Biofunctionalization of Textile Materials. 3. Fabrication of Poly(lactide)-Potassium Iodide Composites with Antifungal Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Fungal Strains
2.2. Methods
2.2.1. Poly(lactide) Nonwoven Fabrics
Melt-Blown Technique
2.2.2. Dip-Coating Modification
2.3. Instrumental Methods
2.3.1. SEM/EDS—Scanning Electron Microscopy/Energy-Dispersive X-ray Spectroscopy
2.3.2. ATR-FTIR—Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy
2.3.3. UV-VIS Analysis
2.3.4. Filtration Parameters
2.3.5. Tensile Testing
2.3.6. Antifungal Activity
3. Results and Discussion
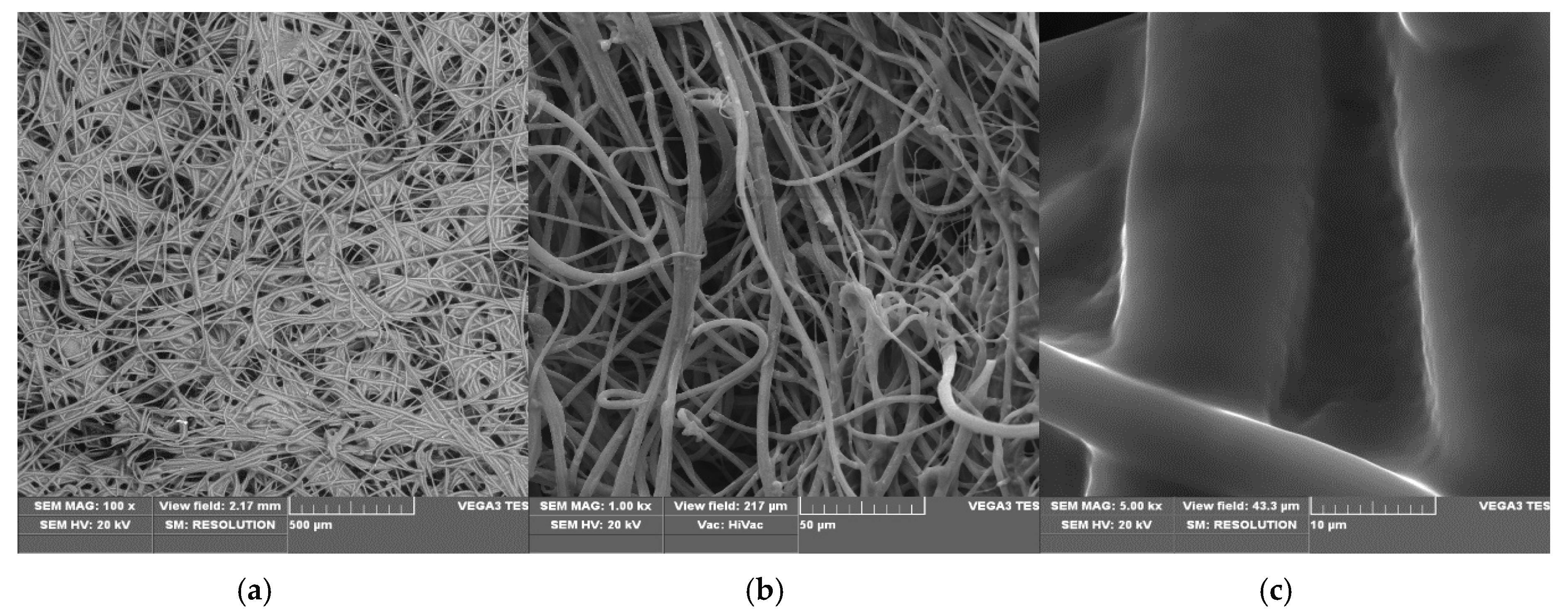
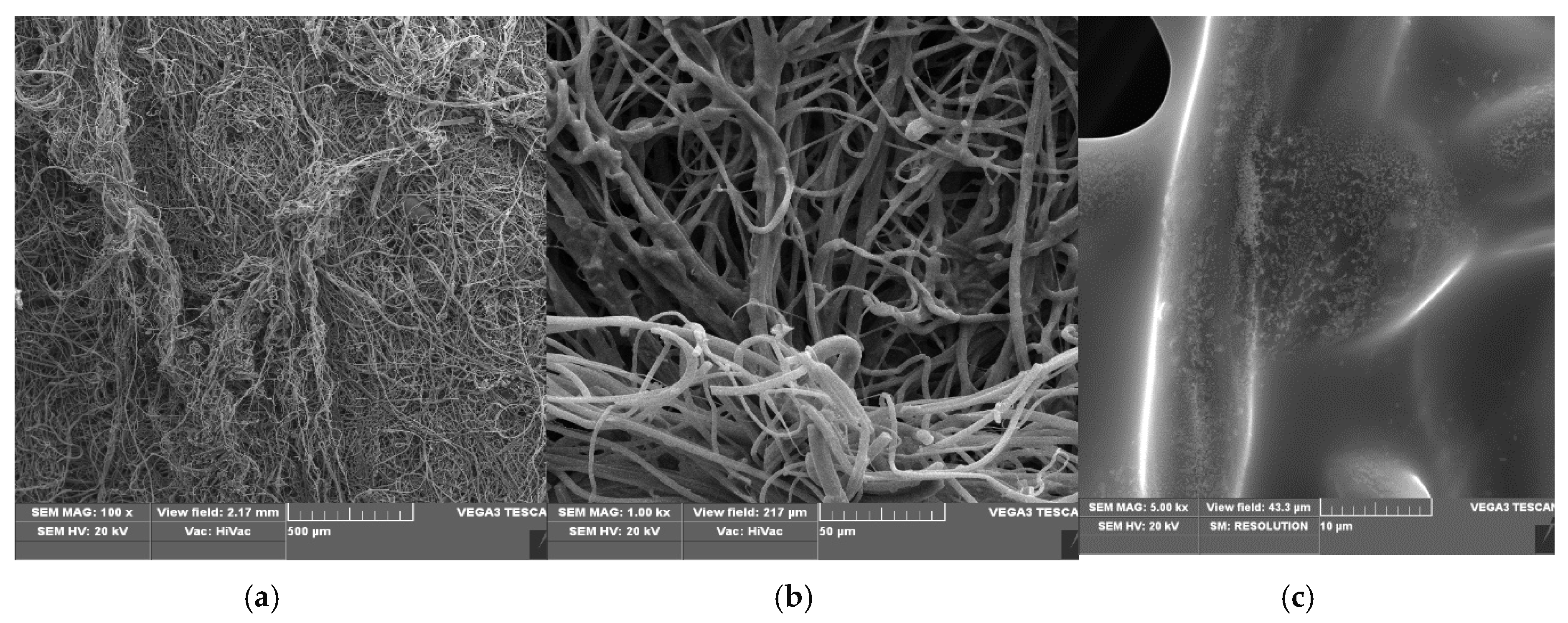
3.1. SEM/EDS—Scanning Electron Microscopy/Energy-Dispersive X-ray Spectroscopy
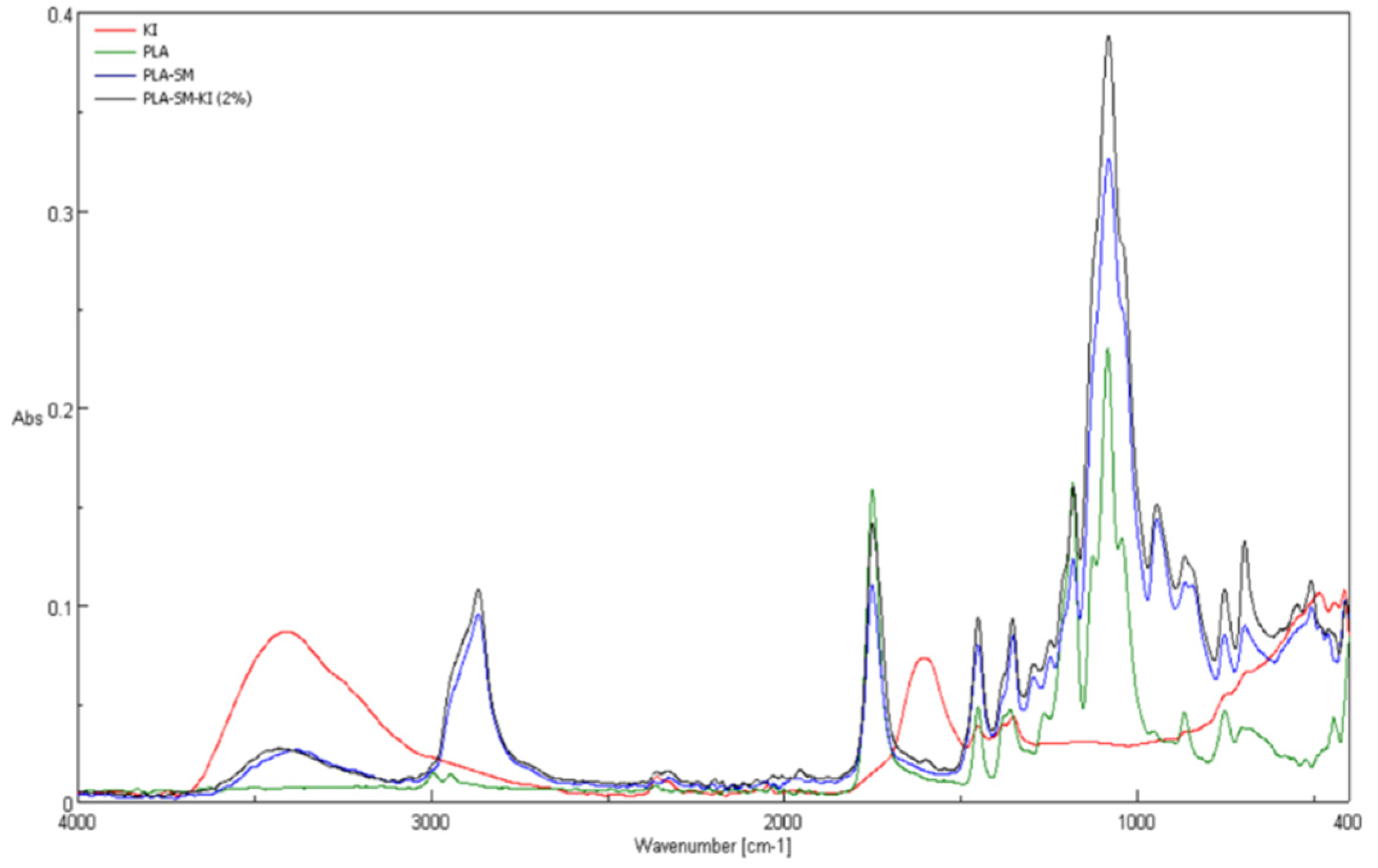
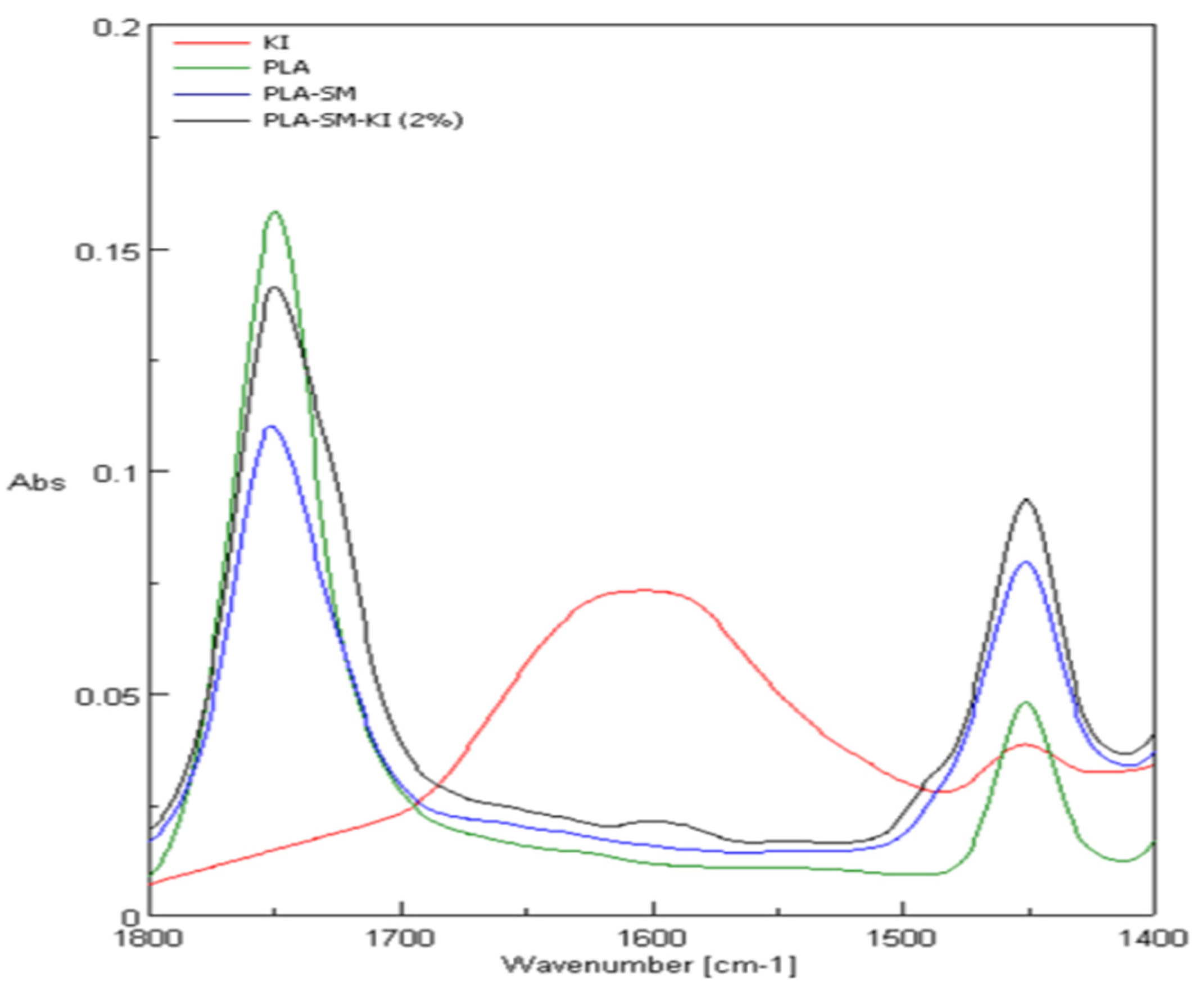
3.2. ATR-FTIR
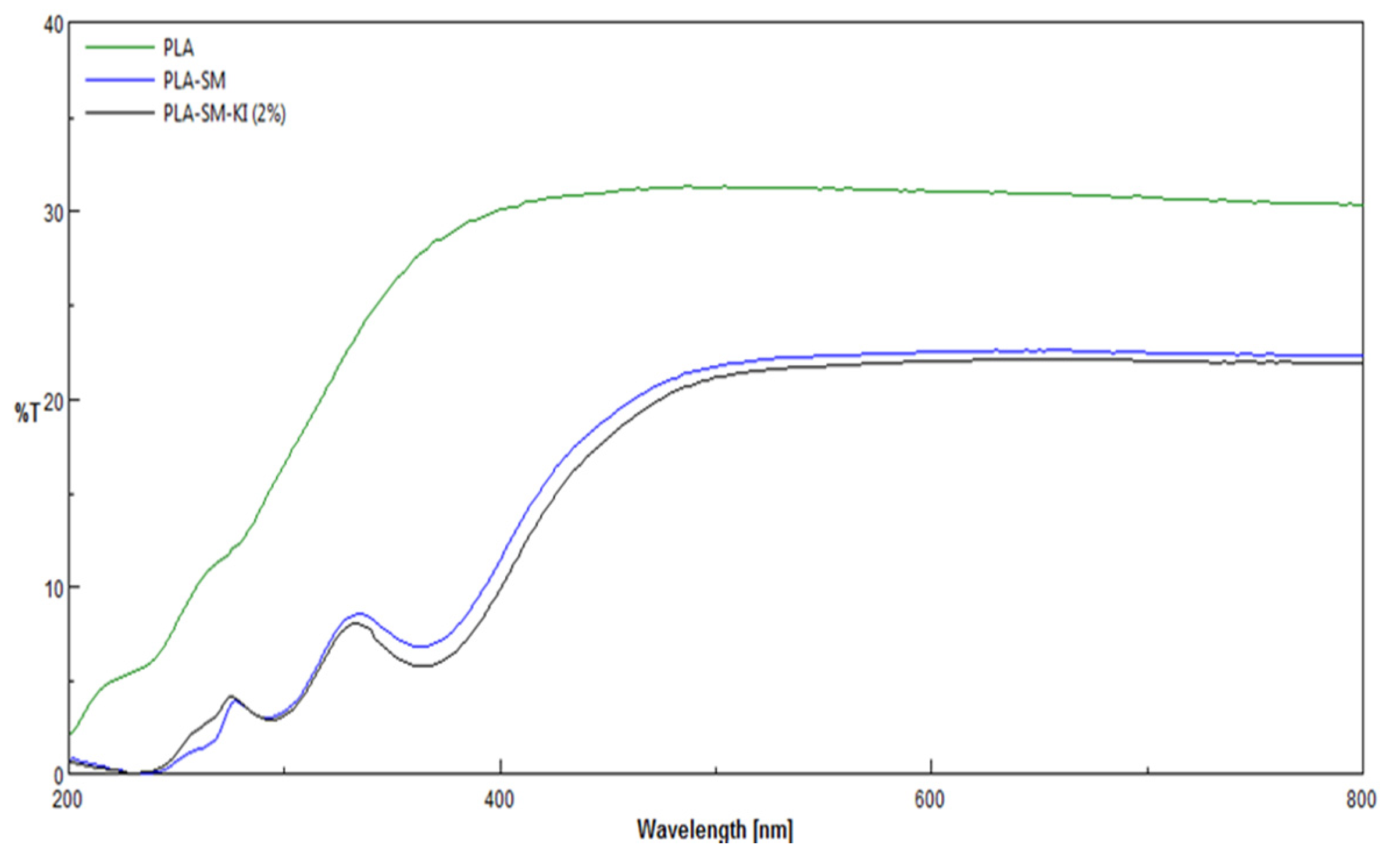
3.3. UV/VIS Transmittance Spectra
3.4. Technical Parameters
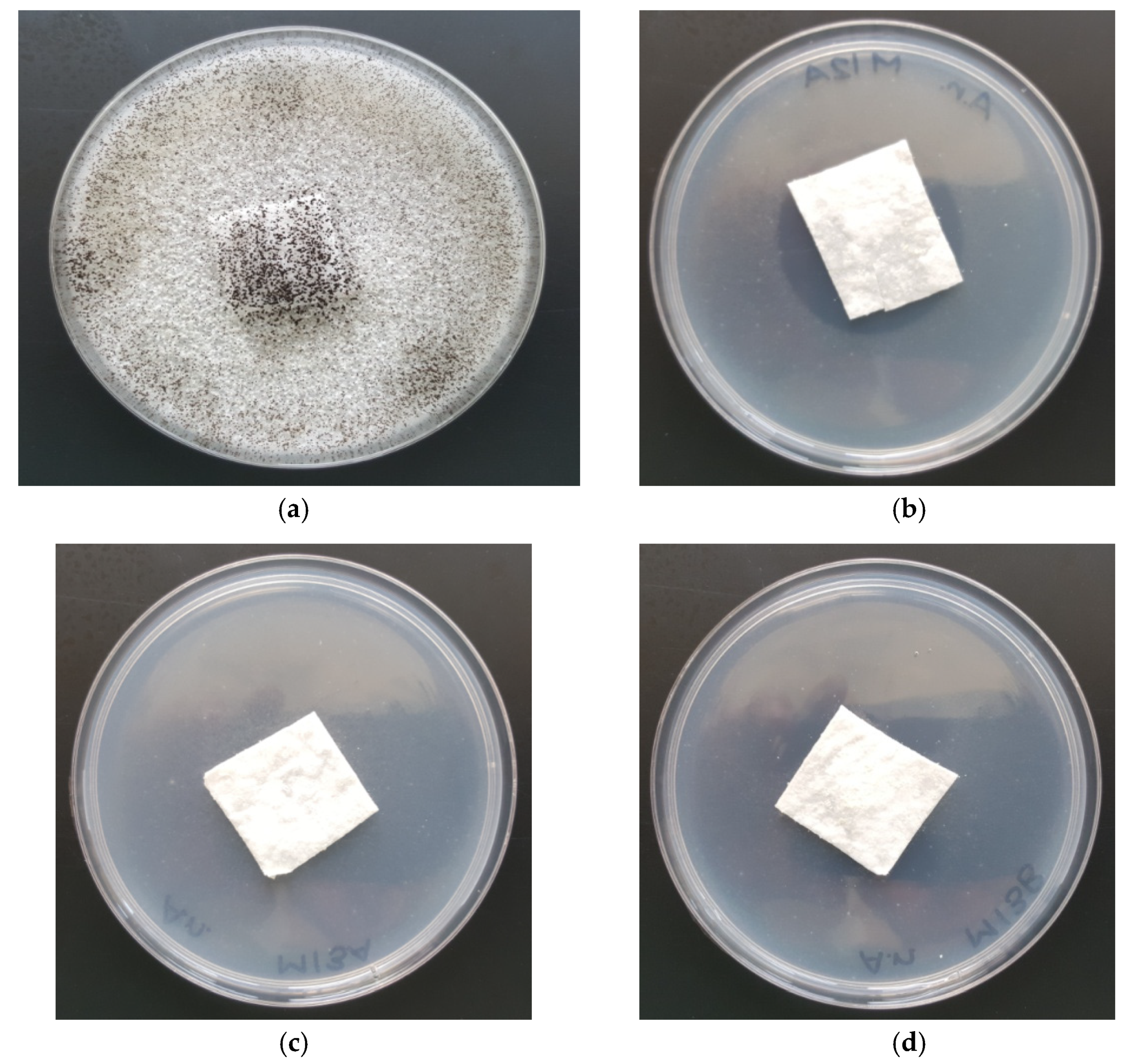
3.5. Antifungal Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Auras, R.; Lim, L.T.; Selke, S.E.M. Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; Tsuji, H., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Zhou, J.; Han, S.; Dou, Y.; Lu, J.; Wang, C.; He, H.; Li, X.; Zhang, J. Nanostructured poly(l-lactide) matrix as novel platform for drug delivery. Int. J. Pharm. 2013, 448, 175–188. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Q.; Wang, X.; Gao, W.; Zhao, X. Antibacterial properties of PLA nonwoven medical dressings coated with nanostructured silver. Fiber Polym. 2008, 9, 556–560. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, C.; Tang, X.; Zhao, J.; Song, Y.; Shao, Z.; Xu, L. Characterization and antibacterial properties of porous fibers containing silver ions. Appl. Surf. Sci. 2016, 387, 828–838. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Ocio, M.J.; Lagaron, J.M. Morphology, physical properties, silver release, and antimicrobial capacity of ionic silver-loaded poly(L-lactide) films of interest in food-coating applications. J. Appl. Polym. Sci. 2014, 131, 41001. [Google Scholar] [CrossRef]
- Sójka-Ledakowicz, J.; Chruściel, J.J.; Kudzin, M.H.; Łatwińska, M.; Kiwała, M. Antimicrobial functionalization of textile materials with copper silicate. Fibres Text. East. Eur. 2016, 24, 151–156. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Electrospinning/electrospraying vs. electrospinning: A comparative study on the design of poly(l-lactide)/zinc oxide non-woven textile. Appl. Surf. Sci. 2014, 311, 842–850. [Google Scholar] [CrossRef]
- Toniatto, T.V.; Rodrigues, B.V.M.; Marsi, T.C.O.; Ricci, R.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Nanostructured poly(lactic acid) electrospun fiber with high loadings of TiO2 nanoparticles: Insights into bactericidal activity and cell viability. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Schkarpetkin, D.; Reise, M.; Wyrwa, R.; Völpel, A.; Berg, A.; Schweder, M.; Schnabelrauch, M.; Watts, D.C.; Sigusch, B.W. Development of novel electrospun dual-drug fiber mats loaded with a combination of ampicillin and metronidazole. Dent. Mater. 2016, 32, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mat. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, X.; Shahid, S.; Cattel, M.J.; Gould, D.J.; Sukhorukov, G.B. Electrospun poly(lactic acid) fibers containing novel chlorhexidine particles with sustained antibacterial activity. Biomater. Sci. 2016, 5, 111–119. [Google Scholar] [CrossRef]
- Olmo, C.; Franco, L.; del Valle, L.J.; Puiggalí, J. Preparation of medicated polylactide micropieces by means of ultrasonic technology. Appl. Sci. 2019, 9, 2360. [Google Scholar] [CrossRef]
- Davachi, S.M.; Kaffashi, B.; Zamanian, A.; Torabinejad, B.; Ziaeirad, Z. Investigating composite systems based on poly L-lactide and poly L-lactide/triclosan nanoparticles for tissue engineering and medical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Karaszewska, A.; Kamińska, I.; Kiwała, M.; Gadzinowski, M.; Gosecki, M.; Slomkowski, S. Preparation and properties of textile materials modified with triclosan-loaded polylactide microparticles. Polym. Adv. Technol. 2017, 28, 1185–1193. [Google Scholar] [CrossRef]
- Goetzendorf-Grabowska, B.; Polus, Z.; Kiwała, M.; Karaszewska, A.; Kamińska, I.; Mączka, I. Antibacterial air filter nonwovens modified by poly(lactide) microspheres containing triclosan. Fibres Text. East. Eur. 2015, 23, 114–119. [Google Scholar]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Cooper, R. A review of the evidence for the use of topical antimicrobial agents in wound care. World Wide Wounds 2004. Available online: http://www.worldwidewounds.com/2004/february/Cooper/Topical-Antimicrobial- Agents.html (accessed on 1 February 2004).
- Sibbald, R.G.; Leaper, D.J.; Queen, D. Iodine Made Easy. Wounds Int. 2011, 2. Available online: http://www.woundsinternational.com (accessed on 1 May 2011).
- Moulay, S. Molecular iodine/polymer complexes. J. Polym. Eng. 2013, 33, 389–443. [Google Scholar] [CrossRef]
- Moulay, S. Macromolecule/polymer-iodine complexes: An update. Rec. Innov. Chem. Eng. 2019, 12, 174–233. [Google Scholar] [CrossRef]
- Immel, S.; Lichtenthaler, F.W. The hydrophobic topographies of amylose and its blue iodine complex. Starch 2000, 52, 1–8. [Google Scholar] [CrossRef]
- Klimaviciute, R.; Bendoraitiene, J.; Rutkaite, R.; Siugzdaite, J.; Zemaitaitis, A. Preparation, stability and antimicrobial activity of cationic cross-linked starch-iodine complexes. Int. J. Biol. Macromol. 2012, 51, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Bendoraitiene, J.; Sarkinas, A.; Danilovas, P.P.; Rutkaite, R.; Klimaviciute, R.; Zemaitaitis, A. Cationic starch iodophores. J. Appl. Polym. Sci. 2013, 128, 4346–4354. [Google Scholar] [CrossRef]
- Tang, Y.; Xie, L.; Sai, S.; Xu, N.; Ding, D. Preparation and antibacterial activity of quaternized chitosan with iodine. Mat. Sci. Eng. C 2015, 48, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Kashef, N.; Huang, Y.Y.; Hamblin, M.R. Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.N.M.; Neves, M.G.P.M.S.; Faustino, A.F.; Almeida, A. An insight into the potentiation effect of potassium iodide on APDT efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef]
- Ghaffari, S.; Sarp, A.S.K.; Lange, D.; Gulsoy, M. Potassium iodide potentiated photodynamic inactivation of Enterococcus faecalis using toluidine blue: Comparative analysis and post-treatment biofilm formation study. Photodiagnosis Photodyn. Ther. 2018, 24, 245–249. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wintner, A.; Seed, P.C.; Braun, T.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial photodynamic therapy mediated by methylene blue and potassium iodide to treat urinary tract infection in a female rat model. Sci. Rep. 2018, 8, 7257. [Google Scholar] [CrossRef]
- Santos, A.R.; Batista, A.F.P.; Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A.; Hioka, N.; Mikcha, J.M.G. The remarkable effect of potassium iodide in eosin and rose bengal photodynamic action against Salmonella typhimurium and Staphylococcus aureus. Antibiotics 2019, 8, 211. [Google Scholar] [CrossRef]
- Kubát, P.; Henke, P.; Mosinger, J. The effect of iodide and temperature on enhancing antibacterial properties of nanoparticles with an encapsulated photosensitizer. Colloids Surf. B Biointerfaces 2019, 176, 334–340. [Google Scholar] [CrossRef]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium iodide potentiates broad-spectrum antimicrobial photodynamic inactivation using photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; He, Y.; Huang, L.; Huang, Y.Y.; Bhayana, B.; Xia, L.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial Photodynamic Inactivation mediated by tetracyclines In Vitro and In Vivo: Photochemical mechanisms and potentiation by potassium iodide. Sci. Rep. 2018, 8, 17130. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Inorganic salts and antimicrobial photodynamic therapy: Mechanistic conundrums. Molecules 2018, 23, 3190. [Google Scholar] [CrossRef] [PubMed]
- Azimzadehirani, M.; Elahifard, M.R.; Haghighi, S.; Gholami, M.R. Highly efficient hydroxyapatite/TiO2 composites covered by silver halides as E. coli disinfectant under visible light and dark media. Photochem. Photobiol. Sci. 2013, 12, 1787–1794. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Choi, H.; Kushida, Y.; Bhayana, B.; Wang, Y.; Hamblin, M.R. Broad-spectrum antimicrobial effects of photocatalysis using titanium dioxide nanoparticles are strongly potentiated by addition of potassium iodide. Antimicrob. Agents Chemother. 2016, 60, 5445–5453. [Google Scholar] [CrossRef]
- Deng, W.; Ning, S.; Lin, Q.; Zhang, H.; Zhou, T.; Lin, H.; Long, J.; Lin, Q.; Wang, X. I-TiO2/PVC film with highly photocatalytic antibacterial activity under visible light. Colloids Surf. B Biointerfaces 2016, 144, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Kida, N.; Mochizuki, Y.; Taguchi, F. An effective iodide formulation for killing Bacillus and Geobacillus spores over a wide temperature range. J. Appl. Microbiol. 2004, 97, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Tonoyan, L.; Fleming, G.T.A.; Mc Cay, P.H.; Friel, R.; O’Flaherty, V. Antibacterial potential of an antimicrobial agent inspired by peroxidase-catalyzed systems. Front. Microbiol. 2017, 8, 680. [Google Scholar] [CrossRef]
- Tonoyan, L.; Boyd, A.; Fleming, G.T.A.; Friel, R.; Gately, C.M.; Mc Cay, P.H.; O’Flaherty, V. In vitro comparative cytotoxicity study of a novel biocidal iodo-thiocyanate complex. Toxicol. Vitro 2018, 50, 264–273. [Google Scholar] [CrossRef]
- Punyani, S.; Singh, H. Preparation of iodine containing quaternary amine methacrylate copolymers and their contact killing antimicrobial properties. J. Appl. Polym. Sci. 2006, 102, 1038–1044. [Google Scholar] [CrossRef]
- Zainul Abid, C.K.V.; Jackeray, R.; Jain, S.; Chattopadhyay, S.; Asif, S.; Singh, H. Antimicrobial efficacy of synthesized quaternary ammonium polyamidoamine dendrimers and dendritic polymer network. J. Nanosci. Nanotechnol. 2016, 16, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.; Rubira, A.F.; Muniz, E.C. Antimicrobial activity of chitosan derivatives containing N-quaternized moieties in its backbone: A review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Depczynski, R.; Kudzin, M.H.; Luczak, J.; Drabowicz, J. 1-(N-Trifluoroacetyl-amino)alkylphosphonic acids: Synthesis and properties. Amino Acids 2007, 33, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Kudzin, Z.H.; Depczyński, R.; Kudzin, M.H.; Drabowicz, J. 1-(N-chloroacetylamino)-alkylphosphonic acids—Synthetic precursors of phosphonopeptides. Amino Acids 2008, 34, 163–168. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jakubowski, H.; Kudzin, M.H.; Kudzin, Z.H. The nomenclature of 1-aminoalkylphosphonic acids and derivatives: Evolution of the code system. Acta Biochim. Pol. 2015, 62, 139–150. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic acids-phosphorus analogues of natural amino acids. Part 1: Syntheses of α-aminophosphonic acids. Curr. Org. Chem. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Drabowicz, J.; Jordan, F.; Kudzin, M.H.; Kudzin, Z.H.; Stevens, C.V.; Urbaniak, P. Reactivity of aminophosphonic acids. Oxidative dephosphonylation of 1-aminoalkylphosphonic acids by aqueous halogens. Dalton Trans. 2016, 45, 2308–2317. [Google Scholar] [CrossRef]
- Cypryk, M.; Drabowicz, J.; Gostynski, B.; Kudzin, M.H.; Kudzin, Z.H. Urbaniak, P.1-(Acylamino)alkyl-phosphonic acids—Alkaline deacylation. Molecules 2018, 23, 859. [Google Scholar] [CrossRef]
- Sójka-Ledakowicz, J.; Kudzin, M.H. Effect of plasma modification on the chemical structure of a polyethylene terephthalate fabrics surface. Fibres Text. East. Eur. 2014, 22, 118–122. [Google Scholar]
- Sójka-Ledakowicz, J.; Łatwińska, M.; Kałuzka, J.; Kudzin, M. Polypropylene nonwovens with natural polymers addition for filtration applications. Polymers 2013, 58, 557–561. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Walawska, A.; Sójka-Ledakowicz, J. Biofunctionalization of textile materials. 1. Biofunctionalization of poly(propylene) (PP) nonwovens fabrics by Alafosfalin. Coatings 2019, 9, 412. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z. Biofunctionalization of Textile Materials. 2. Antimicrobial modification of poly(lactide) (PLA) nonwoven fabrics by fosfomycin. Polymers 2020, 12, 768. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z.; Urbaniak, P.; Drabowicz, J. Phosphorylation of Cellulose, P-128. In Proceedings of the 19th International Symposium Advances in the Chemistry of Heteroorganic Compounds, Łódź, Poland, 12 October 2016. CBMM PAN & CHF UŁ. [Google Scholar]
- EN ISO 9237:1998 standard. Textiles–Determination of Permeability of Fabrics to Air; International Organization for Standardization: Geneva, Switzerland, 1998. [Google Scholar]
- EN ISO 10319:2015-08 standard. Textiles—Test methods for nonwovens—Part 3: Determination of tensile strength and elongation.
- EN 14119: 2005 point 10.5 (B2) standard. Testing of textiles—Evaluation of the action of microfungi—Visual method.
- Jia, L.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Stem cell differentiation on electrospun nanofibrous substrates for vascular tissue engineering. Mater. Sci. Eng. C 2013, 33, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, W.; Kapica, A.; Tański, T.; Dubiel, A. Analysis of the influence of electro spinning process parameters on the morphology of poly(lactic acid) fibres. Archiv. Mater. Sci. Eng. 2019, 96, 73–78. [Google Scholar] [CrossRef]
- Krucińska, I.; Surma, B.; Chrzanowski, M.; Skrzetuska, E.; Puchalski, M. Application of melt-blown technology in the manufacturing of a solvent vapor-sensitive, non-woven fabric composed of poly(lactic acid) loaded with multi-walled carbon nanotubes. Text. Res. J. 2013, 83, 859–870. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Bhat, G.S.; Azari, H.; Pen, H. Electret characteristics of melt-blown polylactic acid fabrics for air filtration application. J. Appl. Polym. Sci. 2020, 137, 48309. [Google Scholar] [CrossRef]
- Zhu, F.; Yu, B.; Su, J.; Han, J. Study on PLA/PA11 bio-based toughening melt-blown nonwovens. Autex Res. J. 2020, 20. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Grabowska, B.; Holtzer, M. Structural examination of the cross-linking reaction mechanism of polyacrylate binding agents. Arch. Metal. Mater. 2009, 54, 427–438. [Google Scholar]
- Vrandečić, N.S.; Erceg, M.; Jakić, M.; Klarić, I. Kinetic analysis of thermal degradation of poly(ethylene glycol) and poly(ethylene oxide)s of different molecular weight. Thermochim. Acta 2010, 498, 71–80. [Google Scholar] [CrossRef]
- Snavely, D.L.; Dubsky, J. Near-IR spectra of polyethylene, polyethylene glycol, and polyvinylethyl ether. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2575–2579. [Google Scholar] [CrossRef]
- Fang, J.F.; Xuan, Y.M.; Li, Q. Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Tech. Sci. 2010, 53, 3088–3093. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.K.; Surana, K.; Bhattacharya, B.; Singh, P.K.; Rhee, H.W. New polymer electrolyte for electrochemical application. J. Ind. Eng. Chem. 2012, 19, 819–822. [Google Scholar] [CrossRef]
- NIST Standard Reference Database 69: NIST Chemistry WebBook; Coblentz Society, Inc.; Coblentz No. 10116. Available online: https://webbook.nist.gov/chemistry/ (accessed on 6 April 2020).
- Jiang, L.; Wang, F.; Han, F.; Prinyawiwatkul, W.; No, H.K.; Ge, B. Evaluation of diffusion and dilution methods to determine the antimicrobial activity of water-soluble chitosan derivatives. J. Appl. Microbiol. 2012, 114, 956–963. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]


| Processing Parameters | ||
|---|---|---|
| Temperature of the extruder in zone 1 | 195 °C | |
| Temperature of the extruder in zone 2 | 245 °C | |
| Temperature of the extruder in zone 3 | 260 °C | |
| Head temperature | 260 °C | |
| Air heater temperature | 260 °C | |
| Air flow rate | 7–8 m3/h | |
| Polymer yields | 6 g/min | |
| Mass per unit area of nonwovens | 102 g/m2 | |
| Assignments for Surface Modifiers SM-KI | Paste Components (g) | Paste Components after Drying (%) c,d | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Polymeric Components a | KI Aqueous Solutions | ||||||||
| PS-PA | PA | PEG | Water | KI b | PS-PA | PA | PEG | KI | |
| SM | 6 | 1 | 3 | 90 | - | 60 | 10 | 30 | - |
| SM-KI(0.1) | 6 | 1 | 3 | 90 | 0.009 | 60 | 10 | 30 | 0.1 |
| SM-KI(1) | 6 | 1 | 3 | 90 | 0.09 | 60 | 10 | 30 | 1 |
| SM-KI(2) | 6 | 1 | 3 | 90 | 0.18 | 60 | 10 | 30 | 2 |
| Fiber/Composite | PLA | PLA-SM | PL-SM-KI (2%) | |||||
|---|---|---|---|---|---|---|---|---|
| Atom | C | O | C | O | C | O | K | I |
| % a | 51.7 | 48.33 | 60.08 | 39.92 | 57.11 | 41.28 | 0.35 | 1.25 |
| Std. deviation | 0.11 | 0.11 | 0.29 | 0.29 | 1.10 | 1.02 | 0.01 | 0.09 |
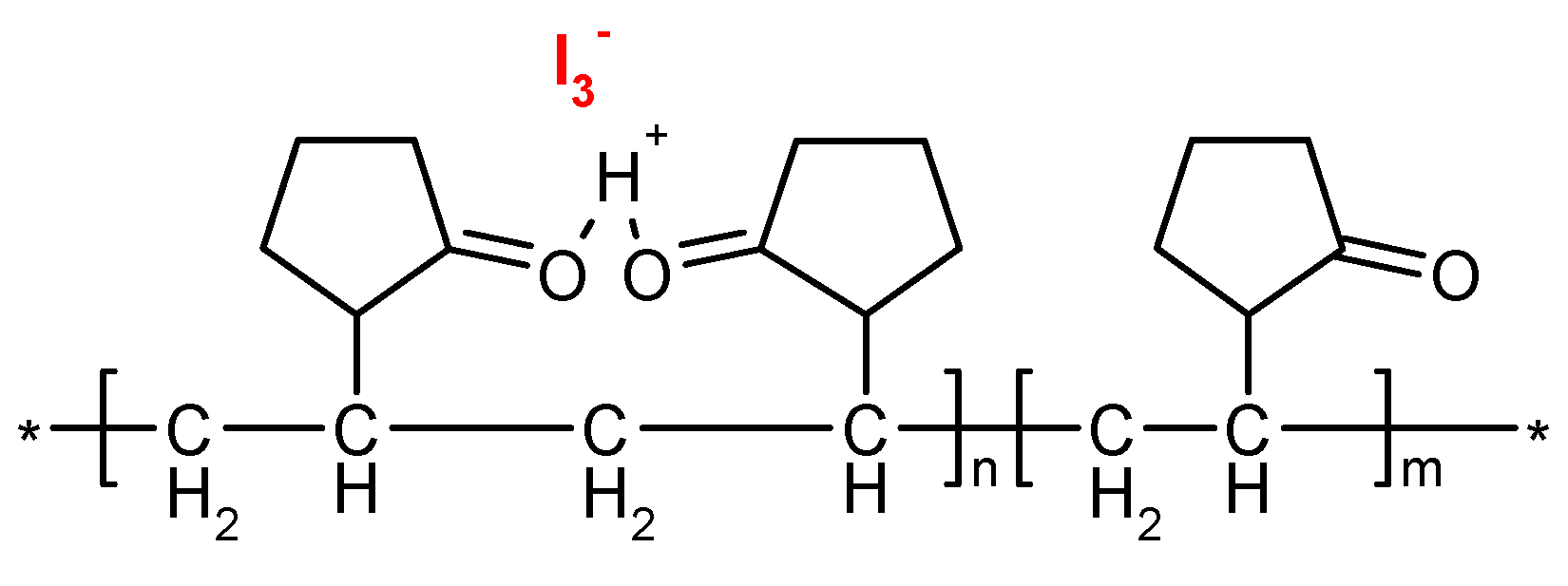
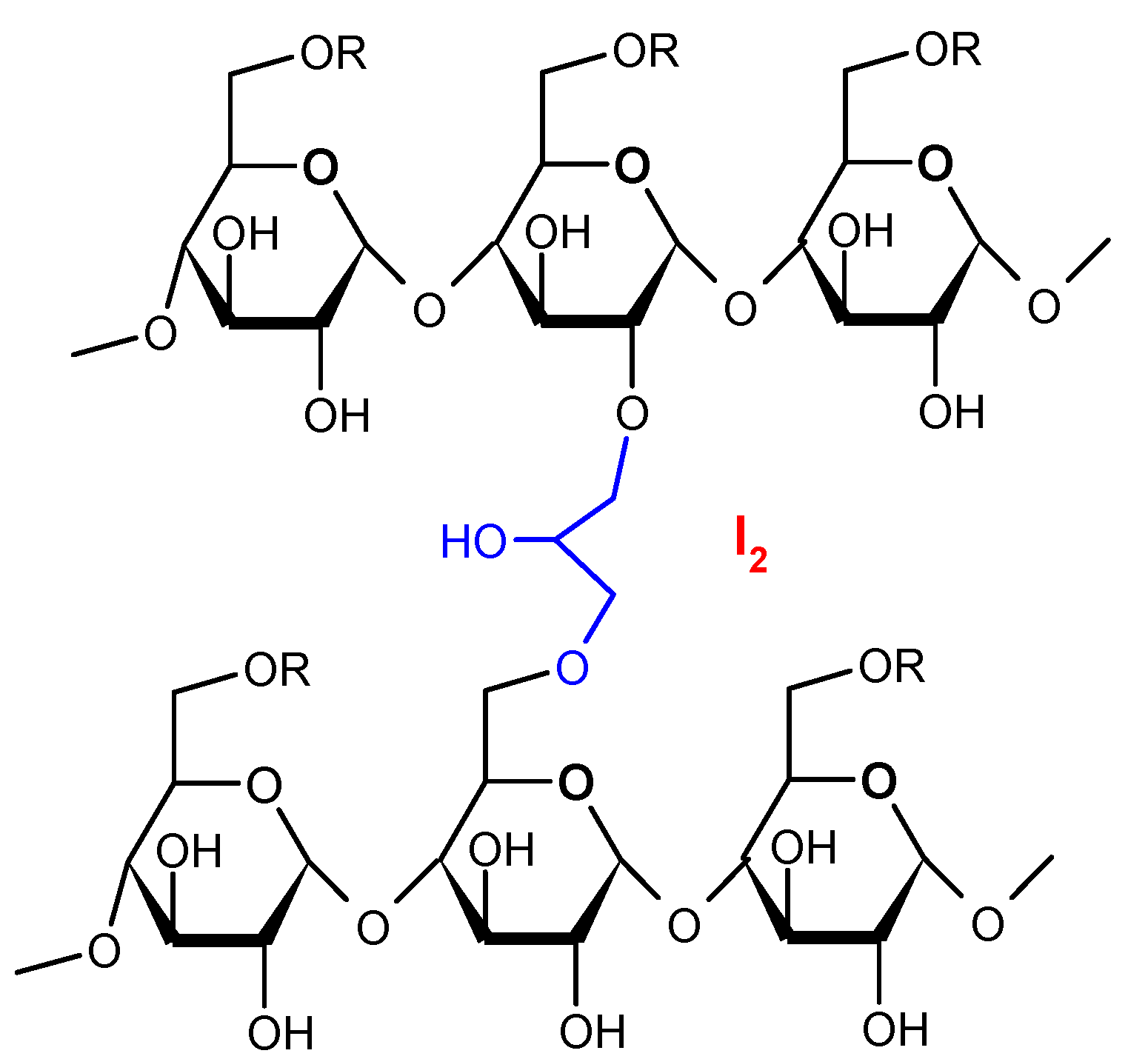
| IR Vibration Bands [ν/cm−1] for PLA-SM-KI Hybrid Components (Name, Abbreviation, Structure [Literature]) a | ||||
|---|---|---|---|---|
| Polylactide (PLA) [63] | Polyacrylate (PA) [64] | Polyglycol (PEG) [65,66] | Polystyrene (PS) [67] | Potassium Iodide (KI) [68] |
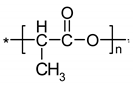 | 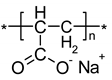 | 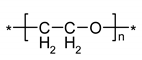 | 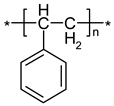 |  |
| - | 3700–2900 | 3500 | 3447 | 3435 |
| - | - | 3060 | - | |
| 2997 | - | - | 3026 | - |
| 2947 | - | 2934 | 2922 | - |
| - | - | - | 2848 | - |
| 1760 | 1750 | - | - | - |
| - | 1651 | - | - | - |
| - | - | - | 1600 | 1623 |
| - | 1577 | - | - | - |
| - | - | - | 1493 | - |
| 1452 | - | 1466 | 1452 | - |
| 1348–1388 | - | - | - | 1399 |
| 1368–1360 | - | 1341 | - | - |
| 1270 | - | 1278 | - | - |
| 1215 | - | 1241 | - | - |
| 1185 | - | 1145 | - | - |
| 1130 | - | - | - | - |
| 1100 | - | 1097 | - | - |
| 1090 | - | - | - | - |
| 1045 | - | 1058 | - | 1042 |
| - | - | 960 | - | - |
| - | 800 | 840 | - | - |
| - | - | 756 | - | |
| - | - | 698 | - | |
| 538 | - | 538 | - | |
| Composites and Components | |||||||
|---|---|---|---|---|---|---|---|
| PLA | KI | PLA-SM | PLA-SM-KI (2%) | ||||
| ν [cm−1] | Absorb. a | ν [cm−1] | Absorb. a | ν [cm−1] | Absorb.a | ν [cm−1] | Absorb.a |
| - | - | 3680–2800 | 0.09 | 3600–3100 | 0.05 | 3600–3100 | 0.06 |
| 2993 | 0.01 | - | - | - | - | - | - |
| 2944 | 0.01 | - | - | - | - | - | - |
| - | - | - | - | 2863 | 0.09 | 2867 | 0.11 |
| - | - | 2362 | 0.01 | - | - | - | - |
| - | - | 2184 | 0.01 | - | - | - | - |
| - | - | 2041 | 0.01 | - | - | - | - |
| 1749 | 0.16 | - | - | 1751 | 0.11 | 1751 | 0.14 |
| - | - | 1602 | 0.07 | - | - | 1602 | 0.02 |
| 1451 | 0.05 | 1455 | 0.04 | 1451 | 0.08 | 1451 | 0.10 |
| 1359 | 0.05 | 1352 | 0.04 | 1353 | 0.08 | 1353 | 0.10 |
| 1186 | 0.16 | - | - | 1184 | 0.12 | 1178 | 0.16 |
| 1084 | 0.23 | - | - | 1083 | 0.33 | 1083 | 0.39 |
| - | - | - | - | 943 | 0.14 | 943 | 0.15 |
| 864 | 0.05 | - | - | 862 | 0.11 | 862 | 0.13 |
| 750 | 0.05 | - | - | 751 | 0.08 | 751 | 0.11 |
| 695 | 0.04 | - | - | 693 | 0.09 | 696 | 0.14 |
| - | - | - | - | 507 | 0.98 | 511 | 0.11 |
| - | - | 485 | 0.11 | - | - | - | - |
| 442 | 0.04 | - | - | - | - | - | - |
| Parameter | PLA | PLA-SM | PLA-SM-KI (KI Paste Concentr. [%]) | |||
|---|---|---|---|---|---|---|
| - | 0.1% | 1% | 2% | |||
| Average air permeability [mm/s], pressure decrease: | 100 Pa | 905 | 428 | 430 | 420 | 435 |
| 200 Pa | 1640 | 825 | 820 | 831 | 825 | |
| Parameter | PLA | PLA-SM | PLA-SM-KI | ||
|---|---|---|---|---|---|
| KI Paste Concentr. [%] | |||||
| 0.1% | 1% | 2% | |||
| Tensile strength [kN/m] | 0.030 | 0.11 | 0.11 | 0.11 | 0.11 |
| Relative elongation at maximum load [%] | 10.0 | 10.6 | 10.6 | 11.7 | 10.8 |
| Fungal Average Inhibition Zone (mm) | |||||||
|---|---|---|---|---|---|---|---|
| KI concentrations in applied SM-KI (%) | |||||||
| 0 | 0.1 | 1 | 2 | ||||
| Side of the sample | |||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| 0 | 0 | 6 | 7 | Nvg | Nvg | Nvg | Nvg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudzin, M.H.; Mrozińska, Z. Biofunctionalization of Textile Materials. 3. Fabrication of Poly(lactide)-Potassium Iodide Composites with Antifungal Properties. Coatings 2020, 10, 593. https://doi.org/10.3390/coatings10060593
Kudzin MH, Mrozińska Z. Biofunctionalization of Textile Materials. 3. Fabrication of Poly(lactide)-Potassium Iodide Composites with Antifungal Properties. Coatings. 2020; 10(6):593. https://doi.org/10.3390/coatings10060593
Chicago/Turabian StyleKudzin, Marcin H., and Zdzisława Mrozińska. 2020. "Biofunctionalization of Textile Materials. 3. Fabrication of Poly(lactide)-Potassium Iodide Composites with Antifungal Properties" Coatings 10, no. 6: 593. https://doi.org/10.3390/coatings10060593
APA StyleKudzin, M. H., & Mrozińska, Z. (2020). Biofunctionalization of Textile Materials. 3. Fabrication of Poly(lactide)-Potassium Iodide Composites with Antifungal Properties. Coatings, 10(6), 593. https://doi.org/10.3390/coatings10060593






