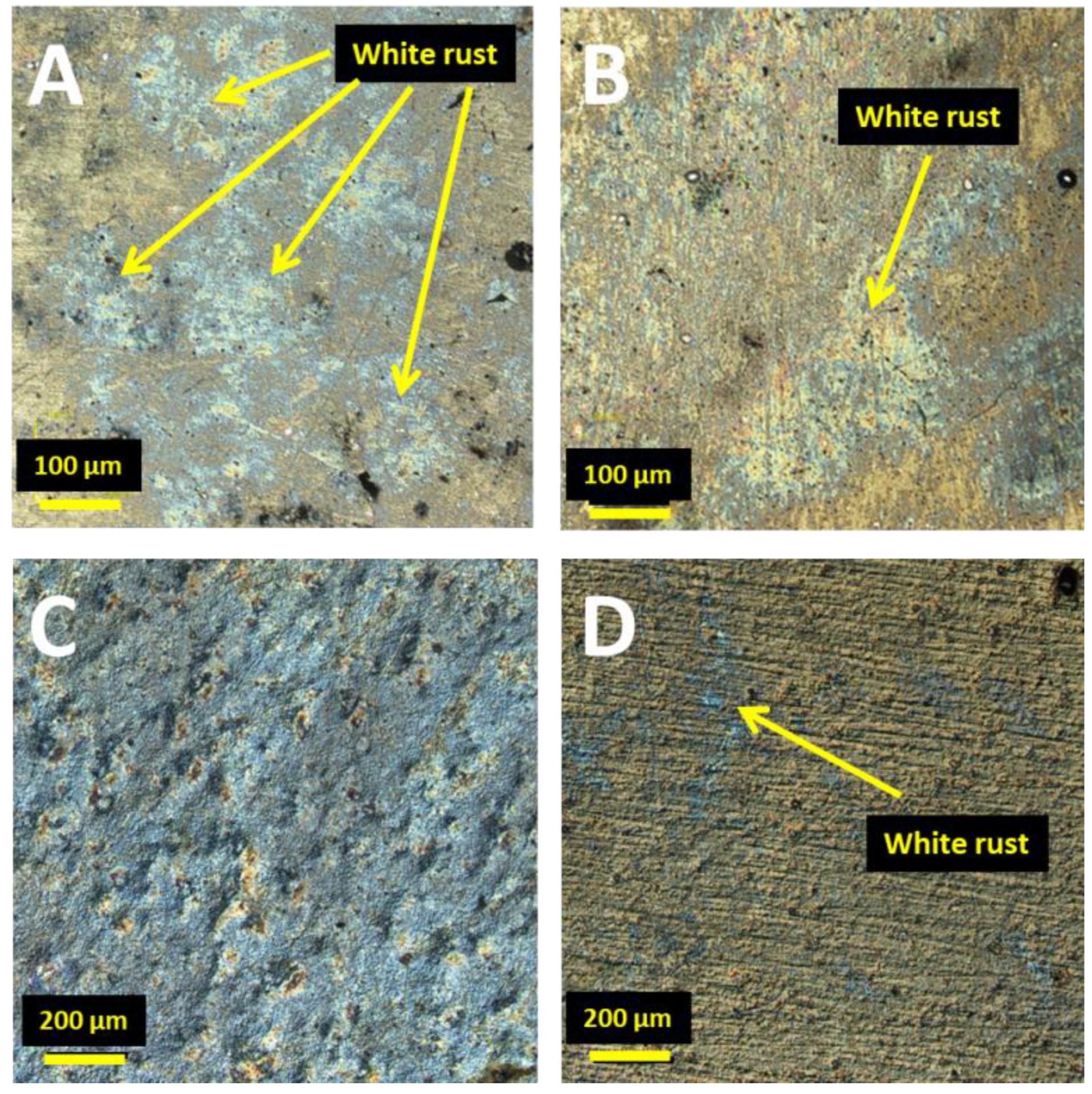
11]. The microscopic images of the surfaces of both coatings show numerous instances of corrosion discoloration, resulting from the area oxidation of the surface layer (
Figure 2). However, further comparison of these images (
Figure 2A,B) clearly reveals white rust on the Zn coatings. The corrosion products of Zn/NP coatings are much less visible. This indicates that the inclusion of nanoparticles can slow down the corrosion process. The delay of white rust formation is the result of the enhanced barrier properties of nanocomposite coatings compared to pure zinc coatings.
Furthermore, coated samples were stored for six months in a dry room at a constant temperature of 19–22 °C. The microscopic analysis presented in
Figure 2C showed that Zn coatings were all covered with white rust. For Zn/NP composite coatings, the white rust phenomenon was much smaller and occurred locally (
Figure 2D).
Analysis of resistance to motion after 300 s of friction shows that the kinematic pairs of Zn-Al
2O
3, under non-lubricated conditions, were characterized by slightly higher friction coefficients at both 1 N and 2 N (
Table 3). Lowering of the friction coefficient after adding the hard nanoparticles to the metallic coating was previously observed [
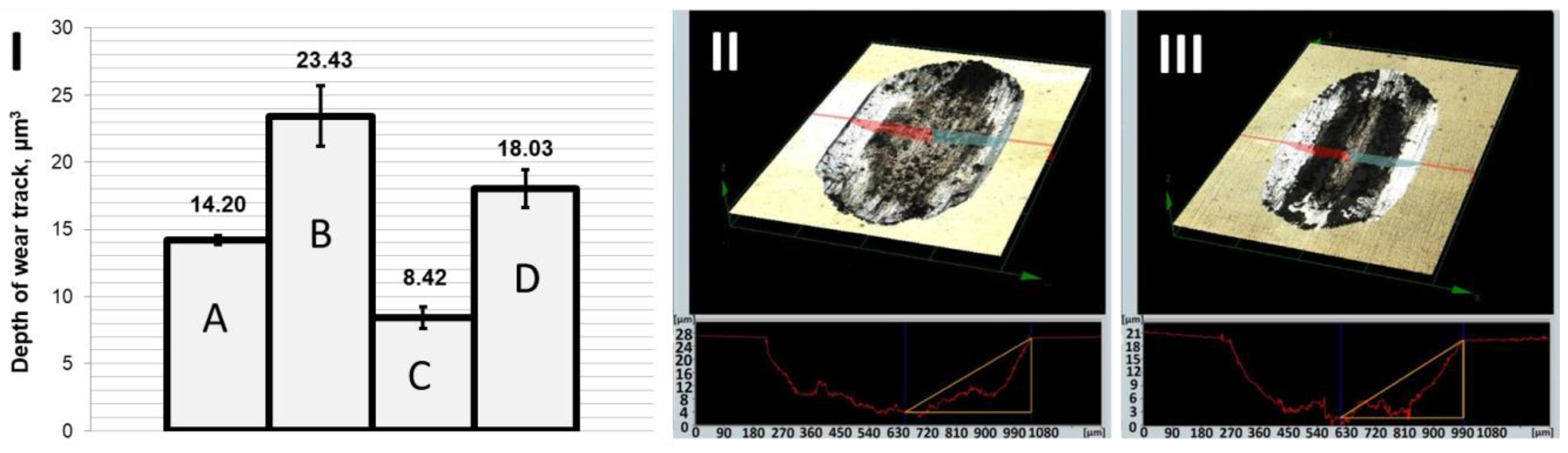
12]. Increased resistance to motion resulted in deeper friction marks (
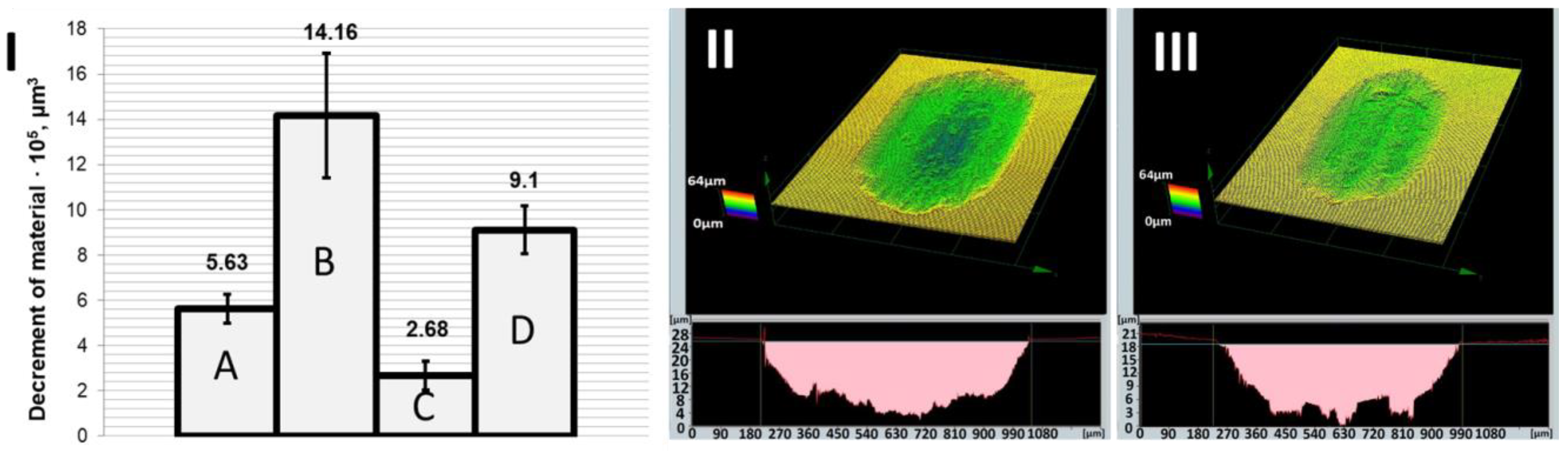
Figure 3) and intensification of tribological wear (
Figure 4). Comparison of the depth and volume of friction marks clearly indicates that the wear resistance of the pure Zn coating was rather weak. Significantly better tribological resistance was obtained when hard nanoparticles were incorporated into the zinc matrix (Zn/NP coatings). This may be due to the increased microhardness of Zn/NP coatings, which was about 12 HV higher than the Zn coatings. Additionally, it is suspected that during the friction process, the hard nanoparticles gradually protrude out of the zinc matrix and carry the load applied to the coating, resulting in the improved wear resistance of the nanocomposite coating. Roughness tests also indicated that Zn/NP coatings were characterized by a higher roughness Ra (0.155 ± 0.010 µm), which may have a slight impact on the higher resistance of motion in the first friction cycles. The results of roughness and microhardness measurements are presented in
Table 4. The increase of around 9% is a significant improvement in future applications of the nanocomposite coatings at an industrial scale. Images of selected friction traces are shown in
Figure 5.
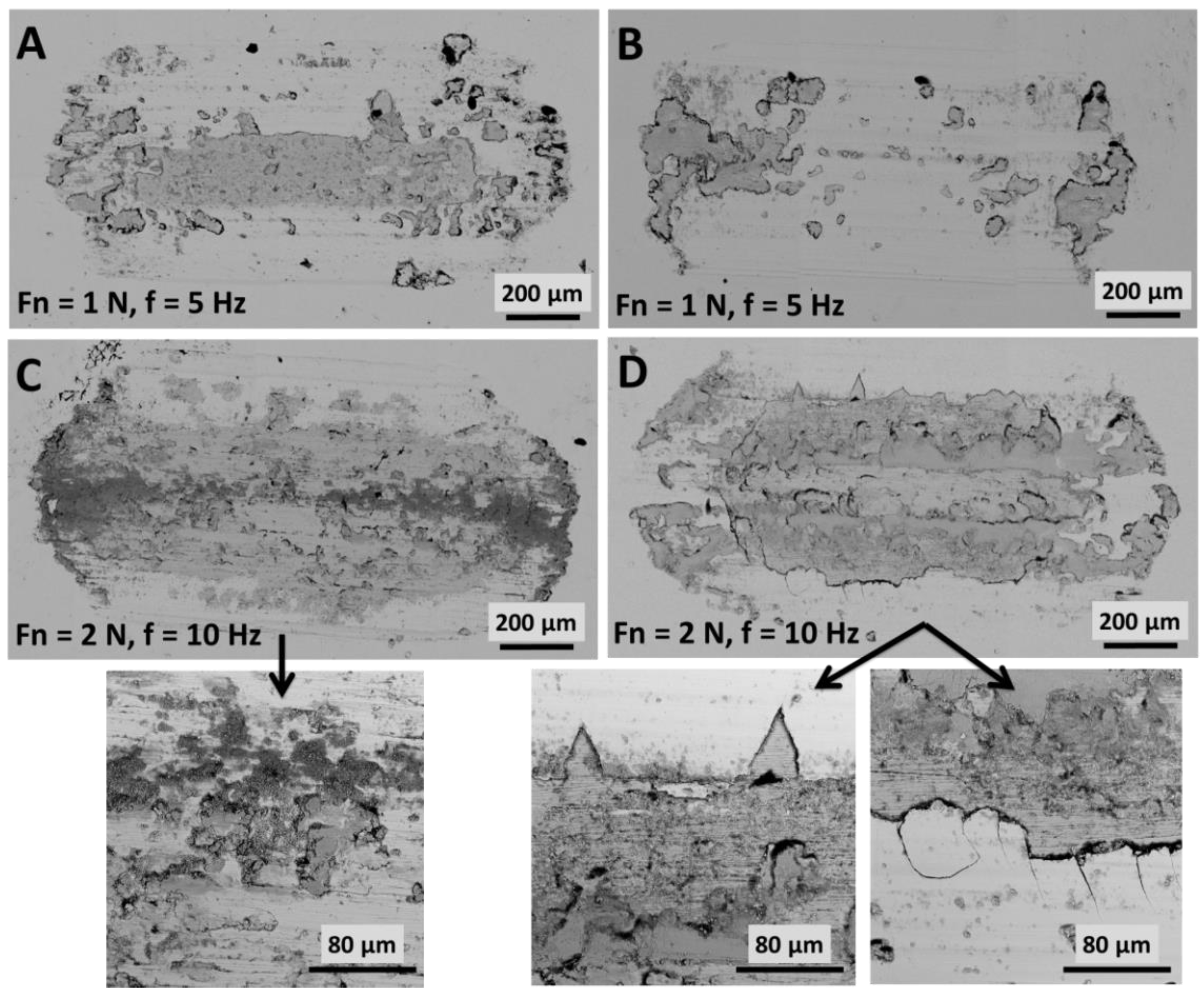
The microscopic analysis of friction marks presented in
Figure 5 shows that wear debris accumulated on the surface as a result of sliding. Therefore, remaining in the wear area, these scraps led to intensification of second wear processes. A larger amount of wear products was generated for Zn coatings, which is particularly evident in the photos taken with the SEM technique (
Figure 5A,C). It should be noted that the higher hardness of Zn/NP coatings affected their brittleness. At the edges of contact of the surface with the alumina ball, delamination, cracking and chipping of the applied coating occurred, especially in the case of samples subjected to friction processes at normal force Fn = 2 N and frequency f = 10 Hz (
Figure 5D). The abrasion of Zn coatings occurred rather uniformly (
Figure 5C).
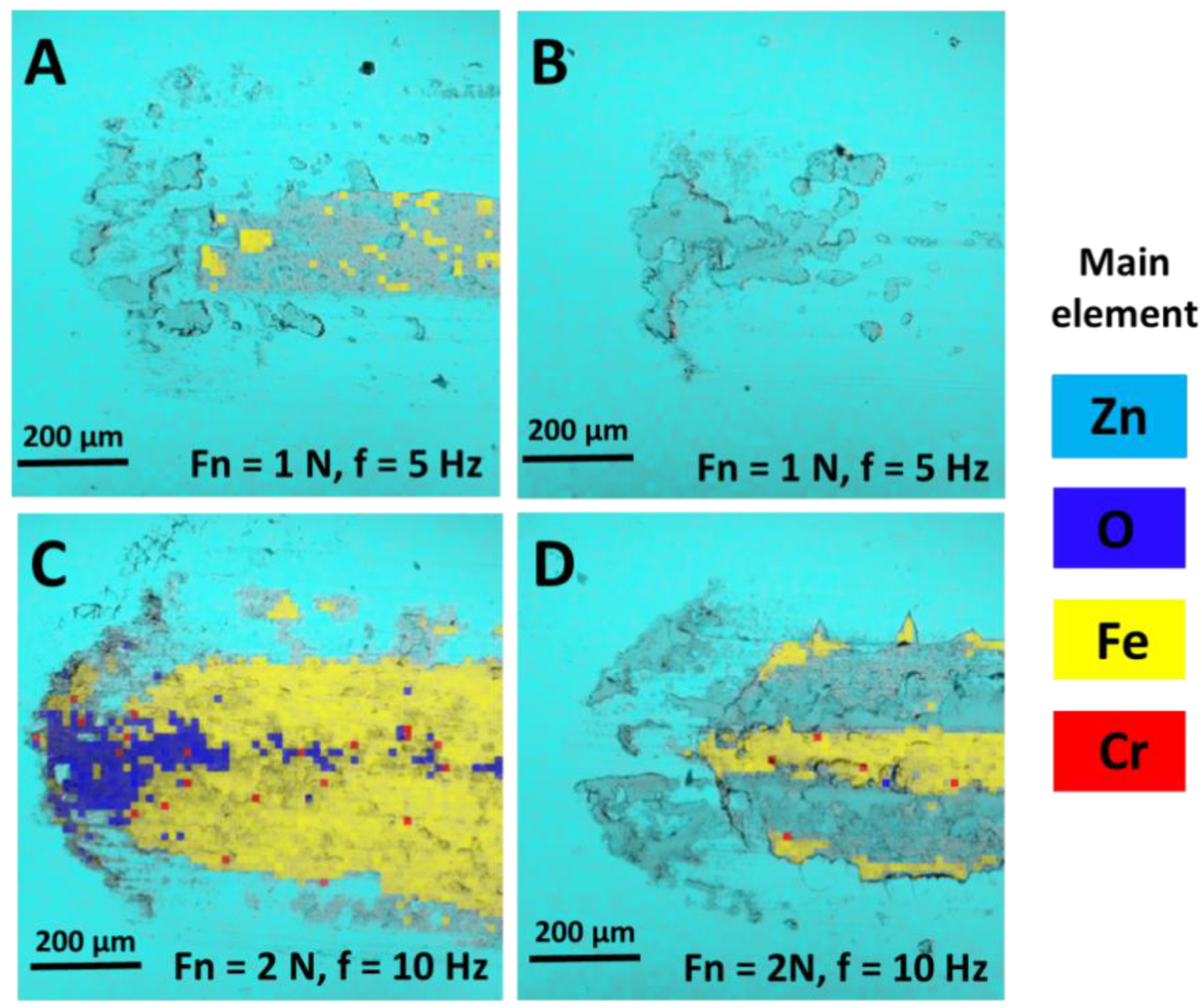
Traces of friction were also tested with energy dispersive X-ray spectroscopy (EDS). Selected test results are presented in
Figure 6.
X-ray analysis of the elemental composition of the surfaces of samples subjected to friction processes confirms the increased tribological strength of Zn/NP coatings. At lower unit loads at the friction site, the coating core was not exposed (
Figure 6B). With similar friction parameters for Zn coatings, places where iron quantitatively predominated were visible (
Figure 6A), which suggests a gradual exposure of the material core. The increase in unit load and the friction frequency intensified tribological wear processes, which was the highest for samples with Zn coating. EDS analysis has shown that wear products consisting mainly of oxygen and iron were moved to the edges of the friction zone (
Figure 6C). The amount of collected particles was smaller for samples with Zn/NP coatings (
Figure 6D).