Architectured Cu–TNTZ Bilayered Coatings Showing Bacterial Inactivation under Indoor Light and Controllable Copper Release: Effect of the Microstructure on Copper Diffusion
Abstract
1. Introduction
2. Experimental Details
2.1. Deposition Procedures of the Nanoarchitected Films of Cu and TNTZ
2.2. Physical and Chemical Characterization of the Sputtered Samples by Energy Dispersive X-Ray Spectroscopy (EDX), SEM, Atomic Force Microscopy (AFM), and XRD
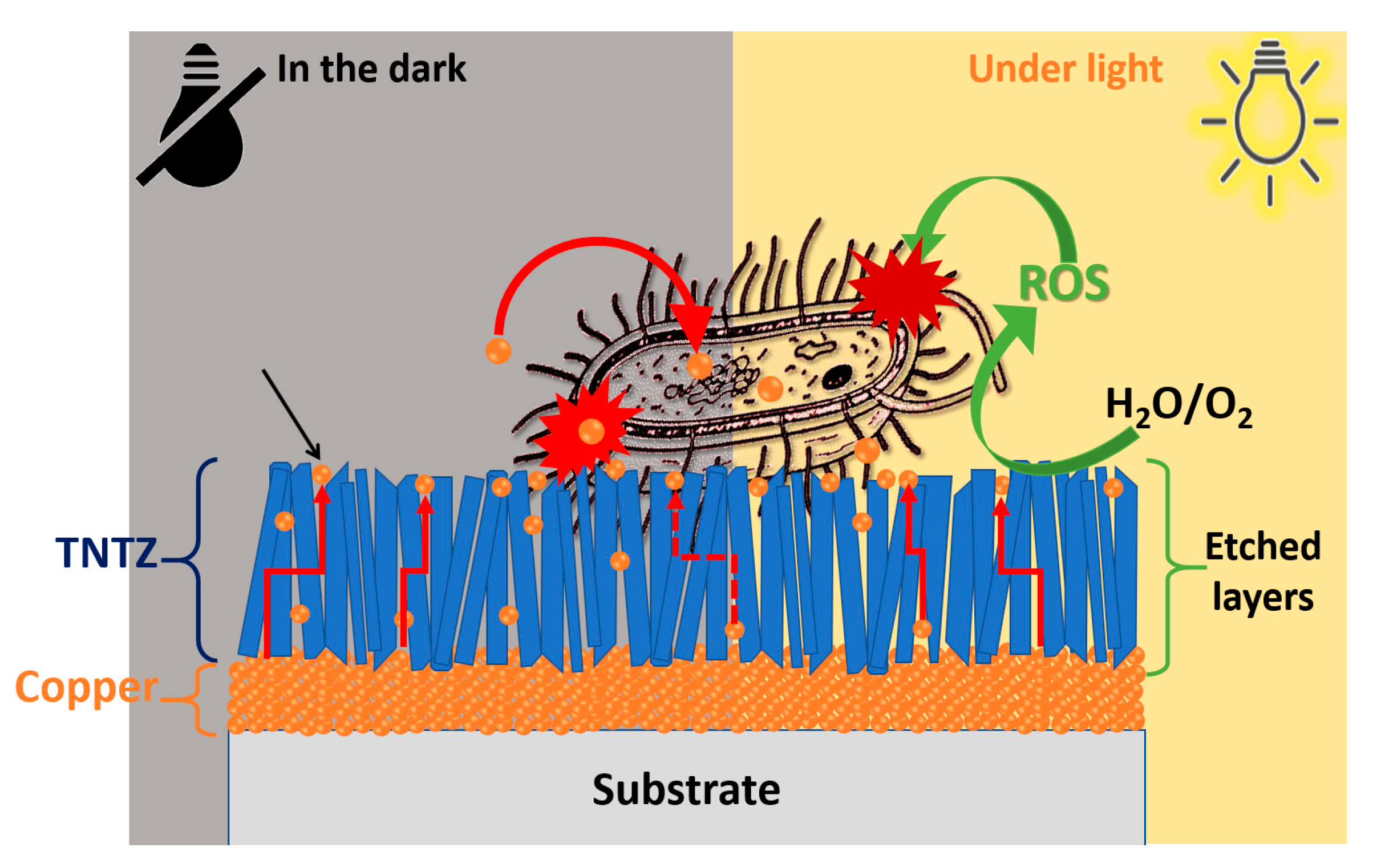
2.3. Bacterial Adhesion to the Coating Surface, Bacterial Inactivation Testing, and the Sustainability of the Coatings
2.4. Copper Release and Atomic Etching of the Architectured Samples
3. Results and Discussion
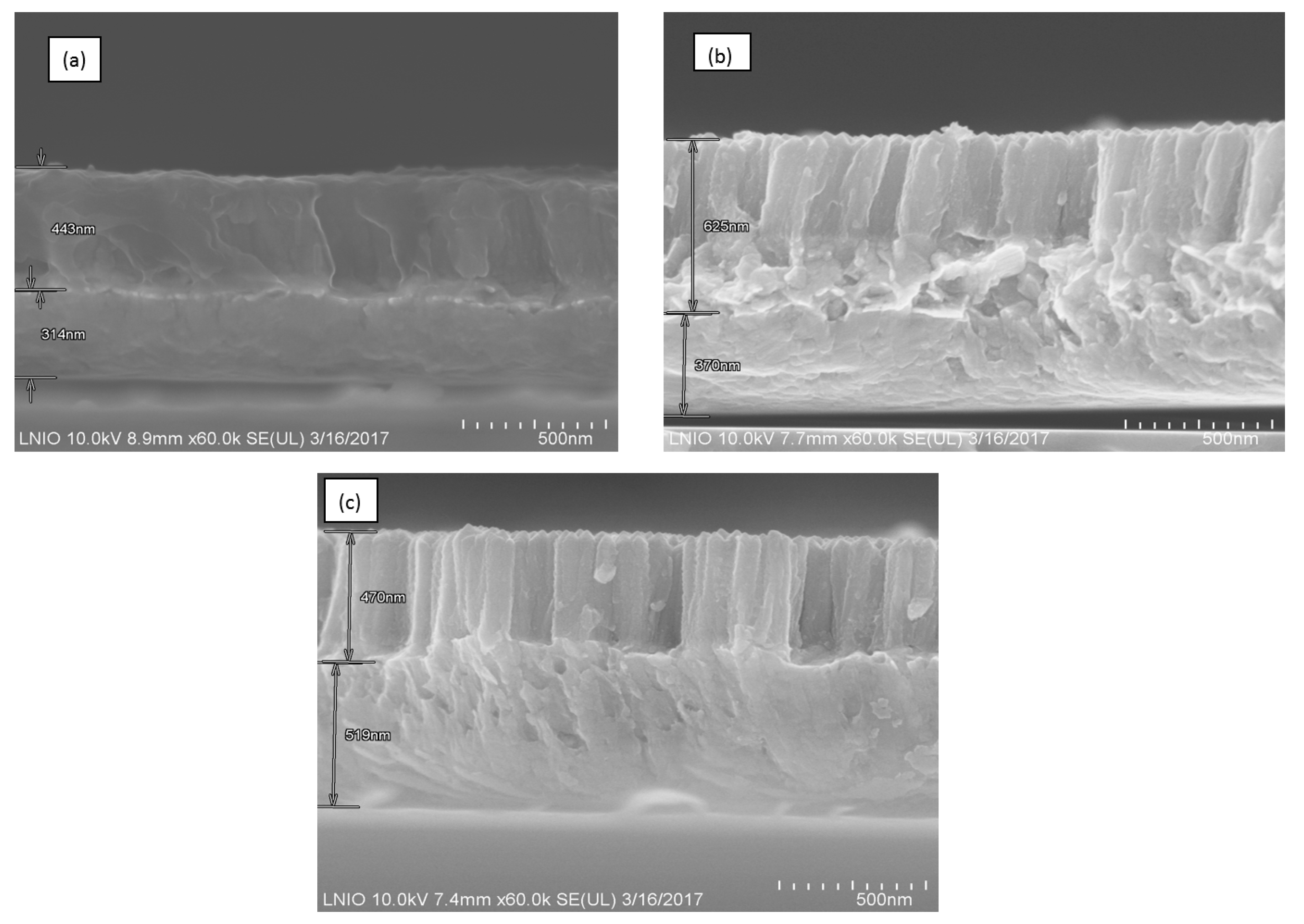
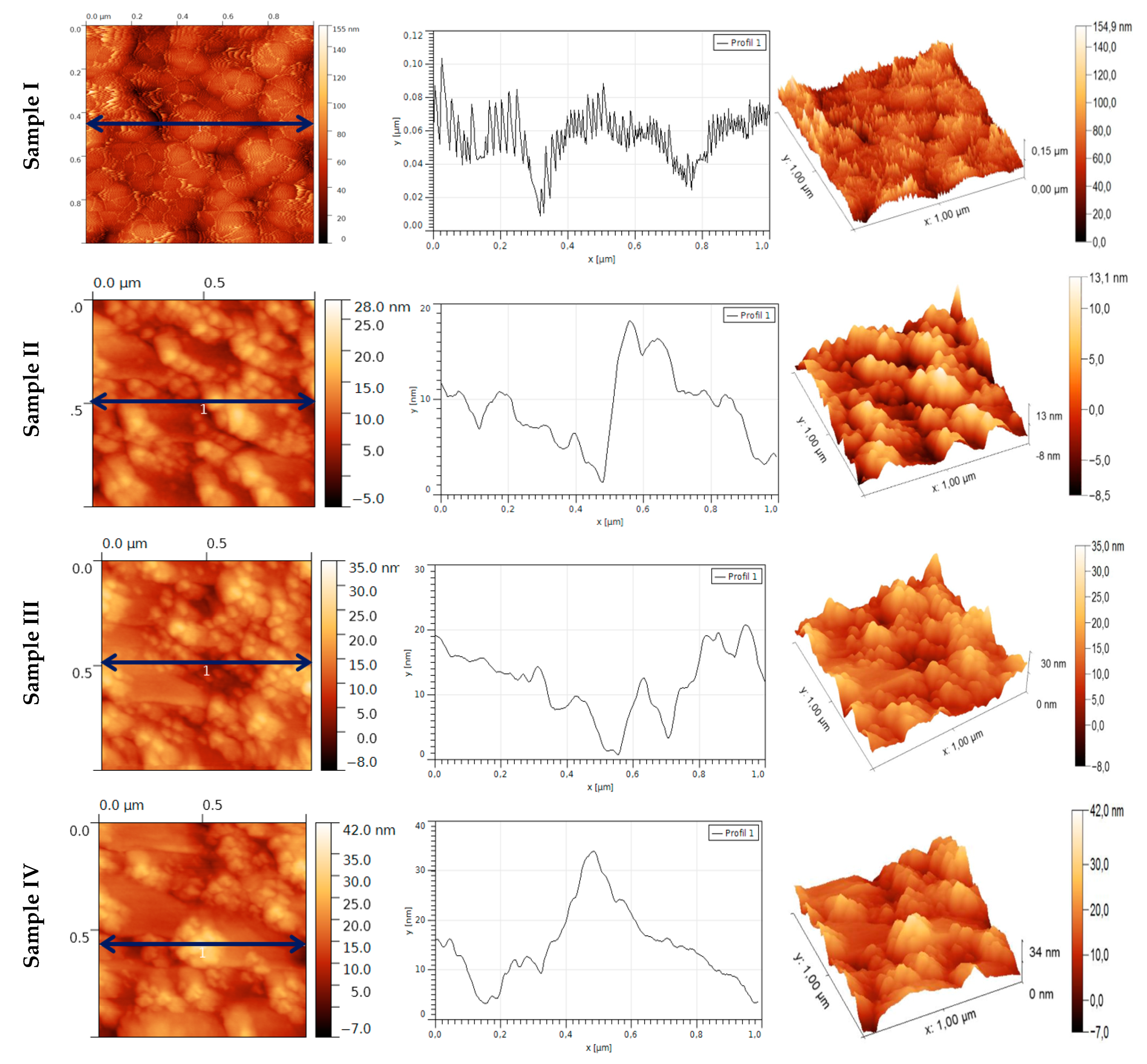
3.1. Elemental Composition, Morphology, and Topography of Cu–TNTZ Thin Films Deposited under Different Pressures
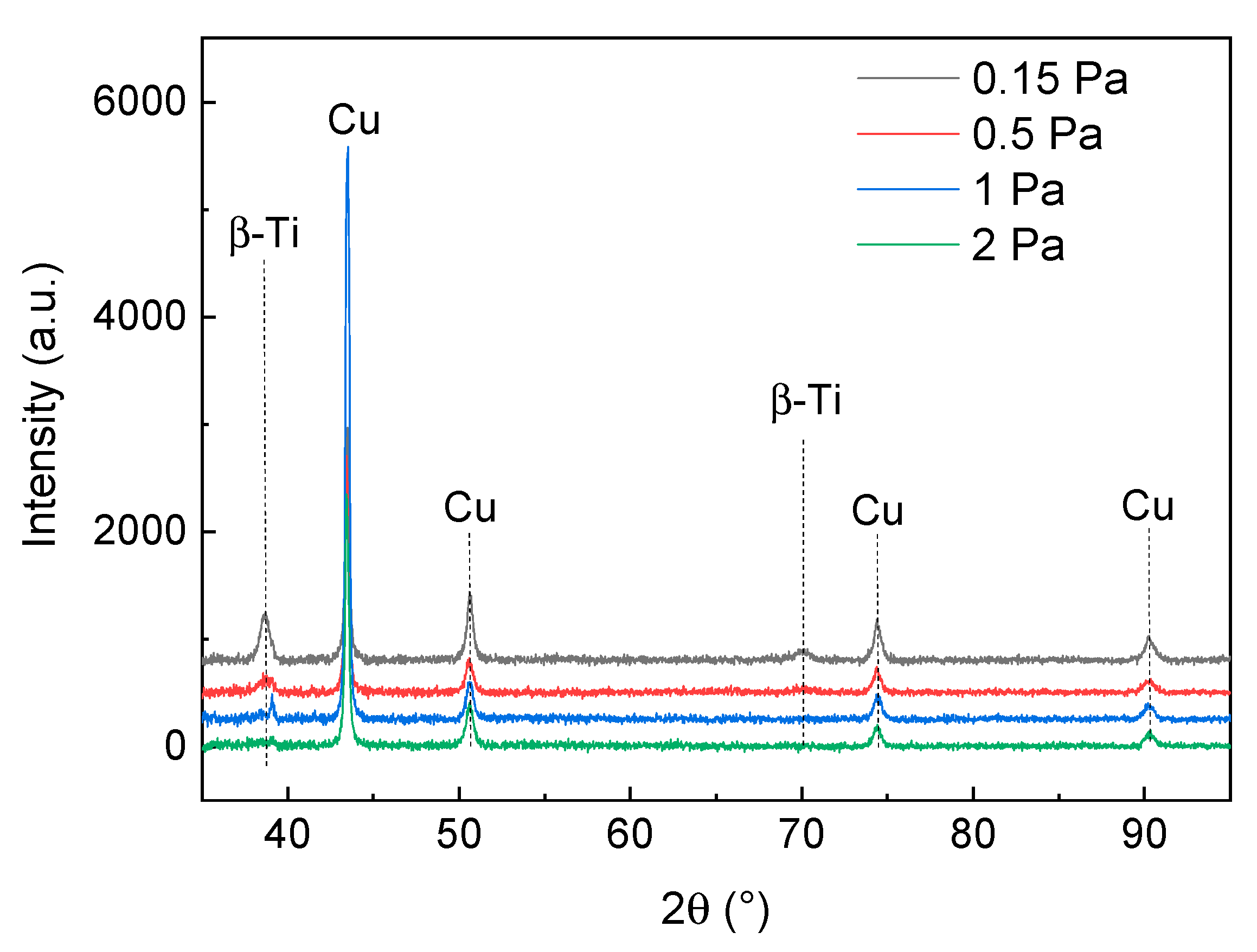
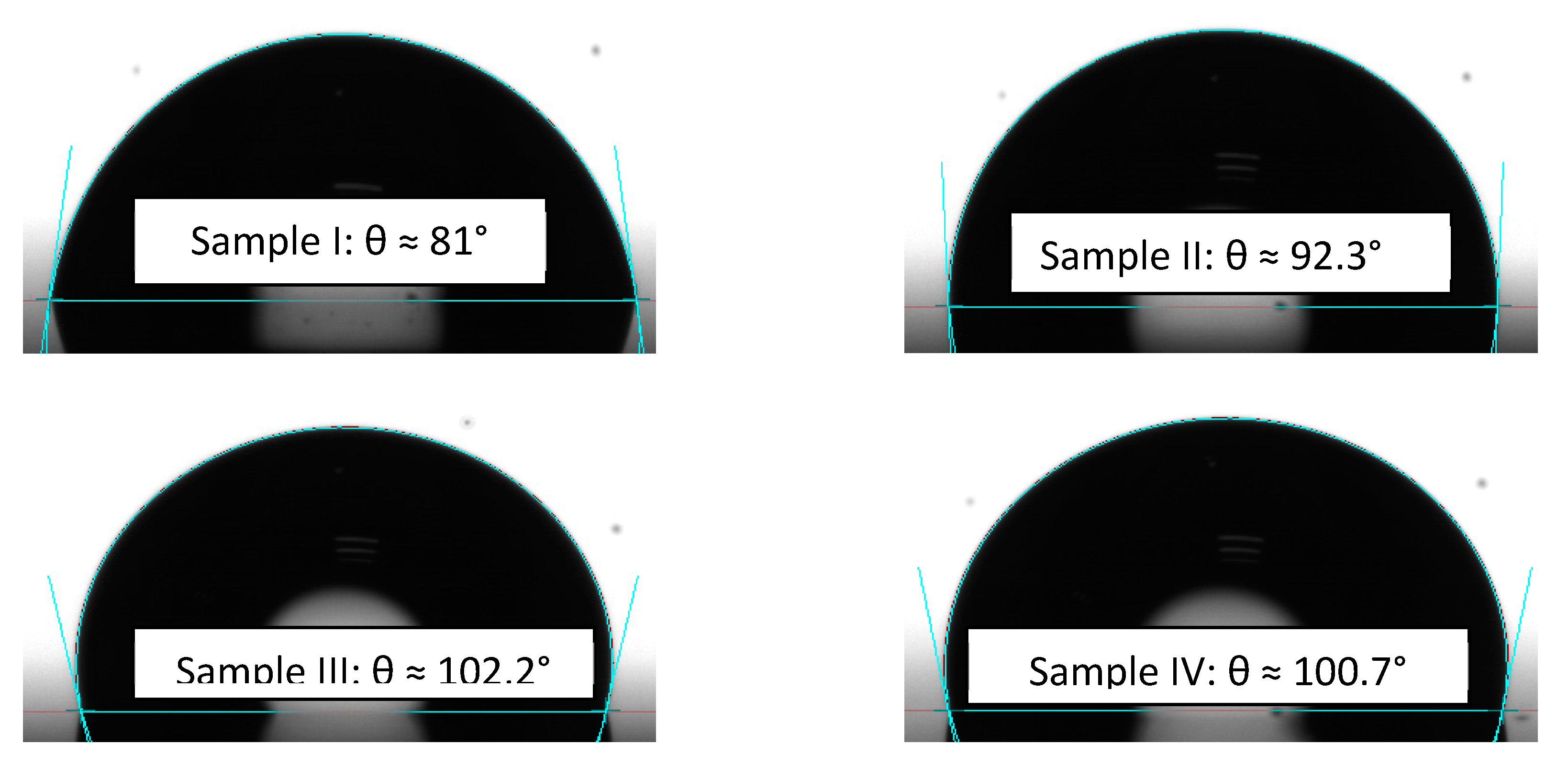
3.2. Crystalline Structure and Hydrophobic/Hydrophilic Properties of the TNTZ Films
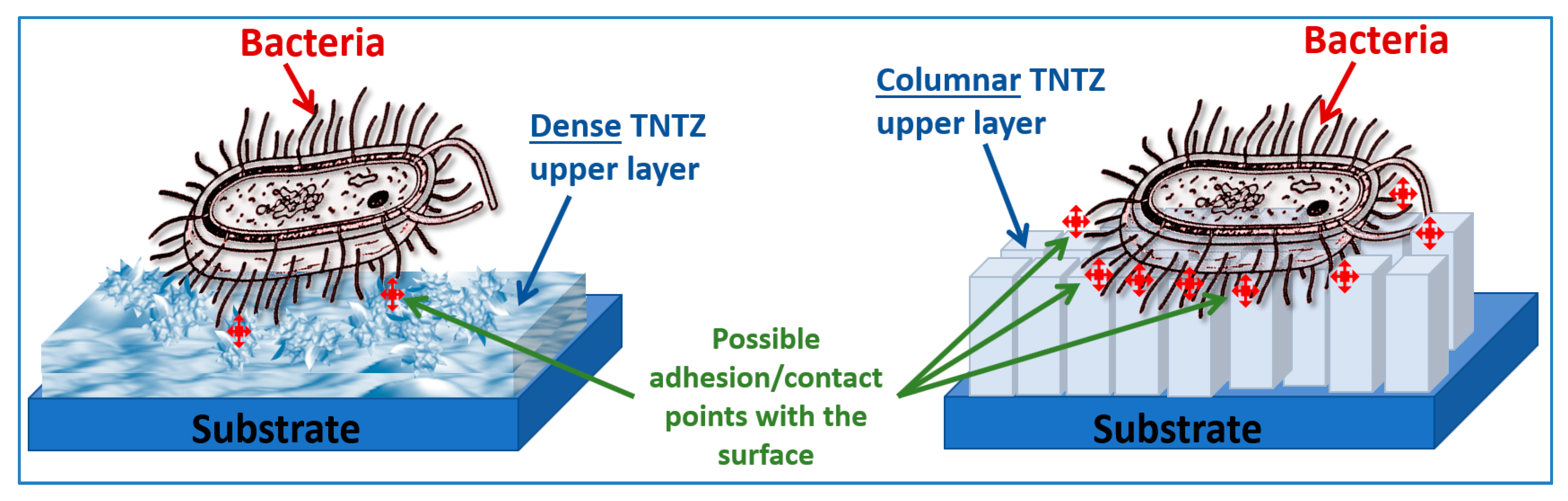
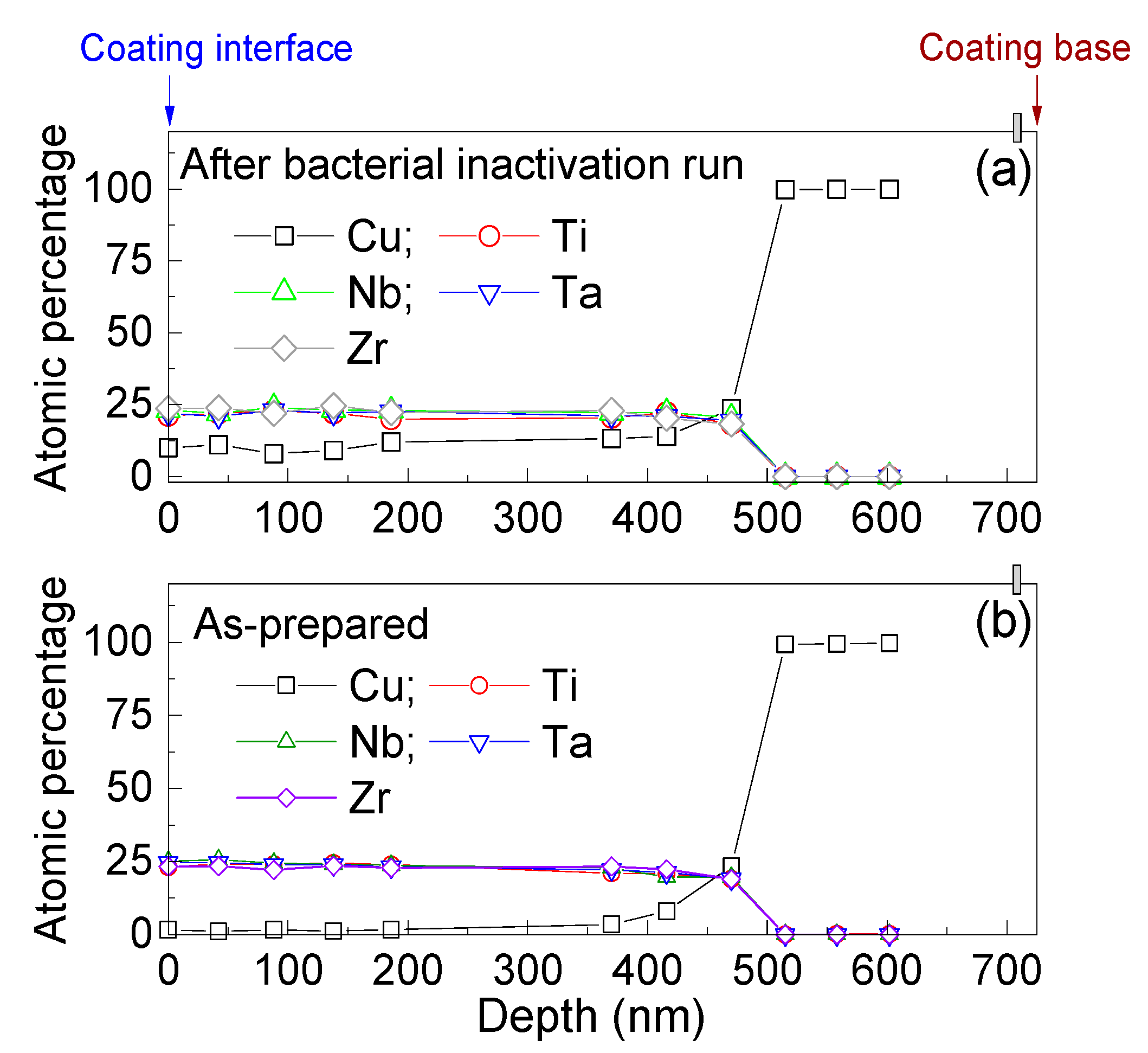
3.3. Bacterial Adhesion and Inactivation, and the Atomic Depth Profiling before and after Bacterial Inactivation
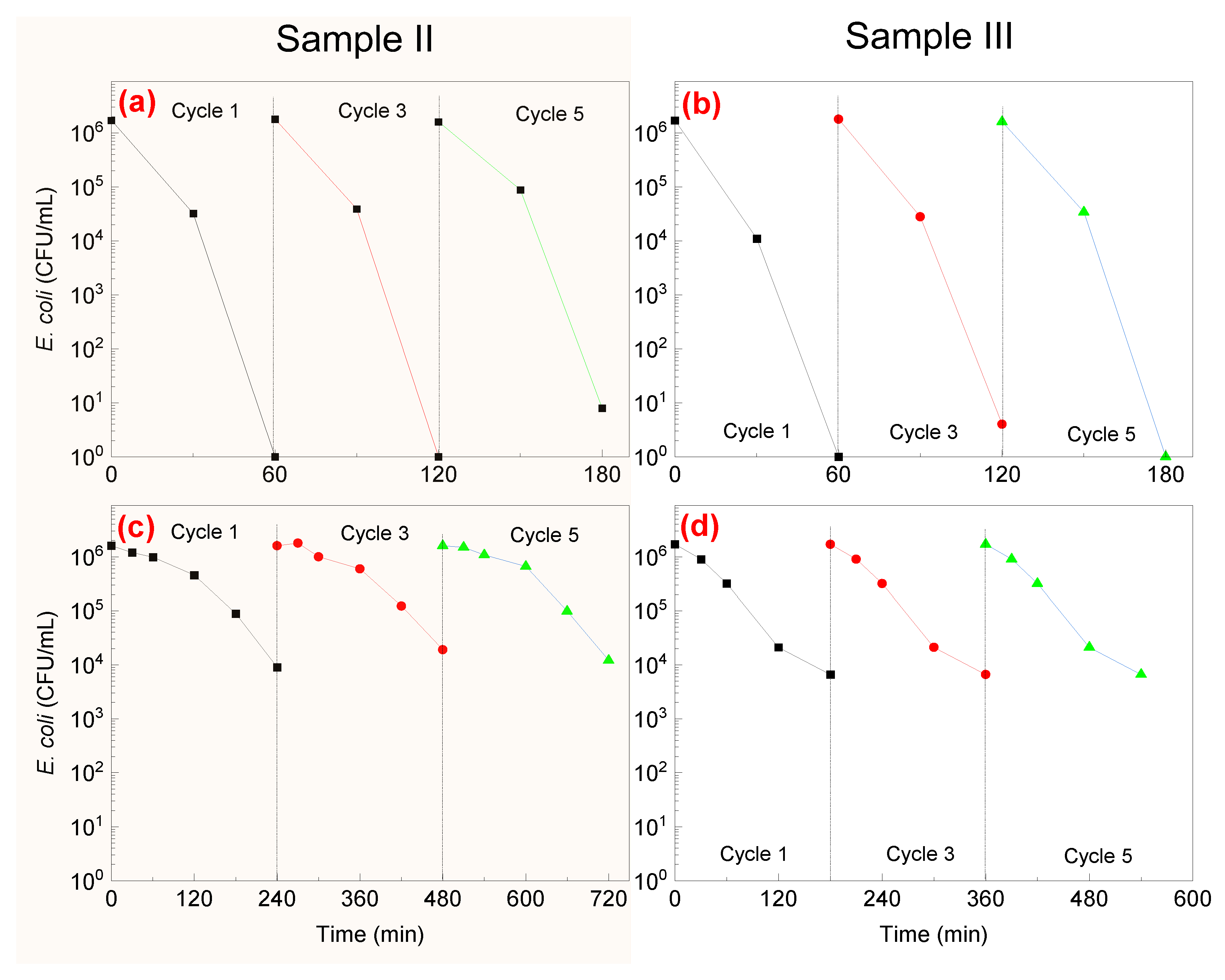
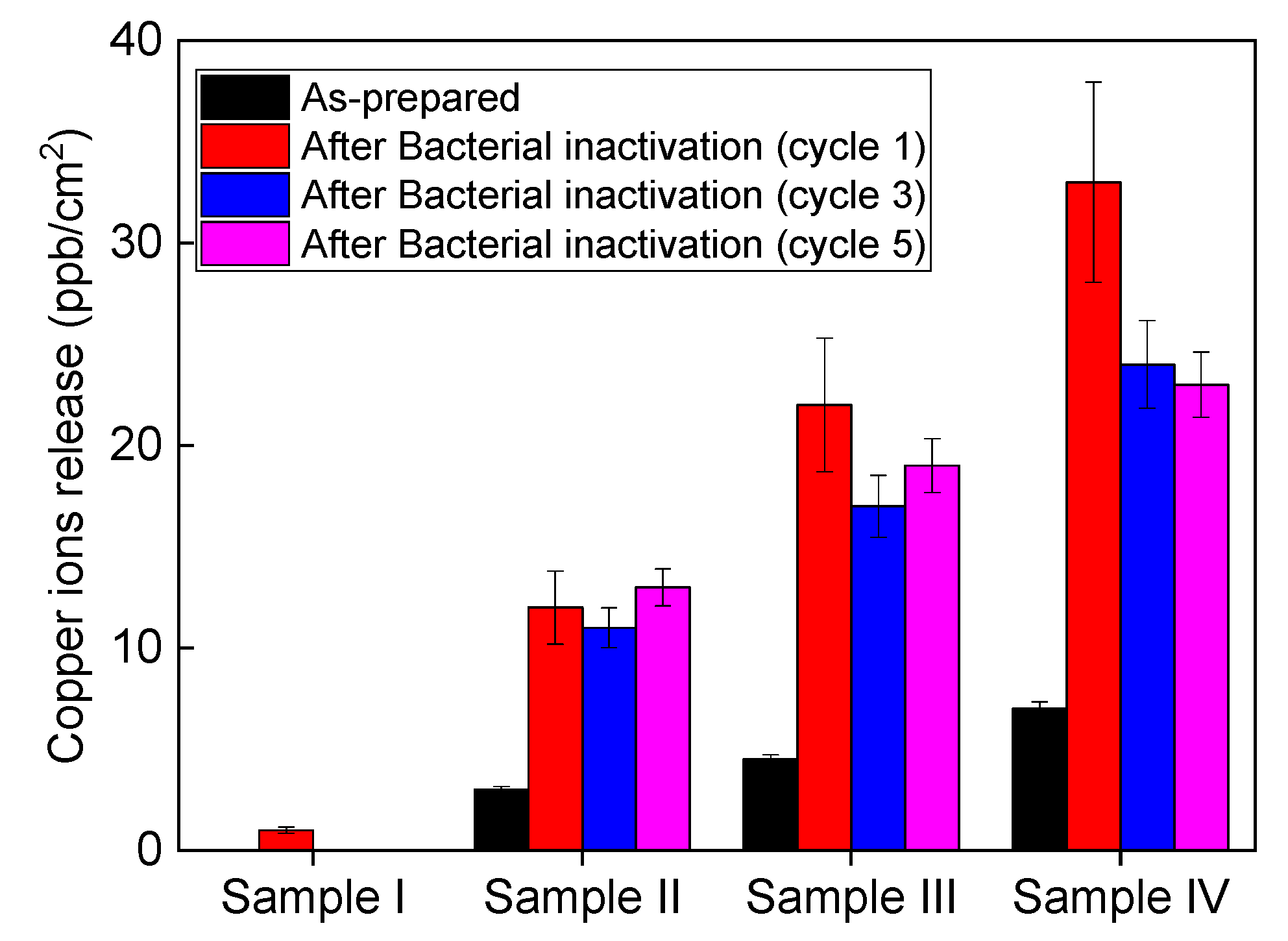
3.4. Recycling and Ion Release during Bacterial Inaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.Y.; Sasaki, T.; Okutsu, K.; Kim, J.I.; Inamura, T.; Hosoda, H.; Miyazaki, S. Texture and shape memory behavior of Ti–22Nb–6Ta alloy. Acta Mater. 2006, 54, 423–433. [Google Scholar] [CrossRef]
- Tane, M.; Akita, S.; Nakano, T.; Hagihara, K.; Umakoshi, Y.; Niinomi, M.; Nakajima, H. Peculiar elastic behavior of Ti–Nb–Ta–Zr single crystals. Acta Mater. 2008, 56, 2856–2863. [Google Scholar] [CrossRef]
- Hu, Q.M.; Li, S.J.; Hao, Y.L.; Yang, R.; Johansson, B.; Vitos, L. Phase stability and elastic modulus of Ti alloys containing Nb, Zr, and/or Sn from first-principles calculations. Appl. Phys. Lett. 2008, 93, 121902. [Google Scholar] [CrossRef]
- Li, S.J.; Cui, T.C.; Hao, Y.L.; Yang, R. Fatigue properties of a metastable β-type titanium alloy with reversible phase transformation. Acta Biomater. 2008, 4, 305–317. [Google Scholar] [CrossRef]
- Sikka, S.K.; Vohra, Y.K.; Chidambaram, R. Omega phase in materials. Prog. Mater. Sci. 1982, 27, 245–310. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue performance and cyto-toxicity of low rigidity titanium alloy, Ti–29Nb–13Ta–4.6 Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef]
- Laheurte, P.; Prima, F.; Eberhardt, A.; Gloriant, T.; Wary, M.; Patoor, E. Mechanical properties of low modulus β titanium alloys designed from the electronic approach. J. Mech. Behav. Biomed. Mater. 2010, 3, 565–573. [Google Scholar] [CrossRef]
- Saito, T.; Furuta, T.; Hwang, J.H.; Kuramoto, S.; Nishino, K.; Suzuki, N. Multifunctional alloys obtained via a dislocation-free plastic deformation mechanism. Science 2003, 300, 464. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Recent developments in the research of shape memory alloys. Intermetallics 1999, 7, 511. [Google Scholar] [CrossRef]
- Rtimi, S.; Giannakis, S.; Sanjines, R.; Pulgarin, C.; Bensimon, M.; Kiwi, J. Insight on the photocatalytic bacterial inactivation by co-sputtered TiO2–Cu in aerobic and anaerobic conditions. Appl. Catal. B Environ. 2016, 182, 277–285. [Google Scholar] [CrossRef]
- Baghriche, O.; Rtimi, S.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Effect of the spectral properties of TiO2, Cu, TiO2/Cu sputtered films on the bacterial inactivation under low intensity actinic light. J. Photochem. Photobiol. A Chem. 2013, 251, 50–56. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Kaczmarek, D.; Szponar, B.; Grobelny, M.; Kalisz, M.; Pelczarska, A.; Szczygiel, I.; Poniedzialek, A.; Osekowska, M. Structural and surface properties of semitransparent and antibacterial (Cu, Ti, Nb) Ox coating. Appl. Surf. Sci. 2016, 380, 159–164. [Google Scholar] [CrossRef]
- Brown, R.D.; Boggs, J.E.; Hilderbrandt, R.; Lim, K.; Mills, I.M.; Niktin, E.; Palmer, M.H. Gum Metal thin films obtained by magnetron sputtering of a Ti-Nb-Zr-Ta target. Mater. Sci. Eng. A 2016, 673, 492–502. [Google Scholar]
- Achache, S.; Lamri, S.; Arab Pour Yazdi, M.; Billard, A.; François, M.; Sanchette, F. Ni-free superelastic binary Ti–Nb coatings obtained by DC magnetron co-sputtering. Surf. Coat. Technol. 2015, 275, 283–288. [Google Scholar] [CrossRef]
- Achache, S.; Alhussein, A.; Guelorget, B.; Salut, R.; François, M.; Sanchette, F. Effect of oxygen addition on microstructure and mechanical properties of quaternary TNTZ superelastic thin films obtained by magnetron sputtering. Mater. Chem. Phys. 2018, 217, 262–269. [Google Scholar] [CrossRef]
- Achache, S.; Alhussein, A.; Lamri, S.; François, M.; Sanchette, F.; Pulgarin, C.; Kiwi, J.; Rtimi, S. Sputtered Gum metal thin films showing bacterial inactivation and biocompatibility. Colloids Surf. B Biointerfaces 2016, 146, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Kanagesan, M.; Hashim, S.; Tamilselvan, A.; Ismail, I.; Ahsanul, K. Synthesis, characterization, and cytotoxicity of iron oxide nanoparticles. Adv. Mat. Sci. Eng. 2013, 2013, 710432. [Google Scholar] [CrossRef]
- Alhussein, A.; Achache, S.; Deturche, R.; Sanchette, F.; Pulgarin, C.; Kiwi, J.; Rtimi, S. Beneficial effect of Cu on Ti-Nb-Ta-Zr sputtered uniform/adhesive gum films accelerating bacterial inactivation under indoor visible light. Colloids Surf. B Biointerfaces 2017, 152, 152–158. [Google Scholar] [CrossRef]
- Seddiki, O.; Harnagea, C.; Levesque, I.; Mantovani, D.; Rosei, F. Evidence ofantibacterial activity on titanium surfaces through nanotextures. Appl. Surf. Sci. 2014, 308, 275–284. [Google Scholar] [CrossRef]
- Kadlec, R.; Jakubec, M.; Jaglic, Z. A novel flotation technique for the separation of Non-Adherent microorganisms from a substrate. Lett. Appl. Microbiol. 2014, 58, 604–609. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction, 2nd ed.; Addison-Wesley: Reading, MA, USA, 1978; p. 292. [Google Scholar]
- Bonnefond, A.; Gonzalez, E.; Asua, J.M.; Leiza, J.R.; Kiwi, J.; Pulgarin, C.; Rtimi, S. New evidence for hybrid acrylic/TiO2 films inducing bacterial inactivation under low intensity simulated sunlight. Colloids Surf. B Biointerfaces 2015, 135, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lamari, F.; Chakroun, I.; Rtimi, S. Assessment of the correlation among antibiotic resistance, adherence to abiotic and biotic surfaces, invasion and cytotoxicity of Pseudomonas aeruginosa isolated from diseased gilthead sea bream. Colloids Surf. B Biointerfaces 2017, 158, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Kiwi, J. Recent advances on sputtered films with Cu in ppm concentrations leading to an acceleration of the bacterial inactivation. Catal. Today 2020, 340, 347–362. [Google Scholar] [CrossRef]
- Rtimi, S. Indoor light enhanced photocatalytic Ultra-Thin films on flexible Non-Heat resistant substrates reducing bacterial infection risks. Catalysts 2017, 7, 57. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Pulgarin, C.; Kiwi, J. Microstructure of Cu–Ag uniform nanoparticulate films on polyurethane 3D catheters: Surface properties. ACS Appl. Mater. Interfaces 2015, 8, 56–63. [Google Scholar] [CrossRef]
- Graf, A.; Finkel, J.; Chauvet, A.A.P.; Rtimi, S. Deciphering the mechanisms of bacterial inactivation on HiPIMS Sputtered CuxO-FeOx-PET Surfaces: From light absorption to catalytic bacterial death. ACS Appl. Mater. Interfaces 2019, 11, 45319–45329. [Google Scholar] [CrossRef]
- Rtimi, S.; Dionysiou, D.D.; Pillai, S.C.; Kiwi, J. Advances in bacterial inactivation by Ag, Cu, Cu-Ag coated surfaces & medical devices. Appl. Catal. B Environ. 2019, 240, 291–318. [Google Scholar]
- Zeghioud, H.; Assadi, A.A.; Khellaf, N.; Djelal, H.; Amrane, A.; Rtimi, S. Photocatalytic performance of CuxO/TiO2 deposited by HiPIMS on polyester under visible light LEDs: Oxidants, ions effect, and reactive oxygen species investigation. Materials 2019, 12, 412. [Google Scholar] [CrossRef]


| Sample | Cu Under Layer | TNTZ Upper Layer | ||
|---|---|---|---|---|
| Argon Flow Rate (sccm) | Working Pressure (Pa) | Argon Flow Rate (sccm) | Working Pressure (Pa) | |
| I | 15 | 0.15 | 15 | 0.15 |
| II | 50 | 0.5 | ||
| III | 90 | 1 | ||
| IV | 140 | 2 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhussein, A.; Achache, S.; Deturche, R.; Rtimi, S. Architectured Cu–TNTZ Bilayered Coatings Showing Bacterial Inactivation under Indoor Light and Controllable Copper Release: Effect of the Microstructure on Copper Diffusion. Coatings 2020, 10, 574. https://doi.org/10.3390/coatings10060574
Alhussein A, Achache S, Deturche R, Rtimi S. Architectured Cu–TNTZ Bilayered Coatings Showing Bacterial Inactivation under Indoor Light and Controllable Copper Release: Effect of the Microstructure on Copper Diffusion. Coatings. 2020; 10(6):574. https://doi.org/10.3390/coatings10060574
Chicago/Turabian StyleAlhussein, Akram, Sofiane Achache, Regis Deturche, and Sami Rtimi. 2020. "Architectured Cu–TNTZ Bilayered Coatings Showing Bacterial Inactivation under Indoor Light and Controllable Copper Release: Effect of the Microstructure on Copper Diffusion" Coatings 10, no. 6: 574. https://doi.org/10.3390/coatings10060574
APA StyleAlhussein, A., Achache, S., Deturche, R., & Rtimi, S. (2020). Architectured Cu–TNTZ Bilayered Coatings Showing Bacterial Inactivation under Indoor Light and Controllable Copper Release: Effect of the Microstructure on Copper Diffusion. Coatings, 10(6), 574. https://doi.org/10.3390/coatings10060574







