Anti-Graffiti Coatings on Stones for Historical Buildings in Turin (NW Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Stones
- (1)
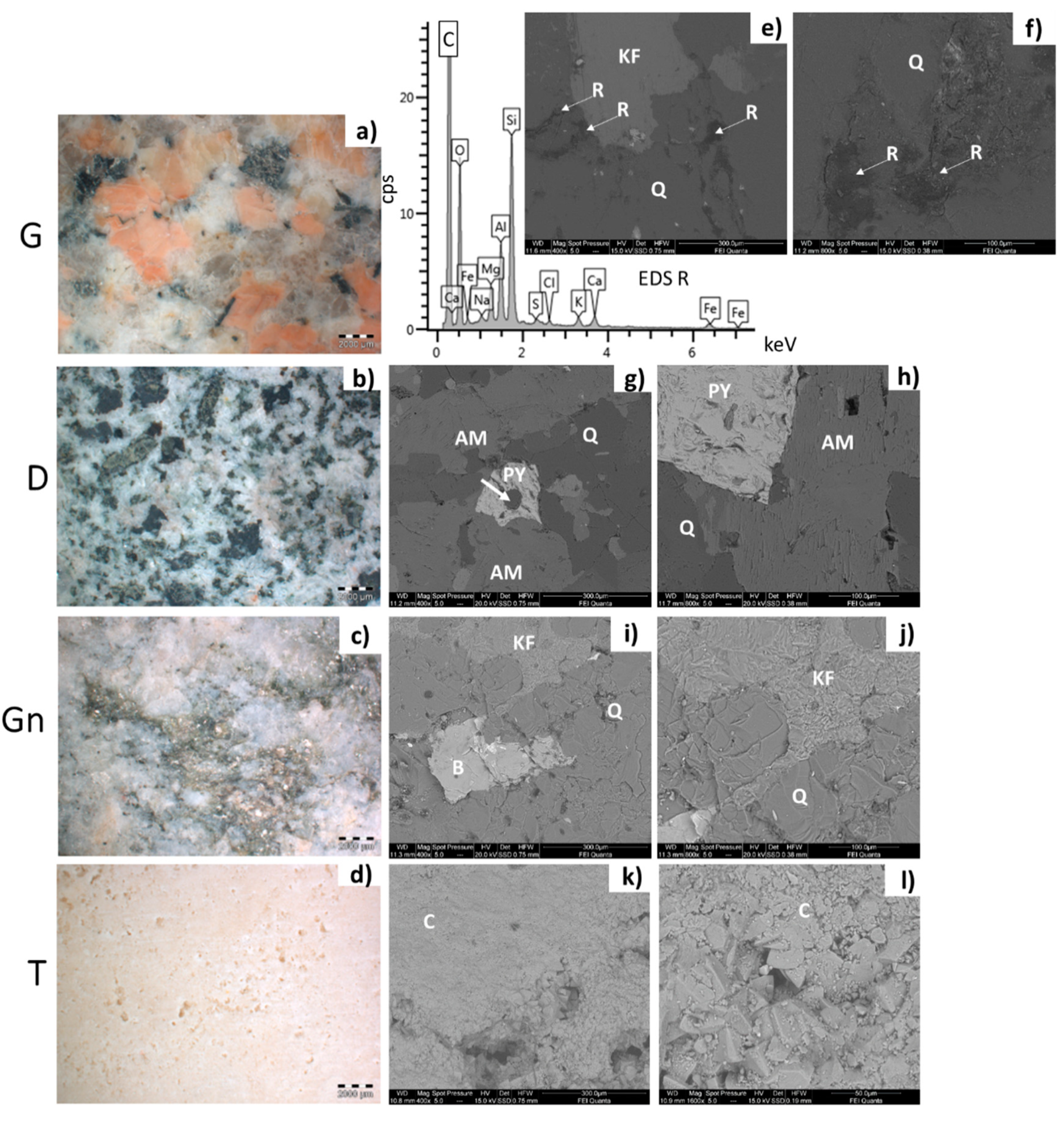
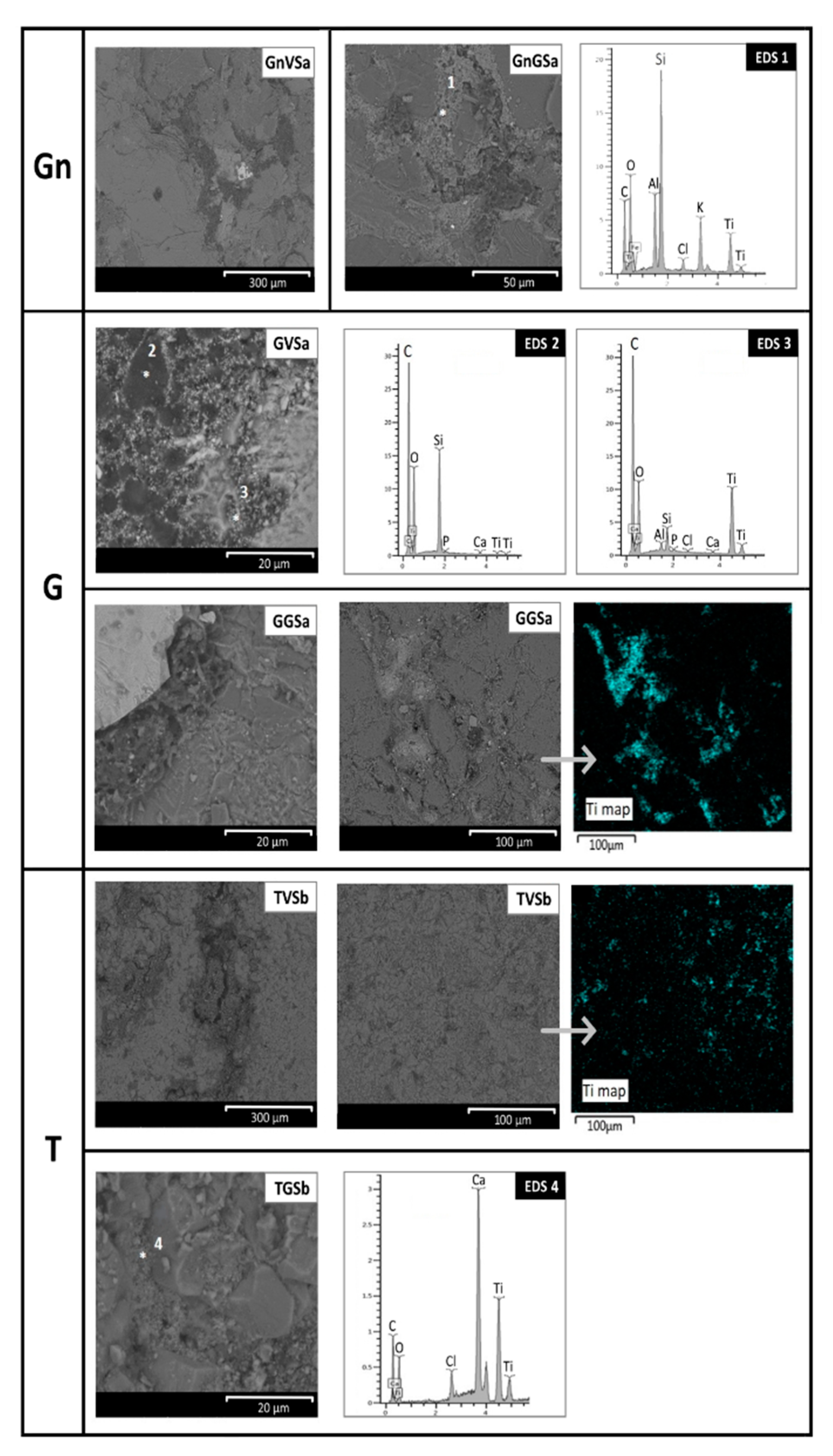
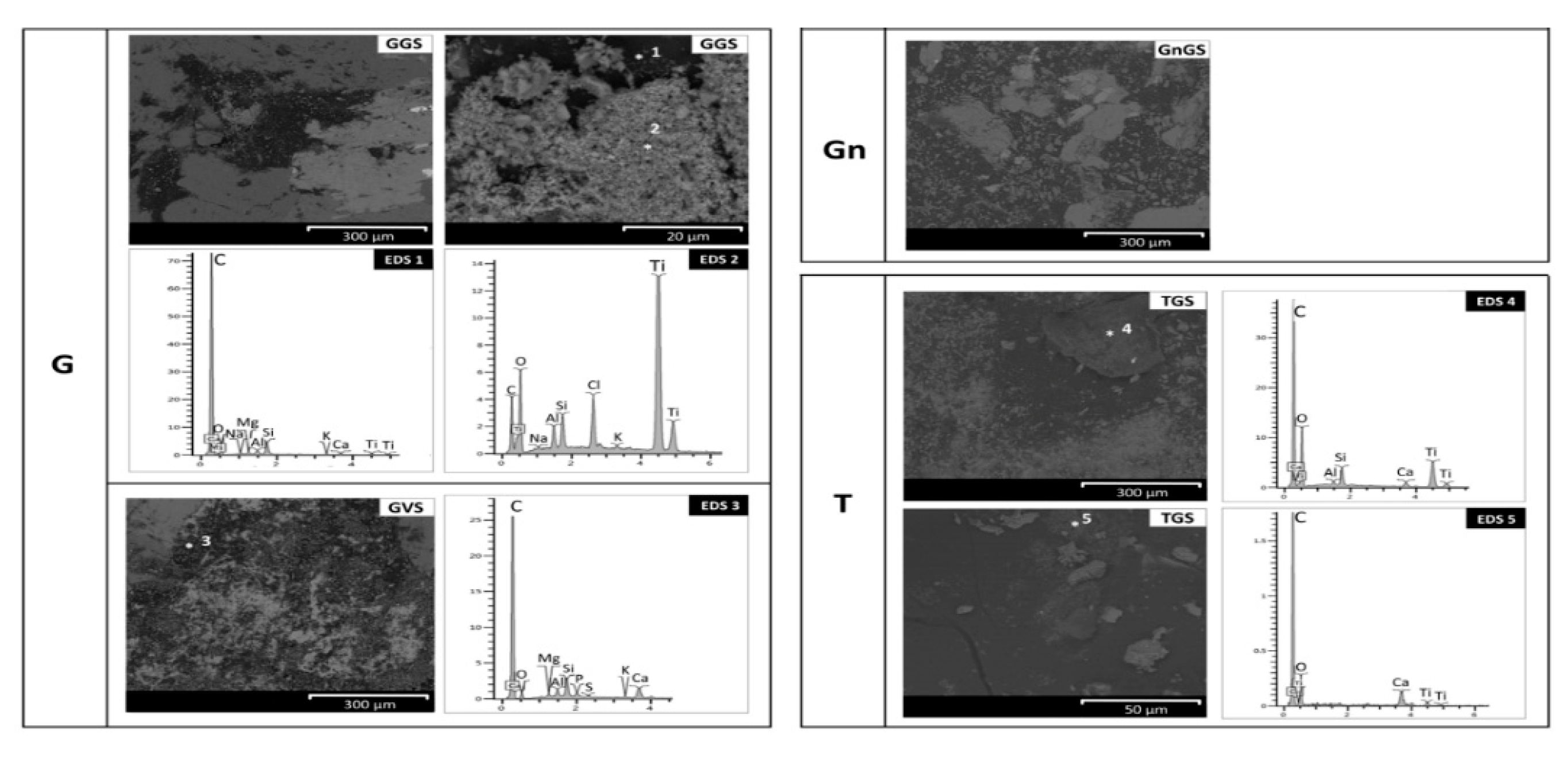
- Baveno granite (hereinafter G, Figure 1 and Figure 2a,e,f), which is a medium-grained and pink granite, composed of white plagioclase, gray quartz, pink K-feldspar, and biotite partially replaced by chlorite [35,36]. It takes its name from the main quarry, located in the small town of Baveno on the west bank of Maggiore Lake (in the Verbano-Cusio-Ossola quarry district, northern sector of the Piedmont region) [31]. In Turin, it has been employed with both a polished and unpolished surface, depending on the application. Within this work, a polished surface finish was considered, as it occurs, for example, for several columns of the typical arcades in the city center. It is known that during the polishing process, an epoxy resin filler is used to fill any micro fissures [37]. The EDX spectra reported in Figure 2 highlight the presence of this resin on the granite (Figure 2e,f), showing C, Al, and Si as major elements and, to a lesser extent, Mg, Na, S, Cl, K, Ca, and Fe;
- (2)
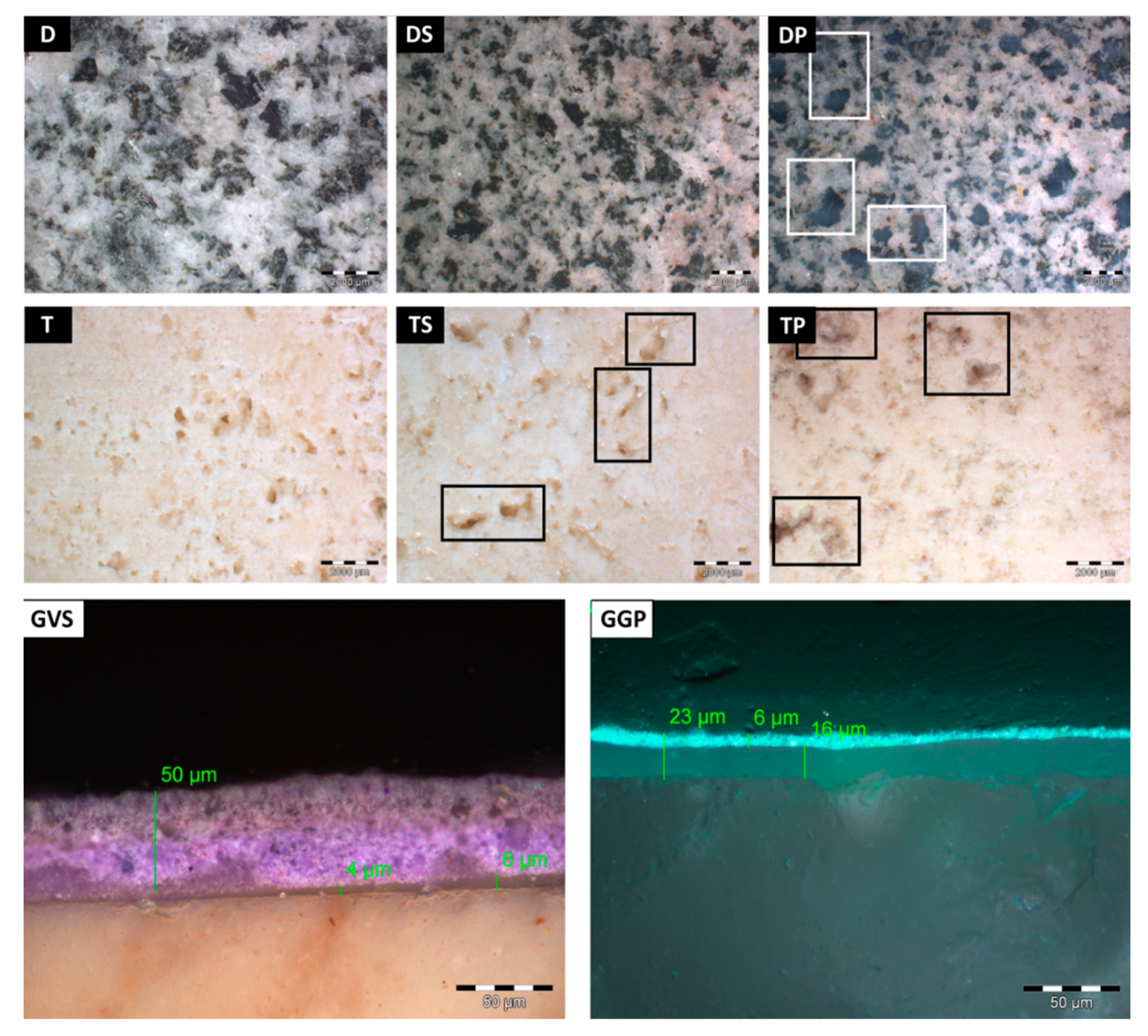
- Vico diorite (hereinafter D, Figure 1 and Figure 2b,g,h) is a quartzdiorite with a granular texture and characterized by a light to very dark gray colour, depending on the grain size and the percentage of femic minerals, represented by amphibole, biotite, and rare pyroxene. With regards to sialic minerals, plagioclase mainly occurs, in addition to K-feldspar and rare quartz [38]. Vico diorite comes from the quarry district of the Chiusella Valley, which is about 70 km from Turin [31]. As for the granite, a polished surface was selected for the Vico diorite, as this surface type was observed for several columns and paving of the arcades of blocks in the city center. Si-rich fillers were detected in the superficial voids and fissures of this stone (as highlighted in Figure 2g with an arrow), as a result of the silicone-based chemical applied to enhance the absorption qualities, increase the resistance to staining, and/or alter the stone´s appearance [37];
- (3)
- Luserna Stone (Pietra di Luserna, hereinafter Gn, Figure 1 and Figure 2c,i,j) is an orthogneiss. It is named after the municipality of Luserna San Giovanni, which is about 65 km from Turin (NW Italy), where the main quarry district was historically located [31]. It is characterized by a micro-augen texture, medium-fine grained size, and good fissibility along the schistosity planes, well-defined by muscovite crystallized under high-pressure conditions [38]. It displays a light gray colour, occasionally turning slight darker or greenish; magmatic porphyroclasts of K-feldspar, in addition to quartz and albite, are clearly visible. As for the forming minerals, it is composed of quartz, plagioclase, K-feldspar, muscovite, and biotite; epidote, chlorite, and sphene are also present as minor minerals [32]. Luserna Stone has been frequently employed in Turin, such as in the case of the slabs of the dome of the Mole Antonelliana (a major landmark building in the city) and the façade of the Automobile Museum. As for numerous real applications, a flamed surface finish was considered for this stone within the present work;
- (4)
- Travertine (hereinafter T, Figure 1 and Figure 2d,k,l) is a very porous orthochemical sedimentary rock, with fine grains. It is completely composed of a calcite matrix and often contains plant prints. Macroscopically, it is characterized by a more or less intense beige color, with thin bands highlighted by different contents of impurities [39]. Among the four lithotypes selected, it is the only one not from the Piedmont region, with the main mining area being located in the region of Rome [31]. Nevertheless, it was included in the present work since it has been frequently employed in Turin, such as in the case of the façade and arcades of the Santissima Annunziata church, as well as several façades of buildings and shops in the city center. As is mostly seen in Turin, a smooth disc-cutting finish was selected for the travertine.
2.2. Anti-Graffiti Products
2.3. Graffiti Paints and Application
2.4. Cleaning
2.5. Analytical Techniques
- (1)
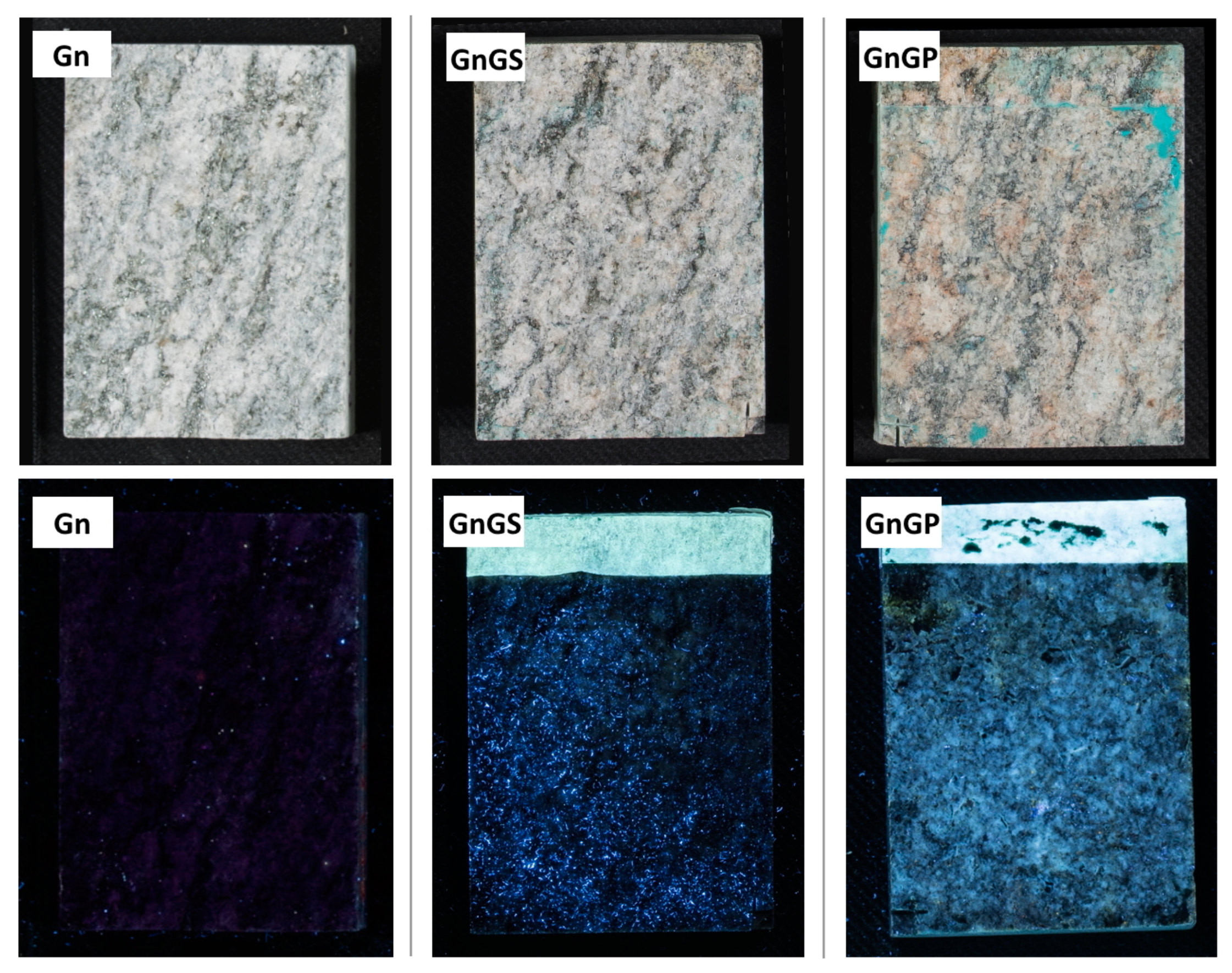
- The coated surfaces were observed with an OLYMPUS SZ ×10 stereomicroscope, with an OLYMPUS Color View I digital camera, in order to observe any visible effect of the anti-graffiti product applied on the different stones. Moreover, all surfaces, after having been treated with the different cleaning methods, were evaluated by stereomicroscopy to identify the graffiti remains. Moreover, in order to determine the thickness of the anti-graffiti coatings and the graffiti paints, fragments of 1 cm × 1 cm × 1 cm were embedded in resin to be visualized by means of stereomicroscopy;
- (2)
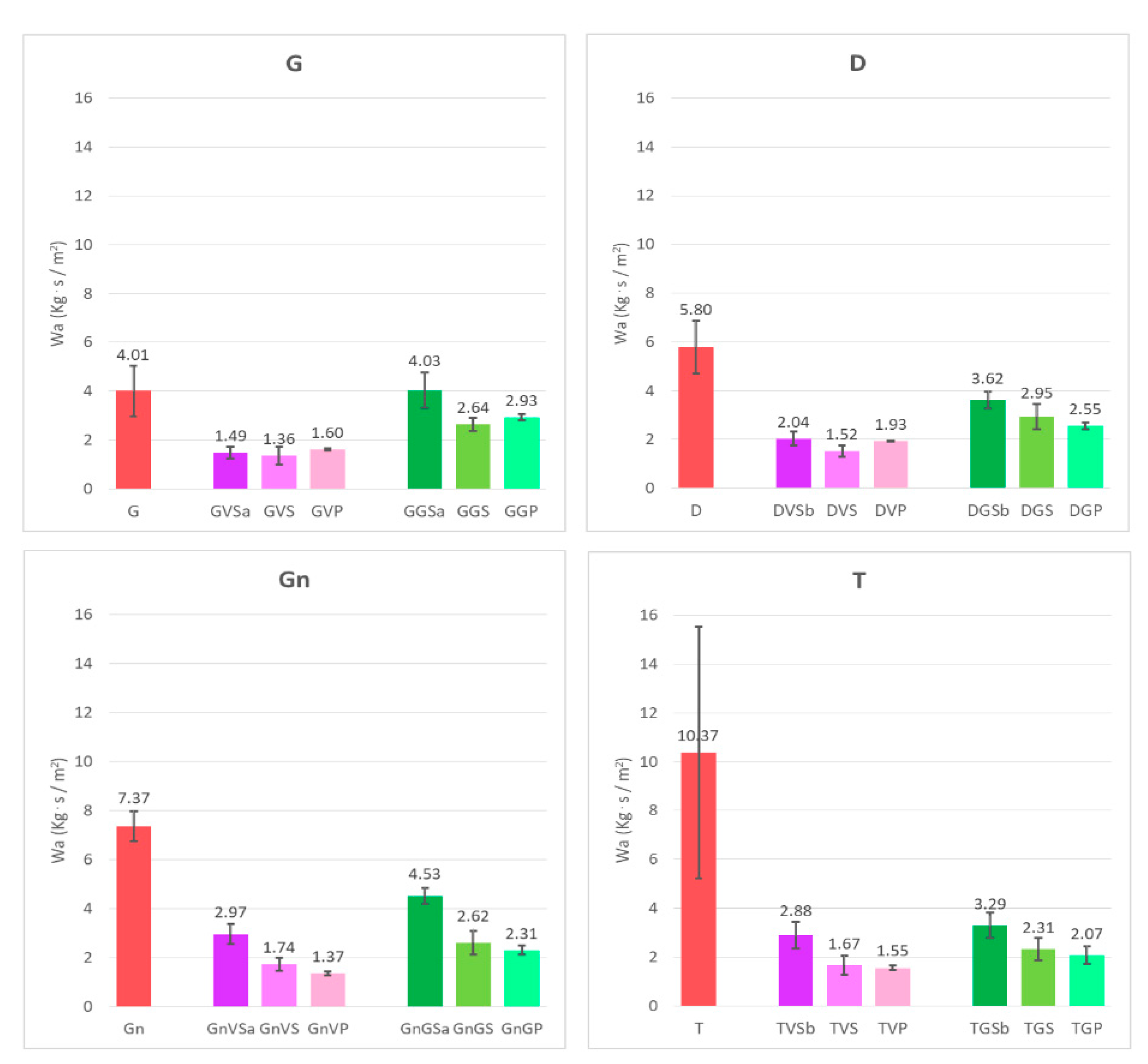
- The water absorption of both the coated surfaces and the surfaces, after having been treated with the different removers, was evaluated by the contact sponge method. A CTS contact sponge kit was used, following [47]. It is a non-destructive water absorption test, which may be performed in both the laboratory and in situ and has been proven to be directly comparable with other water uptake laboratory measurements (e.g., the capillary rise method) [48]. A Spontex1 Calypso-type sponge, in natural fibers, having pre-determined characteristics and dimensions, was charged with an adequate amount of water (2 mL within the present work), after preliminary tests to verify that the water did not leak when the sponge was put in contact with the surface. The sponge was applied on the stone sample surface for 180 s, by using a Falcon 1034 Rodac® circular plastic plate: by placing the borders of the plate in contact with the examined surface, the maximum applicable manual pressure was determined. The amount of water absorbed by the surface was calculated by the difference, by weighing the sponge before and after the application. The difference in weight corresponds to the amount of water which has been absorbed by the material through the surface. The results (water absorption, Wa) were then expressed by the mass difference as a function of area and time. For each surface, three measurements were performed, by drying the sample between measurements;
- (3)
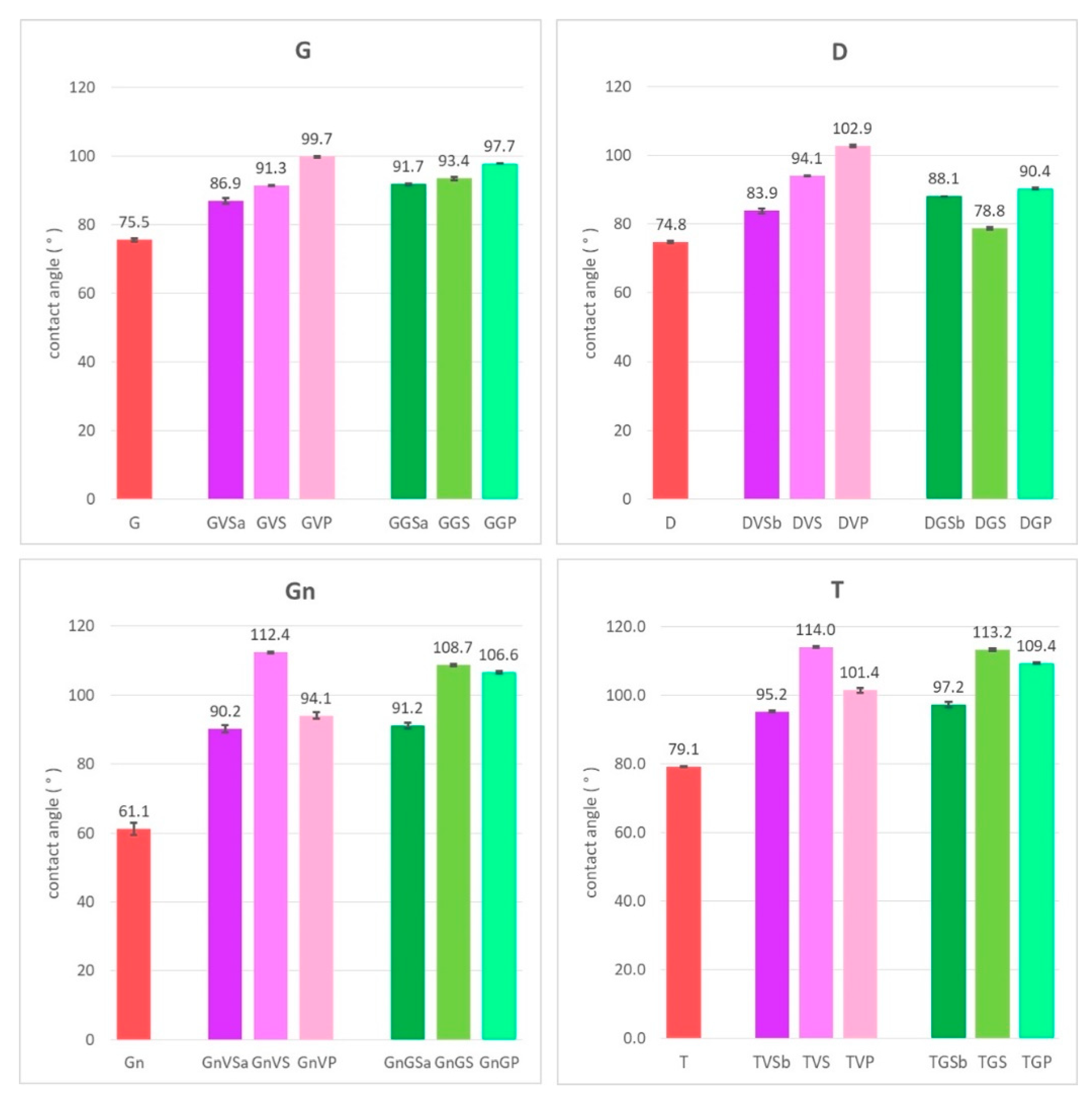
- The hydrophobicity of the surfaces after cleaning was evaluated by means of a SEO Phoenix-300 Touch goniometer (Surface Electro Optics Co., Suwon, Korea) following BS [49] by applying the sessile drop method. Three drops of 6 µL of deionized water per sample were applied;
- (4)
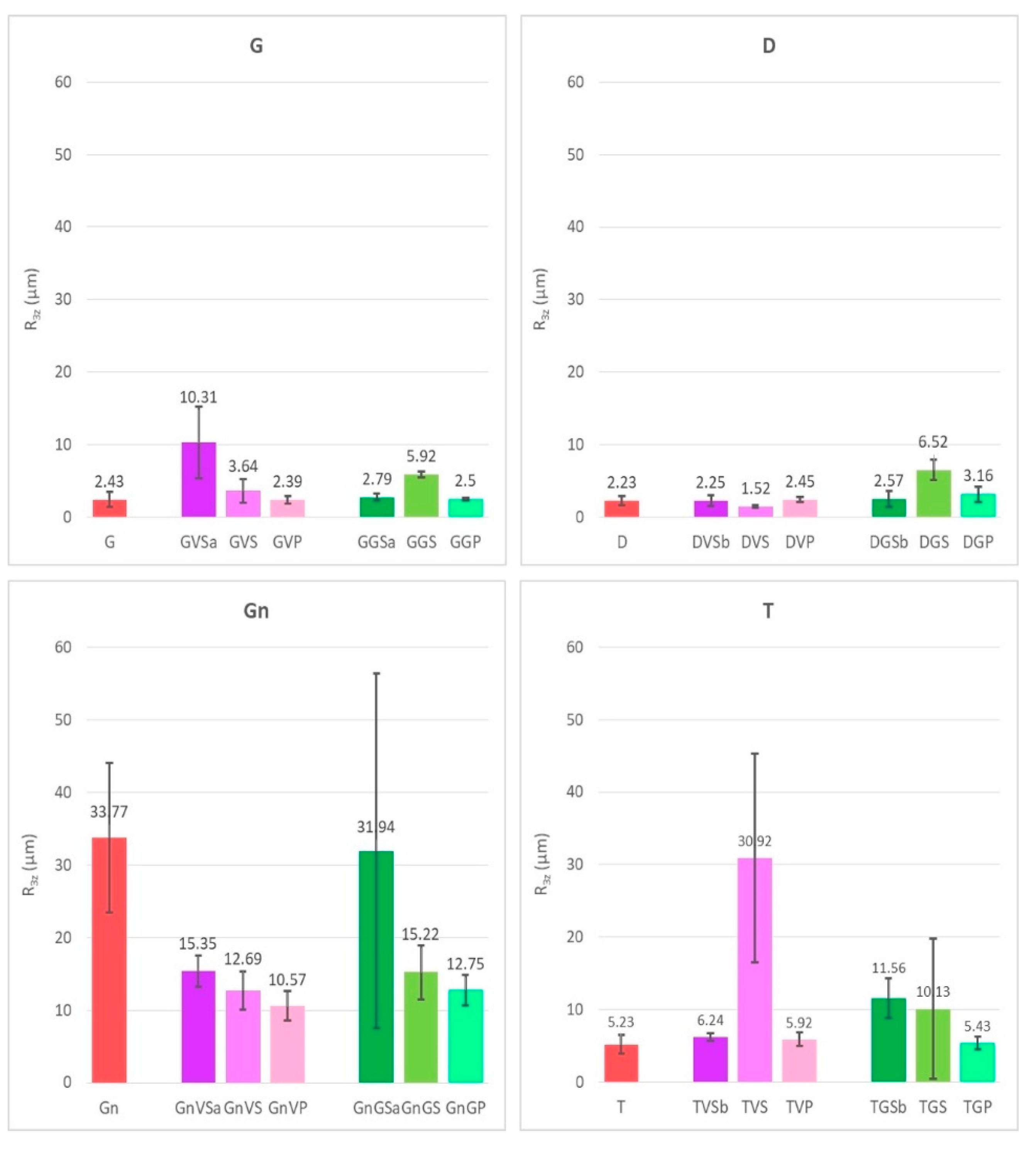
- The variation of roughness of the surfaces after having been treated with the different cleaning methods were evaluated using a PLu 2300 Sensofar® optical imaging profiler (Sensofar, Barcelona, Spain). The images with the optical imaging profiler were collected with an EPI 10X-N objective, an overlap of 25%, a depth range of 2 mm, and a lateral resolution of 1 nm. The system allowed us to obtain 3D images of the cleaned surfaces and therefore, the roughness parameter ([50]), R3z (third maximum peak-to-valley height), was obtained using the Gwyddion 2.47 software. For each sample, three images of 4 mm × 4 mm areas were taken and used to obtain the roughness parameter;
- (5)
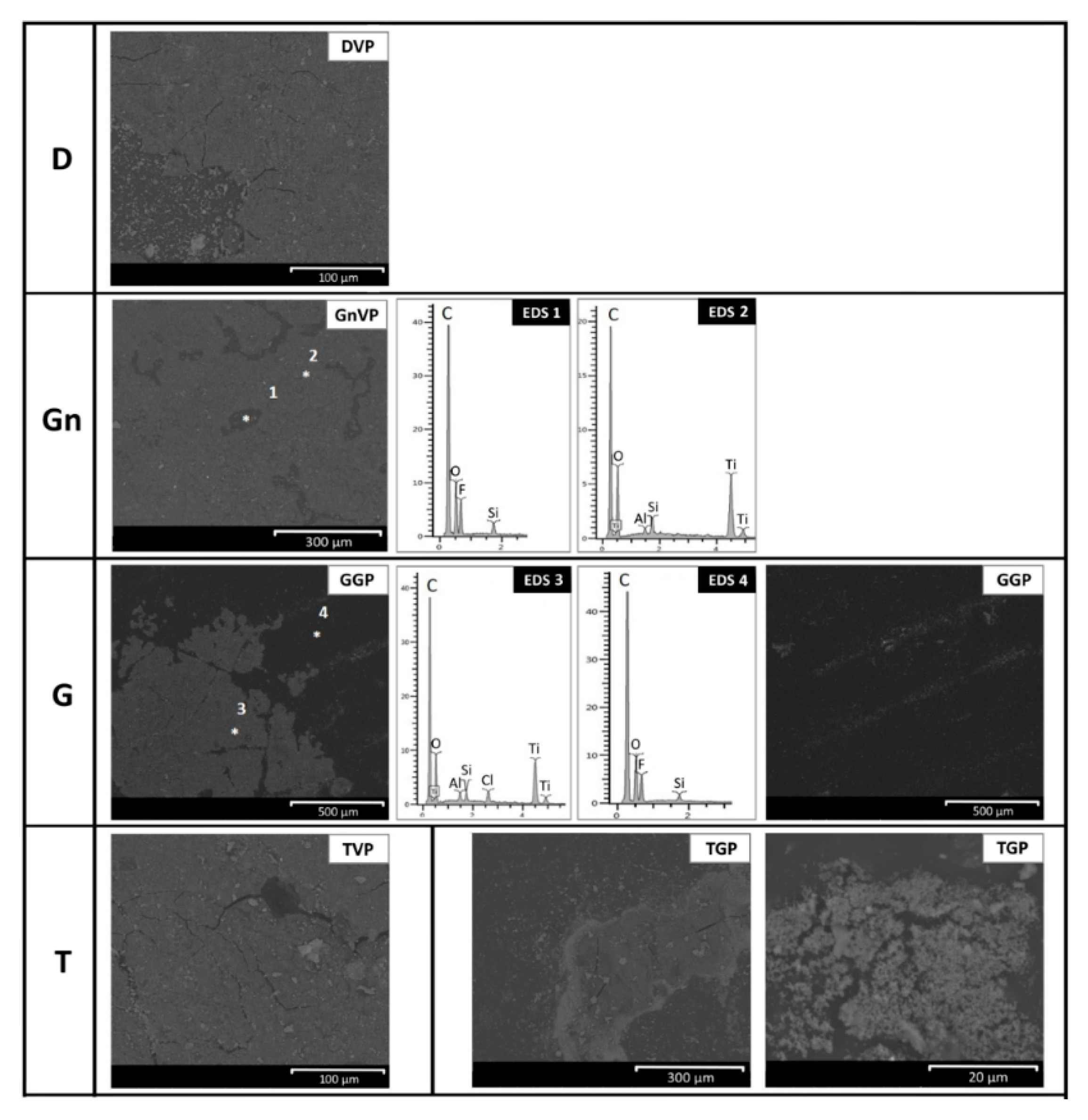
- A microscopic evaluation of the cleaned surfaces was performed using a scanning electron microscope (FEI Quanta 200) in BSE mode to find the location of the graffiti and anti-graffiti remains and the damage induced on the surfaces. The optimal conditions of observation were the same as used to characterize the paint layers on the stone.
3. Results
4. Discussion
- The removal of the green acrylic paint was satisfactory from all four stones. Nevertheless, as for the samples with sacrificial anti-graffiti product, paint remains filling the voids were found on travertine surfaces. Therefore, the commercial graffiti remover employed (a blend of alcohol and terpene solvents) proved to be effective in the dissolution of this paint;
- The cleaning of the violet alkyd paint was totally unsatisfactory, leaving the graffiti layer apparently unaffected. However, an observation by SEM revealed a few fissures and cracks in the paint layer, possibly showing the initial step of the detaching process from the protective coating.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations. About the Sustainable Development Goals (Agenda 2030). Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals (accessed on 20 March 2020).
- Hosagrahar, J.; Soule, J.; Fusco Girard, L.; Potts, A.; ICOMOS. Concept Note for the United Nations Agenda 2030 and the Third United Nations Conference on Housing and Sustainable Urban Development (HABITAT). Available online: http://www.usicomos.org/wp-content/uploads/2016/05/Final-Concept-Note.pdf (accessed on 20 March 2020).
- Sanmartín, P.; Cappitelli, F.; Mitchell, R. Current methods of graffiti removal: A review. Constr. Build. Mater. 2014, 71, 363–374. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Rivas, T.; Fiorucci, M.P.; López, A.J.; Ramil, A. Effectiveness and harmfulness evaluation of graffiti cleaning by mechanical, chemical and laser procedures on granite. Microchem. J. 2016, 125, 1–9. [Google Scholar] [CrossRef]
- Gomes, V.; Dionísio, A.; Pozo-Antonio, J.S. Conservation strategies against graffiti vandalism on cultural heritage stones: Protective coatings and cleaning methods. Prog. Organ. Coat. 2017, 113, 90–109. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M. Surface characterization and effectiveness evaluation of anti-graffiti coatings on highly porous stone materials. Appl. Surf. Sci. 2014, 288, 466–477. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Blanco-Varela, M.T. Fluorinated anti-graffiti coating for natural stones. Mater. De Constr. 2008, 58, 233–246. [Google Scholar]
- Carmona-Quiroga, P.M.; Rubio, J.; Sánchez, M.J.; Martínez-Ramírez, S.; Blanco-Varela, M.T. Surface dispersive energy determined with IGC-ID in anti-graffiti-coated building materials. Prog. Organ. Coat. 2011, 71, 207–212. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Jacobs, R.M.J.; Martínez-Ramírez, S.; Viles, H.A. Durability of anti-graffiti coatings on stone: Natural vs. accelerated weathering. PLoS ONE 2017, 12, e0172347. [Google Scholar] [CrossRef]
- Licchelli, M.; Malagodi, M.; Weththimuni, M.; Zanchi, C. Anti-graffiti nanocomposite materials for surface protection of a very porous stone. Appl. Phys. A Mater. Sci. Process. 2014, 116, 1525–1539. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Rivas, T.; Jacobs, R.M.J.; Viles, H.A.; Carmona-Quiroga, P.M. Effectiveness of commercial anti-graffiti treatments in two granites of different texture and mineralogy. Prog. Organ. Coat. 2018, 116, 70–82. [Google Scholar] [CrossRef]
- Urquhart, D. The Treatment of Graffiti on Historic Surfaces: Advice on Graffiti Removal Procedures, Anti-Graffiti Coatings and Alternative Strategies; Historic Scotland technical advice note no.18; TCRE Division/Scottish Conservation Bureau, Hist.: Edinburgh, Historic Scotland, UK, 1999. [Google Scholar]
- Giusti, C.; Colombini, M.P.; Lluveras-Tenorio, A.; la Nasa, J.; Striova, J.; Salvadori, B. Graphic vandalism: Multi-analytical evaluation of laser and chemical methods for the removal of spray paints. J. Cult. Herit. 2020. [Google Scholar] [CrossRef]
- Ortiz, P.; Antúnez, V.; Ortiz, R.; Martín, J.M.; Gómez, M.A.; Hortal, A.R.; Martínez-Haya, B. Comparative study of pulsed laser cleaning applied to weathered marble surfaces. Appl. Surf. Sci. 2013, 283, 193–201. [Google Scholar] [CrossRef]
- Carvalhão, M.; Dionísio, A. Evaluation of mechanical soft-abrasive blasting and chemical cleaning methods on alkyd-paint graffiti made on calcareous stones. J. Cult. Herit. 2015, 16, 579–590. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; López, L.; Dionísio, A.; Rivas, T. A Study on the suitability of mechanical soft-abrasive blasting methods to extract graffiti paints on ornamental stones. Coatings 2018, 8, 335. [Google Scholar] [CrossRef]
- Rivas, T.; Pozo, S.; Fiorucci, M.P.; López, A.J.; Ramil, A. Nd: YVO4 laser removal of graffiti from granite. Influence of paint and rock properties on cleaning efficacy. Appl. Surf. Sci. 2012, 263, 563–572. [Google Scholar] [CrossRef]
- Samolik, S.; Walczak, M.; Plotek, M.; Sarzynski, A.; Pluska, I.; Marczak, J. Investigation into the removal of graffiti on mineral supports: Comparison of nanosecond Nd:YAG laser cleaning with traditional mechanical and chemical methods. Stud. Conserv. 2015, 60, S58–S64. [Google Scholar] [CrossRef]
- Atanassova, V. Laser cleaning of graffiti spray paints on marble, limestone and granite. In Graffiti: Vandalism, Street Art and Cultural Significance; Paradis, X., Matthew, M., Eds.; Nova Science Publishers: New York, NY, USA, 2018; pp. 117–144. [Google Scholar]
- Pozo-Antonio, J.S.; Papanikolaou, A.; Melessanaki, K.; Rivas, T.; Pouli, P. Laser-assisted removal of graffiti from granite: Advantages of the simultaneous use of two wavelengths. Coatings 2018, 8, 124. [Google Scholar] [CrossRef]
- Sanmartín, P.; DeAraujo, A.; Vasanthakumar, A.; Mitchell, R. Feasibility study involving the search for natural strains of microorganisms capable of degrading graffiti from heritage materials. Int. Biodeterior. Biodegrad. 2015, 103, 186–190. [Google Scholar] [CrossRef]
- Sanmartín, P.; Bosch-Roig, P. Biocleaning to remove graffiti: A real possibility? Advances towards a complete protocol of action. Coatings 2019, 9, 104. [Google Scholar] [CrossRef]
- Macchia, A.; Ruffolo, S.A.; Rivaroli, L.; Malagodi, M.; Licchelli, M.; Rovella, N.; Randazzo, L.; la Russa, M.F. Comparative study of protective coatings for the conservation of urban art. J. Cult. Herit. 2020, 41, 232–237. [Google Scholar] [CrossRef]
- García, O.; Rz-Maribona, I.; Gardei, A.; Riedl, M.; Vanhellemont, Y.; Santarelli, M.L.; Suput, J.S. Comparative study of the variation of the hydric properties and aspect of natural stone and brick after the application of 4 types of anti-graffiti. Mater. De Constr. 2010, 60, 69–82. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Sánchez-Cortés, S.; Oujja, M.; Castillejo, M.; Blanco-Varela, M.T. Effectiveness of anti-graffiti treatments in connection with penetration depth determined by different techniques. J. Cult. Herit. 2010, 11, 297–303. [Google Scholar] [CrossRef]
- Grossi, D.; del Lama, E.A. Avaliação da eficácia de hidrofugantes e anti-graffiti no arenito itararé. geologia USP. Série Cient. 2018, 18, 43–55. [Google Scholar] [CrossRef]
- Moura, A.R.; Flores-Colen, I.; de Brito, J. Analysis of graffiti removal techniques and anti-graffiti products for porous materials. In Proceedings of the Hydrophobe VII—7th International Conference on Water Repellent Treatment and Protective Surface Technology for Building Materials, Lisboa, Portugal, 11–12 September 2014. [Google Scholar]
- García, O.; Malaga, K. Definition of the procedure to determine the suitability and durability of an anti-graffiti product for application on cultural heritage porous materials. J. Cult. Herit. 2012, 13, 77–82. [Google Scholar] [CrossRef]
- Gardei, G.; Garcia, O.; Riedl, M.; Vanhellemond, I.; Suput, J.S.; Santarelli, M.L.; Rodriguez-Maribona, I.; Müller, U. Performance and durability of a new anti-graffiti system for cultural heritage–The EC project GRAFFITAGE. In Proceedings of the 11th International Congress on Conservation and Deterioration of Stone, Torun, Poland, 15–20 September 2008; pp. 889–897. [Google Scholar]
- Ricci, C.; Gambino, F.; Nervo, M.; Piccirillo, A.; Scarcella, A.; Zenucchini, F.; Pozo-Antonio, J.S. Developing new cleaning strategies of cultural heritage stones: Are synergistic combinations of a low-toxic solvent ternary mixtures followed by laser the solution? Coatings 2020, 10, 466. [Google Scholar] [CrossRef]
- Gambino, F.; Borghi, A.; D’Atri, A.; Gallo, L.M.; Ghiraldi, L.; Giardino, M.; Martire, L.; Palomba, M.; Perotti, L. TOURinSTONES: A free mobile application for promoting geological heritage in the city of Turin (NW Italy). Geoheritage 2018, 11, 3–17. [Google Scholar] [CrossRef]
- Borghi, A.; Cadoppi, P.; Dino, G.A. The dora-maira unit (Italian cottian alps): A reservoir of ornamental stones locally and worldwide employed since Roman age. In EGU General Assembly Conference Abstracts; European Geosciences Union General Assembly: Vienna, Austria, 2015; Volume 17. [Google Scholar]
- Berra, V.; Borghi, A.; Gallo, L.M. I portici di via Roma a Torino: Rilievo architettonico e caratterizzazione petrografica. Riv. Piemont. Di Stor. Nat. 2013, 34, 3–80. [Google Scholar]
- Poretti, G.; Borghi, A.; Dino, G.A.; Ferrando, S.; Groppo, C.; Martire, L.; Accattino, E.; Favero Longo, S.E.; Piervittori, R.; Rolfo, F. The stone bridges on the Po River at Turin (NW Italy): A scientific dissemination approach for the development of urban geological heritage. In Engineering Geology for Society and Territory; Springer: Cham, Switzerland, 2015; Volume 8, pp. 207–211. [Google Scholar]
- Cavallo, A.; Bigioggero, B.; Colombo, A.; Tunesi, A. The verbano cusio ossola province: A land of quarries in northern Italy (Piedmont). Period. Mineral. 2004, 73, 197–210. [Google Scholar]
- Boriani, A.; Burlini, L.; Caironi, V.; Origoni, E.G.; Sassi, A.; Sesana, E. Geological and petrological studies on the hercynian plutonism of serie dei laghi–geological map of its occurrence between Valsesia and Lago Maggiore (N-Italy). Rend. Soc. It. Mineral. Petrol. 1988, 43, 367–384. [Google Scholar]
- Natural Stone Institute. Restoration and Maintenance. In An Excerpt from the Dimension Stone Design Manual, version VIII; Marble Institute of America: Oberlin, OH, USA, 2016; 123p. [Google Scholar]
- Sandrone, R.; Colombo, A.; Fiora, A.; Fornaro, L.; Lovera, E.; Tunesi, A.; Cavallo, A. Contemporary natural stones from the Italian western Alps (Piedmont and Aosta Valley Regions). Per. Mineral. 2004, 73, 211–226. [Google Scholar]
- Ciriaco, G.; Aldega, L. Il travertino: La Pietra di Roma; Società Geologica Italiana: Roma, Italy, 2013; Volume 27, pp. 98–109. [Google Scholar]
- Available online: www.san-marco.com (accessed on 31 January 2018).
- Available online: www.docchem.com (accessed on 31 January 2018).
- Available online: www.montanacolors.com (accessed on 31 January 2018).
- U.S. National Library of Medicine. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 31 January 2018).
- TriSolv Software, ISCR. Triangolo Interattivo dei Solventi e Delle Solubilità®. Available online: http://icr.beniculturali.it/flash/progetti/TriSolv/TriSolv.html (accessed on 31 January 2018).
- Cremonesi, P. L’uso dei Solventi Organici Nella Pulitura di Opere Policrome; Il Prato: Villatora, Italy, 2004. [Google Scholar]
- Kanegsberg, B.; Kanegsberg, E. (Eds.) Handbook for Critical Cleaning: Cleaning Agents and Systems; CRC Press: Cleveland, OH, USA, 2011. [Google Scholar]
- UNI 11432:2011. Beni Culturali—Materiali Lapidei Naturali ed Artificiali—Misura della Capacità di Assorbimento di Acqua Mediante Spugna di Contatto; Ente Italiano di Normazione: Rome, Italy, 2011; Available online: http://store.uni.com/catalogo/uni-11432-2011?josso_back_to=http://store.uni.com/josso-security-check.php&josso_cmd=login_optional&josso_partnerapp_host=store.uni.com (accessed on 21 June 2020).
- Vandevoorde, D.; Pamplona, M.; Schalm, O.; Vanhellemont, Y.; Cnudde, V.; Verhaeven, E. Contact sponge method: Performance of a promising tool for measuring the initial water absorption. J. Cult. Herit. 2009, 10, 41–47. [Google Scholar] [CrossRef]
- BS, EN. 828. Adhesives. Wettability. Determination by Measurement of Contact Angle and Surface Free Energy of Solid Surface; European Committee for Standardization: Brussels, Belgium, 2013. [Google Scholar]
- UNI-EN; ISO. Surface texture: Profile method, terms, definitions and surface texture parameters. In ISO 4288, Geometrical Product Specifications (GPS); Ente Italiano di Normazione: Rome, Italy, 1999. [Google Scholar]
- Mosquera, M.J.; Rivas, T.; Prieto, B.; Silva, B. Capillary rise in granitic rocks: Interpretation of kinetics on the basis of pore structure. J. Colloid Interface Sci. 2000, 222, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Bico, J.; Thiele, U.; Quéré, D. Wetting of textured surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 41–46. [Google Scholar] [CrossRef]
- de Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]
- Abel, A. Pigments for paints. In Paints in Surface Coatings: Theory and Practice; Lambourne, R., Strivens, T.A., Eds.; Woodhead Publishing: Cambridge, UK, 1999. [Google Scholar]
- Coladonato, M.; Scarpitti, P. Note sul Triangolo Interattivo dei Solvent e Delle Solubilità ©. 2008. Available online: http://www.icr.beniculturali.it/flash/progetti/TriSolv/dati/IT/TriSolv_IT.pdf (accessed on 31 January 2018).
- Marrion, A. The Chemistry and Physics of Coatings; The Royal Society of Chemistry: London, UK, 2004; 396p, ISBN 0-85404-656-9. [Google Scholar]
- Al-Kattan, A.; Wichser, A.; Zuin, S.; Arroyo, Y.; Golanski, L.; Ulrich, A.; Nowack, B. Behavior of TiO2 released from nano-TiO2-containing paint and comparison to pristine nano-TiO2. Environ. Sci. Technol. 2014, 48, 6710–6718. [Google Scholar] [CrossRef] [PubMed]
- Charter, V. The Venice Charter for the Conservation and Restoration of Monuments and Sites. 1964. Available online: http://www.icomos.org/venicecharter2004/index.html (accessed on 31 January 2018).

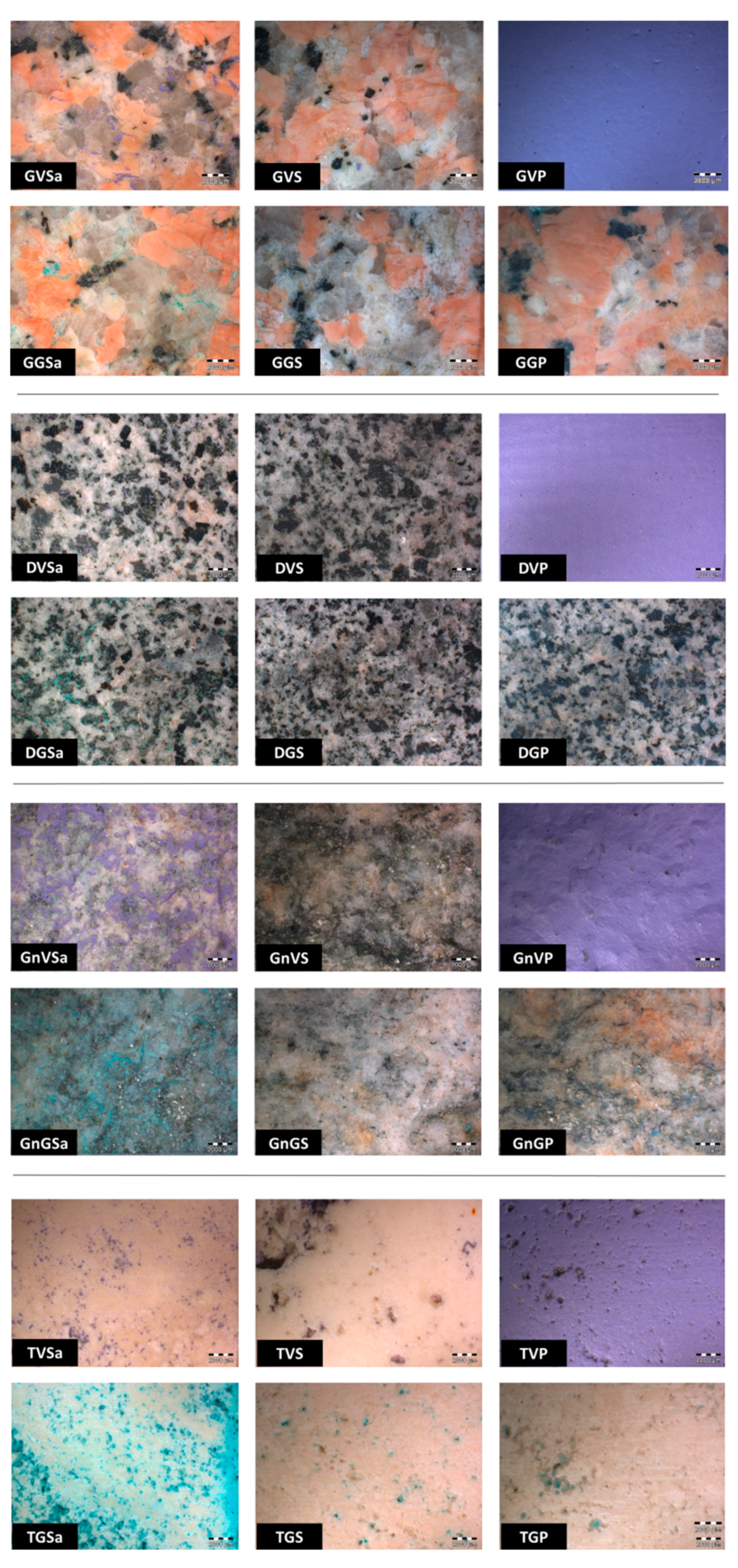
| Stone | Paint Layer | Description | ID |
|---|---|---|---|
| Granite | – | Reference stone | G |
| – | Sacrificial anti-graffiti | GS | |
| – | Permanent anti-graffiti | GP | |
| Violet | Solvent mixture A | GVSa | |
| Sacrificial anti-graffiti | GVS | ||
| Permanent anti-graffiti | GVP | ||
| Green | Solvent mixture A | GGSa | |
| Sacrificial anti-graffiti | GGS | ||
| Permanent anti-graffiti | GGP | ||
| Diorite | – | Reference stone | D |
| – | Sacrificial anti-graffiti | DS | |
| – | Permanent anti-graffiti | DP | |
| Violet | Solvent mixture B | DVSb | |
| Sacrificial anti-graffiti | DVS | ||
| Permanent anti-graffiti | DVP | ||
| Green | Solvent mixture B | DGSb | |
| Sacrificial anti-graffiti | DGS | ||
| Permanent anti-graffiti | DGP | ||
| Gneiss | – | Reference stone | Gn |
| – | Sacrificial anti-graffiti | GnS | |
| – | Permanent anti-graffiti | GnP | |
| Violet | Solvent mixture A | GnVSa | |
| Sacrificial anti-graffiti | GnVS | ||
| Permanent anti-graffiti | GnVP | ||
| Green | Solvent mixture A | GnGSa | |
| Sacrificial anti-graffiti | GnGS | ||
| Permanent anti-graffiti | GnGP | ||
| Travertine | – | Reference stone | T |
| – | Sacrificial anti-graffiti | TS | |
| – | Permanent anti-graffiti | TP | |
| Violet | Solvent mixture B | TVSb | |
| Sacrificial anti-graffiti | TVS | ||
| Permanent anti-graffiti | TVP | ||
| Green | Solvent mixture B | TGSb | |
| Sacrificial anti-graffiti | TGS | ||
| Permanent anti-graffiti | TGP |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, C.; Gambino, F.; Nervo, M.; Piccirillo, A.; Scarcella, A.; De Stefanis, A.; Pozo-Antonio, J.S. Anti-Graffiti Coatings on Stones for Historical Buildings in Turin (NW Italy). Coatings 2020, 10, 582. https://doi.org/10.3390/coatings10060582
Ricci C, Gambino F, Nervo M, Piccirillo A, Scarcella A, De Stefanis A, Pozo-Antonio JS. Anti-Graffiti Coatings on Stones for Historical Buildings in Turin (NW Italy). Coatings. 2020; 10(6):582. https://doi.org/10.3390/coatings10060582
Chicago/Turabian StyleRicci, Chiara, Francesca Gambino, Marco Nervo, Anna Piccirillo, Arianna Scarcella, Alessandra De Stefanis, and Jose Santiago Pozo-Antonio. 2020. "Anti-Graffiti Coatings on Stones for Historical Buildings in Turin (NW Italy)" Coatings 10, no. 6: 582. https://doi.org/10.3390/coatings10060582
APA StyleRicci, C., Gambino, F., Nervo, M., Piccirillo, A., Scarcella, A., De Stefanis, A., & Pozo-Antonio, J. S. (2020). Anti-Graffiti Coatings on Stones for Historical Buildings in Turin (NW Italy). Coatings, 10(6), 582. https://doi.org/10.3390/coatings10060582







