Abstract
A series of self-matting waterborne polyurethanes (WPUs) were successfully prepared by introducing hydrophilic units into both soft and hard segments. By employing a polycaprolactone polyol containing carboxylate groups within the polymer chains to provide hydrophilicity directly, the matting performance of WPU films was greatly improved. The chemical structures of the WPU resins were confirmed by FTIR spectroscopy, and the morphology of WPU films was observed by SEM. The parameters of WPU preparation were investigated in detail. It was found that the surface gloss of WPU films as well as the particle sizes of WPU dispersions were closely associated with the content of hydrophilic units. As the content of carboxylates or sulfonates increased, the particle sizes of WPU decreased, while the gloss increased gradually. When the particle sizes of dispersions were greater than 3 μm, the gloss of WPU films coated on a leather surface was lower than 1. The results of TG showed that, the initial decomposition temperatures of WPU films were higher than 280 °C, which indicated these films also had good thermal stability. The prepared self-matting WPU coatings would have potential application prospects in the field of leather finishing.
1. Introduction
Waterborne polyurethane (WPU) is a kind of environmentally friendly material, which performance is easy to tailor or modify [1,2,3,4,5]. WPU can be used as a leather finishing agent to impart special glosses to the leather surface, such as high gloss and low gloss [6,7]. The demands for low-gloss coatings have grown fast in recent years because of their soft color and harmlessness to human eyes.
Generally, low gloss can be achieved by adding matting agent to form a rough surface [8]. A large number of matting agents have been introduced into waterborne polyurethane, and the most commonly used therein is known as silica powder with different particle size [9,10]. However, due to incompatibility with the organic polyurethane, the matting agents were poorly dispersed in WPU, which resulted in gloss inhomogeneity on the coating surface. In addition, the incompatible matting agents easily fall off the coating surface, thus leading to the loss of matting effect after intermittent use [11].
As a new type of low-gloss material, self-matting waterborne polyurethanes usually have wider particle distribution, where large particles can play a role similar to matting agents, and small particles will impart better adhesion to the coating film [12,13,14,15]. Since no matting agent was contained in the dispersion, the film performance can be greatly improved, especially in the anti-scratch property. There have been some reports on self-matting waterborne polyurethanes. The patent showed a non-ionically hydrophilized polyurethane dispersion [16]. Films with a low degree of gloss can be obtained by regulating the mass of water added during chain extension. Li et al. prepared low-glossed waterborne polyurethane using poly(tetramethylene glycol) (PTMG) as soft segment, isophorone diisocyanate (IPDI) as hard segment, using sulfonic acid sodium salt (AAS) and 2,2-bis(hydroxymethyl) propionic acid (DMPA) as hydrophilic chain-extending agents [17]. By adjusting the ratio of the two hydrophilic monomers AAS and DMPA, the prepared WPU exhibited a broad particle size distribution combined with a relatively large particle size. The roughness of the film surface was increased to achieve matting effect, in which the minimum gloss was 1.5. Cao et al. used TMP as a crosslinking agent and the same hydrophilic chain extenders DMPA and AAS as reported by Li et al. The obtained waterborne polyurethane had lower gloss of 1 [18]. However, a high boiling point solvent NMP was introduced to dissolve DMPA during the preparation of the dispersion.
As mentioned above, most of the matting waterborne polyurethanes were prepared by introducing hydrophilic DMPA into the hard segment. As a hydrophilic chain extender, DMPA played a very important role in the preparation of polyurethane dispersions [16,17,18,19]. High boiling point solvents such as NMP, DMF etc. were used to dissolve DMPA during the reaction [18,20,21]. Su et al. used dimethylolbutanoic acid (DMBA) with a low melt point instead of DMPA to prepare free-organic solvent waterborne polyurethane [22]. But the price of DMBA was much higher than that of DMPA which limited its application in this field.
In this paper, a macromolecular polyol containing carboxylic acid unit in polycaprolactone (PCL) chain was employed to provide carboxylate groups instead of DMPA. A series of self-matting WPUs were successfully prepared by introducing hydrophilic units into both soft segment and hard segment. The matting performance was greatly improved with adjusting the content of hydrophilic groups in the soft and hard segments. In this case, not only WPUs with good matting performance were obtained, but also high boiling solvents such as NMP, DMF, etc., were avoided. Moreover, this method simplified the experimental preparation steps and shortened the reaction time. The influences of PCLD, AAS, R value and hard segment on the properties of waterborne polyurethane were investigated in detail.
2. Materials and Methods
2.1. Materials
Polycaprolactone polyol (PCL, Mn = 2000 g/mol) was supplied from Hunan Juren Chemical Hitechnology Co., Ltd. (Hunan, China). Polycaprolactone polyol with dimethylol propionic acid on the molecular chain (PCLD, Mn = 2000 g/mol) was prepared according to literature method [23]. Isophorone diisocyanate (IPDI) was purchased from Bayer (Leverkusen, Germany), neopentyl glycol (NPG) was purchased from Macklin Shanghai, China). 2-[(2-Aminoethyl)amino]ethanesulfonic acid sodium salt (AAS) was purchased from Shenyuan (Xiaogan, China). Isophorone diamine (IPDA) and triethylamine (TEA) were purchased from Aladdin (Shanghai, China). ethylenediamine (EDA) and acetone were purchased from Beijing Tongguang Fine Chemical Company (Beijing, China). Dibutyltin dilaurate (DBTDL) was obtained from Xilong Fine Chemical Co., Ltd. (Guangzhou, China).
2.2. Synthesis of Waterborne Polyurethane and Preparation of Film
A certain amount of PCL and PCLD were placed in a three-necked flask and evacuated under 110 °C for 1 h. After cooling to 85 °C, IPDI and NPG with DBTDL as the catalyst were added and followed by stirring for 5 h. The mixture was cooled to 40 °C while the viscosity was adjusted with an appropriate amount of acetone. Subsequently, the appropriate amount of IPDA, EDA and AAS were added dropwise in 20 min. TEA was added to neutralize the carboxyl group for 5 min. Finally, at room temperature, an aqueous polyurethane emulsion was obtained after emulsification for 30 min with 100 g of deionized water. The WPU synthesis scheme is shown in Scheme 1, and the main components of the WPU samples are summarized in Table 1. The R value is the molar ratio of the total amount of –NCO groups to –OH groups in the raw materials, which equals n(NCO)/n(OH)). The R value was adjusted by changing the ratio of IPDI to NPG while fixing the content of soft segment, and the hard segment content (Hs) was the proportion of other molecules involved in the reaction except macromolecular polyol.
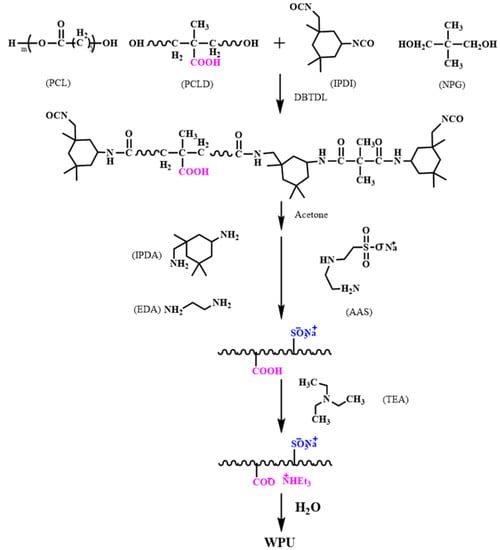
Scheme 1.
Synthesis of waterborne polyurethane dispersions.

Table 1.
The main compositions of prepared waterborne polyurethane.
The aqueous polyurethane dispersions were poured into a PTFE plate, dried at room temperature for 7 days, and then dried in a vacuum oven at 80 °C for 24 h to obtain the WPU films.
2.3. Characterization
2.3.1. FTIR-ATR
The infrared spectrum of the WPU film was measured using a Thermol 8700 infrared spectrometer from Thermo (Waltham, MA, USA). The sample was scanned 48 times at a resolution of 4 cm−1 over the frequency range of 4000–400 cm−1.
2.3.2. Particle Size
After emulsification, the particle size of WPU dispersion was carried out via a Zetasizer Nano ZS90 laser particle size tester from Malvern (Malvern, UK). The emulsion was diluted with deionized water to a mass fraction of 0.01% and measured at room temperature.
2.3.3. Gloss
Ten g WPU dispersion and 0.1 g wetting agent (BYK381) were mixed for 10 min, coated on the PVC leather using a 40 μm wire rod, and subsequently placed in an oven at 120 °C. After drying for 2 min, the leather samples were taken out for gloss test. Using the Ref 101N photometer from Sheen (Essex, UK), according to GB/T9754-2007 “Color paint and varnish − determination of 20°, 60° and 85° specular gloss of metallic paint-free paint film”, the 60° gloss was determined. The average value of three tests was used as the final result.
2.3.4. Transmittance
A U-3010 UV-Vis spectrophotometer (Hitachi, Tokyo, Japan) was used for the transmittance analysis of the WPU film. In the mode of T, the scanning range was 400 nm to 800 nm, with scanning speed of 600 nm/min. The film thickness was about 0.9 mm.
2.3.5. TG
In a N2 atmosphere, the thermal decomposition of the film was tested by a TGA/DSC1 type weight loss analyzer from Mettler Toledo (Zurich, Switzerland). The test range was 30–600 °C and the heating rate was 10 K/min. Weight loss was recorded as a function of temperature.
2.3.6. DSC
Under the protection of N2, the glass transition temperature (Tg) of the film was obtained by using a DSC1 differential scanning calorimeter from Mettler Toledo. The test range was −100~150 °C and the heating rate was 10 K/min.
2.3.7. SEM
A SU8020 scanning electron microscope (Hitachi) was used to observe the surface morphology of the polyurethane coating film on the leather. The operating voltage was 3 kV, and gold was sprayed before the sample test to improve the conductivity.
3. Results and Discussion
3.1. Structural Characterization of Polyurethane
The ATR-FTIR spectra of PCLD and WPU-A1 film are shown in Figure 1. The peak at 3700–3300 cm−1 was caused by the stretching vibration of hydroxyl (OH) in PCLD. There was no absorption peak at 2270 cm−1, indicating that all of the NCO groups were reacted completely in the system. The absorption peaks centered around 1728 cm−1, 3356 cm−1 and 1037 cm−1, were attributed to the C = O, N–H, and –SO3− groups, respectively. The absorption peak at 1234 cm−1 and 1157 cm−1 were corresponding to the C–O–C stretching vibration. The stretching vibration of C–H located at 2945 cm−1 and 2862 cm−1. There was a coupling absorption of the stretching vibration of C–N and the in-plane deformation vibration of N–H at 1556 cm−1. The IR spectrum demonstrated the aqueous polyurethane was synthesized.
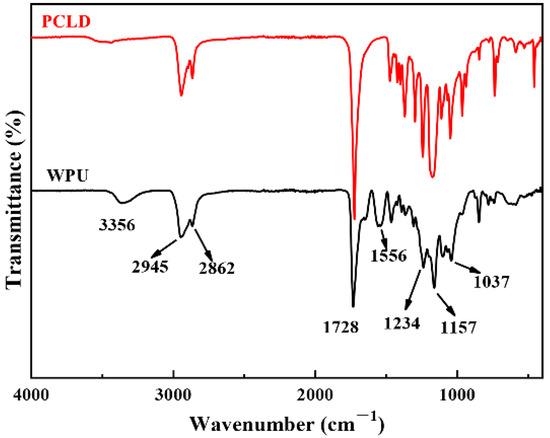
Figure 1.
ATR-FTIR spectra of WPU-A1 and PCLD.
3.2. Effect of AAS
AAS is a strong acid and alkali salt with high ionization strength, which provides hydrophilicity to the hard segment. And the existence of AAS salt can increase thickness of diffuse electric double layer, which had a promotional function in maintaining dispersion stability for longer time [18]. To investigate the effect of AAS content, a series of WPU-A dispersions were prepared varying the initial amount of AAS from 0.5% to 1.0%, while the hard segment content was 35%, the R value was 1.70. As shown in Figure 2, the average particle size decreased from 5815 nm to 459.2 nm, corresponding to an increase in AAS content from 0.5% (WPU-A1) to 1.0% (WPU-A6). The increase of AAS content led to a significant increase in gloss. This was attributed to the greatly improved hydrophilicity of polyurethane by AAS. As the AAS content decreased, the hydrophilic groups were not enough to cover the whole surface of particles in dispersion, and these particles tended to aggregate together, leading to the formation of larger particles, which greatly reduced the gloss values of coatings.
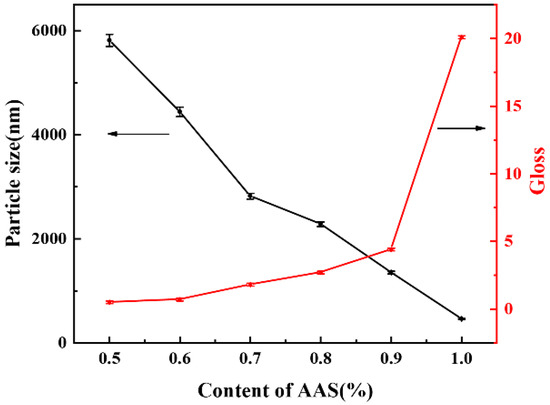
Figure 2.
The particle sizes of WPU-A dispersions and gloss of WPU-A films with different AAS contents.
The SEM characterization results were consistent with the particle size test. The SEM micrographs of WPU-A films are shown in Figure 3. It can be obviously seen that as the AAS increased, the roughness of WPU-A film surface gradually decreased. The degree of gloss was closely related to the surface roughness. When the light hit a rough surface, most of the light was scattered, resulting in a matte feeling visually. When the AAS content was 0.5%, the gloss of WPU-A1 film was as low as 0.5. This result suggested that different gloss levels can be achieved by regulating the content of AAS.
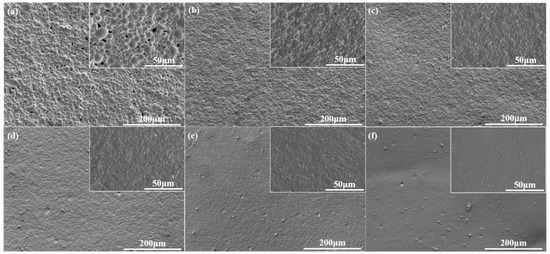
Figure 3.
SEM micrographs of WPU-A films with different AAS contents: (a) 0.5%; (b) 0.6%; (c) 0.7%; (d) 0.8%; (e) 0.9%; (f) 1.0%.
Figure 4 is a comparative photograph of different waterborne polyurethanes applied to leather. It is clear from the picture that the aspect of the polyurethane-coated leathers appeared softer and closer to the texture of the natural leather, comparing to the original leather. As shown in Figure 5, there was a disadvantage of WPU-A1 with large particle size. As the rough surface scattered a lot of light with AAS content at 0.5%, the film appeared opaque in white, leading to the lowest transmittance. However, this type of sample mixed with a little amount of small particle size polyurethane can improve this disadvantage without reducing the matting effect. The transmittance of the WPU-A6 film was as high as 98% with 1.0% of AAS, which was attributed to the reduced surface diffuse reflection caused by the relatively smooth surface. Therefore, WPU-A2 film with good matting effect and appearance was obtained when the AAS content was 0.6%.

Figure 4.
Digital images of leather coated by WPU-A with different AAS contents.
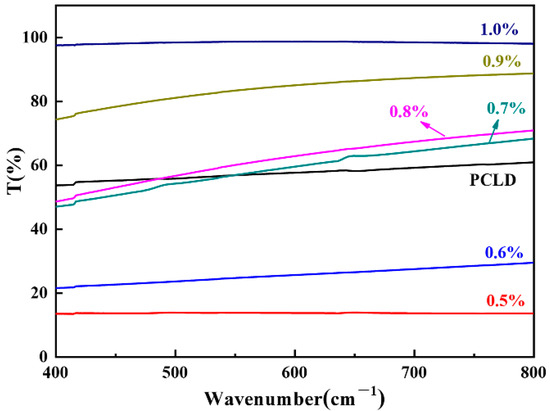
Figure 5.
The transmittance of WPU-A films with different AAS contents.
3.3. Influence of R Value
The n(NCO)/n(OH) is defined as R value, which is significant for the properties of WPU dispersions and films. When the content of AAS was 0.6%, a series of waterborne polyurethanes with different R values were synthesized. The effect of R value on particle size and film gloss of WPU-R was investigated as shown in Figure 6, which shows that when the R value was between 1.65 and 1.85, the particle size of the WPU-R dispersion increased with the increase of the R value. As the R value increased, the hydrophobicity of molecular chain increased, leading to larger particle size. While the particle size of the dispersion decreased with R value from 1.85 to 1.90. The viscosity and molecular weight of the prepolymer decrease because of the increase in R value, which caused a certain reduction in particle size. The SEM of WPU-R films with different R values was shown in Figure 7. The change in the particle size of WPU-R corresponded to different surface micro-roughness. However, the gloss of WPU-R films was generally at the same level (around 0.5). All samples had a particle size of more than 3 µm. 3 µm may be the dividing point of particle size. When the average particle size of the emulsion reached 3 µm or more, the gloss of the corresponding film was below 1.
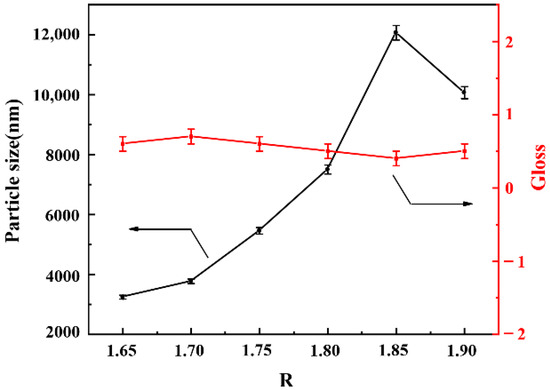
Figure 6.
The particle sizes of WPU-R dispersions and gloss of WPU-R films with different R values.
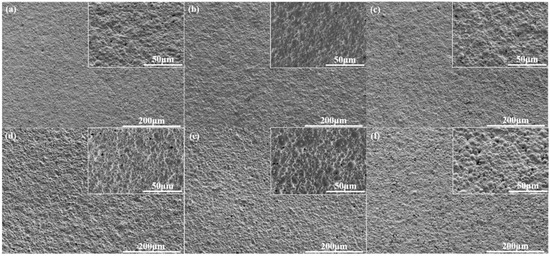
Figure 7.
SEM micrographs of WPU-R films with different R values: (a) 1.65; (b) 1.70; (c) 1.75; (d) 1.80; (e) 1.85; (f) 1.90.
3.4. Influence of Hard Segment Contents
Figure 8 shows the particle size and film gloss as a function of the hard segment content. For these samples, the NCO/OH ratio (1.70) and AAS content (0.6%) was kept constant in formulation, while only the hard segment content was changed. As shown in Figure 8, when the hard segment content increased from 33% to 41%, the particle size of WPU-H dispersion became larger, and the gloss of the coating film floated at around 0.5 rather than change substantially. The increase of hard segment content implies an increase of the rigidity of the molecular chains, which were not easy to disperse under the action of shear force, resulting in an increase in particle size. In addition, the remaining –NCO wrapped in the particles needs some time to react completely with water, which may cause a particle size increase after emulsification. Figure 9 also indicates that the particle size of WPU-H dispersion changed with content of the hard segment, which in turn directly affected the change in the surface roughness of the film. In Figure 9d,e, micro surface protrusions of WPU-H4 and WPU-H5 that were formed by tightly packed spherical particles were clearly exhibited. In the case of high hard segment content, the polyurethane particles became harder, leading to brittle WPU films. While in low content of hard segment, the polyurethane particles were easily deformed, reducing the roughness of coating surface. Therefore, the performance of WPU-H3 with moderate hard segment content was better.
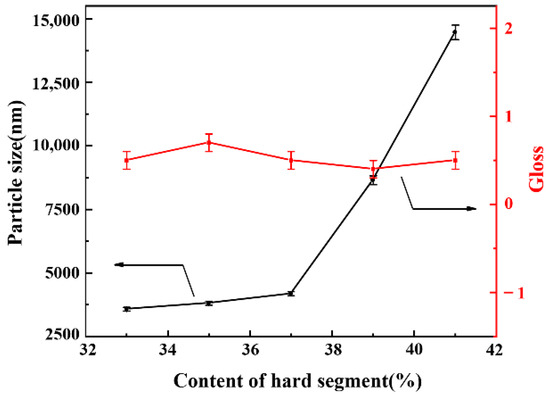
Figure 8.
The particle sizes of WPU-H dispersions and gloss of WPU-H films with different hard segment contents.
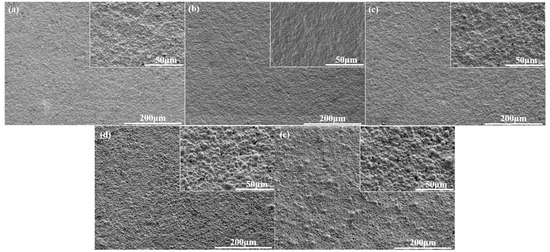
Figure 9.
SEM micrographs of WPU-H films with different hard segment contents: (a) 33%; (b) 35%; (c) 37%; (d) 39%; (e) 41%.
3.5. Effect of PCLD
3.5.1. Polyurethane Dispersion Particle Size and Film Gloss, Transmittance, Scanning Characterization
The reaction was carried out by replacing PCL with equivalent PCLD. The hydrophilic group on the PCLD chain was carboxyl group, which increased as the PCLD increased. The hard segment content was set to 37%. To probe the effect of PCLD on the particle size of the WPU dispersion and the gloss of film, the PCLD content was varied from 12 to 24% based on total content, corresponding to WPU-P1-WPU-P5. The result is shown in Figure 10. As the PCLD content increased, the particle size of WPU-P dispersion gradually decreased. A larger amount of salt groups COO− HN+Et3 presented on the particle surface with a higher PCLD content. The increase of salt groups enhanced the hydrophilicity of polyurethane molecules, which improved the hydration of polyurethane chain. It reduced the intertwining of molecular chain contributing to the dispersion of the waterborne polyurethane, causing particle size of the waterborne polyurethane obtained to become smaller. As we all know, particle size was closely related to gloss. At a bigger particle size of WPU, the particle deposition on the surface of film became more uneven when the WPU films formed, so that the surface roughness of films was larger, resulting in low gloss surfaces. Thus, as the PCLD content went on increasing gloss also increased which can be observed in Figure 10.
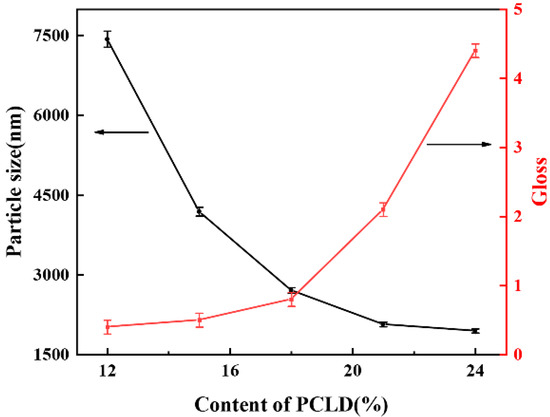
Figure 10.
The particle sizes of WPU-P dispersions and gloss of WPU-P films with different PCLD contents.
SEM images of different WPU-P films are displayed in Figure 11. All of the images display approximately spherical granules on their film surfaces. As the PCLD content was increased from 12% to 24%, the SEM images showed that the surface became relatively smooth from rough. The roughness of the surface affected gloss by means of scattering of diffuse light on the film surface. When the PCLD content was 12%(WPU-P1), the spherical particle was the largest with a wide particle size distribution. Compared with the Figure 11a, the particle size distribution of WPU-P2 was relatively narrow. When the PCLD content was low, the electrostatic repulsion between molecular chains was weak, and the molecular chains tended to aggregate to form a larger particle size. In Figure 12, the WPU-P1 and WPU-P2 films had the same matting effect, but the WPU-P1 film appeared partially whitish, which may be due to a wider particle distribution of WPU-P1. The optimal content of PCLD was indicated 15%. While the relatively smooth surface lead to a bright film with gloss as high as 4.4 with the content of PCLD at 24%. It revealed the matting performance of the film can be improved by controlling the particle size. The picture in Figure 12 also was in agreement with the gloss change of the coating film. The lower the content of PCLD, the better the matting effect.
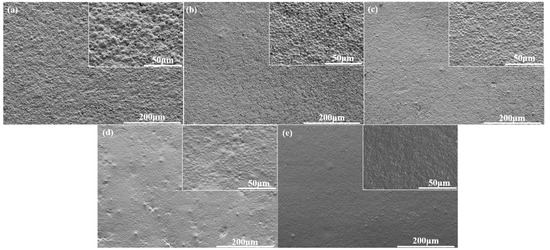
Figure 11.
SEM micrographs of WPU-P films with different PCLD contents: (a) 12%; (b) 15%; (c) 18%; (d) 21%; (e) 24%.

Figure 12.
Digital images of leather coated by WPU-P with different PCLD contents.
3.5.2. Thermal Analysis
TG, DTG and DSC curves of WPU-P films with different PCLD contents are shown in Figure 13. The initial decomposition temperatures of WPU-P films were above 280 °C. The temperatures were higher than those of other self-matting waterborne polyurethane reported in literature [15,18]. The DTG curve demonstrated that the decomposition of WPU films was roughly divided into two stages. The first stage was mainly the decomposition of the urethane bond and the urea bond in the hard segment. While the second stage was the decomposition of the carbon chain of the soft segment main chain. The change of trace carboxylate content had little effect on the thermal decomposition of WPU films. The TG results proved that this polyurethane coating can withstand high temperature embossing above 280 °C.
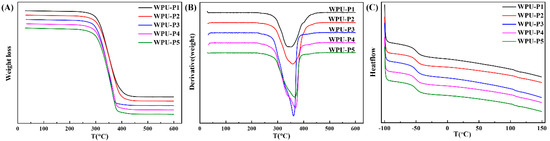
Figure 13.
(A) TG, (B) DTG and (C) DSC curves of WPU-P films with different PCLD contents.
Based on the DSC curves shown in Figure 13C, all WPU films had two glass transition temperatures in the test range of −100~150 °C, which were the glass transition temperatures of the soft segment and the hard segment, respectively. Soft segment glass transition temperature (TgS) as well as hard segment (TgH) are summarized in Table 2. The peak at temperature from −49.52 to −51.68 °C can be assigned to the relaxation processes of the soft segment domains, while the peak at temperature from 106.58 to 108.40 °C was related to the relaxation of the hard segment domains. The result proved that a heterogeneous system was formed and its internal structure was composed of microphase separation of soft and hard segments. The ΔTg decreased due to reduced degree of microphase separation caused by PCLD with side groups.

Table 2.
Tg of WPU-P films with different PCLD contents.
4. Conclusions
PCLD was used for the first time in the preparation of self-matting WPU coating, which introduced hydrophilic units into the soft segment. The obtained WPU films exhibited an excellent matting effect while the WPU preparation procedures can be performed conveniently and avoid high boiling-point solvents. The effect of PCLD on the particle size and film gloss was similar to that of DMPA. As the PCLD decreased, the particle size of the dispersion increased, which induced an increase in the roughness of the films, thus leading to a better matting effect. When PCLD and AAS contents were 15% and 0.6%, respectively, the gloss of WPU film reached as low as 0.5. Moreover, TG results illustrated the self-matting WPU had high temperature embossing resistance, and DSC results showed that self-matting WPU was composed of soft and hard segments with different glass transition temperatures. The prepared self-matting WPU has potential application prospects in the field of low-gloss leather finishing.
Author Contributions
Conceptualization, X.W. and W.X.; Data curation, J.C. and J.S.; Formal analysis, J.C.; Methodology, J.C.; Project administration, X.W., W.X., and Y.L.; Resources, X.W.; Supervision, X.L.; Validation, J.S., X.L., and Y.L.; Writing—original draft, J.C.; Writing—review & editing, X.W. and W.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Honarkar, H. Waterborne Polyurethanes: A Review. J. Dispers. Sci. Technol. 2017, 39, 507–516. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Basri, M.; Jusoh, E.R.; Mamat, S. Colloidal Stability and Rheology of Jatropha Oil-Based Waterborne Polyurethane (JPU) Dispersion. Prog. Org. Coat. 2018, 125, 348–357. [Google Scholar] [CrossRef]
- Santamaria, A.; Fernandes, I.P.M.; Saralegi, A.; Costa, M.R.P.F.N.; Barreiro, M.F.; Corcuera, M.A.; Eceiza, A. Synthesis of Waterborne Polyurethane-Urea Dispersions with Chain Extension Step in Homogeneous and Heterogeneous Media. J. Colloid Interface Sci. 2016, 476, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Xin, W.; Luo, Y. Synthesis and Application of a Cationic Waterborne Polyurethane Fixative Using Quaternary Ammonium Diol as a Chain Extender. RSC Adv. 2018, 8, 42041–42048. [Google Scholar] [CrossRef]
- Liu, X.; Zou, X.; Ge, Z.; Zhang, W.; Luo, Y. Novel Waterborne Polyurethanes Containing Long-Chain Alkanes: Their Synthesis and Application to Water Repellency. RSC Adv. 2019, 9, 31357–31369. [Google Scholar] [CrossRef]
- Saeed, A.; Shabir, G. Synthesis of Thermally Stable High Gloss Water Dispersible Polyurethane/Polyacrylate Resins. Prog. Org. Coat. 2013, 76, 1135–1143. [Google Scholar] [CrossRef]
- Han, Y.; Hu, J.; Xin, Z. Facile Preparation of High Solid Content Waterborne Polyurethane and Its Application in Leather Surface Finishing. Prog. Org. Coat. 2019, 130, 8–16. [Google Scholar] [CrossRef]
- Cuppo, F.L.S.; Garcia-Valenzuela, A.; Olivares, J.A. Influence of Surface Roughness on the Diffuse to near-Normal Viewing Reflectance Factor of Coatings and Its Consequences on Color Measurements. Color Res. Appl. 2012, 38, 177–187. [Google Scholar] [CrossRef]
- Chen, J.-J.; Zhu, C.-F.; Deng, H.-T.; Qin, Z.-N.; Bai, Y.-Q. Preparation and Characterization of the Waterborne Polyurethane Modified with Nanosilica. J. Polym. Res. 2008, 16, 375–380. [Google Scholar] [CrossRef]
- Feng, L.-B.; Wang, Y.-P.; Qiang, X.-H.; Wang, S.-H. Effect of Silica Nanoparticles on Properties of Waterborne Polyurethanes. Chin. J. Polym. Sci. 2012, 30, 845–852. [Google Scholar] [CrossRef]
- Fletcher, T.E. A Simple Model to Describe Relationships between Gloss Behaviour, Matting Agent Concentration and the Rheology of Matted Paints and Coatings. Prog. Org. Coat. 2002, 44, 25–36. [Google Scholar] [CrossRef]
- Bhattarai, S.; Lee, S.; Lee, D.; Lee, Y. Effect of Molecular Weight of Poly (Tetramethylene Glycol) on Waterborne Polyurethane Dispersion Coating Gloss. Bull. Korean Chem. Soc. 2019, 40, 1046–1049. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, H.; Chen, Y.; Huang, J. Synthesis of Self-Matting Waterborne Polyurethane Coatings with Excellent Transmittance. Polym. Int. 2017, 67, 78–84. [Google Scholar] [CrossRef]
- Yong, Q.; Liao, B.; Huang, J.; Guo, Y.; Liang, C.; Pang, H.; Caizhen, L. Preparation and Characterization of a Novel Low Gloss Waterborne Polyurethane Resin. Surf. Coat. Technol. 2018, 341, 78–85. [Google Scholar] [CrossRef]
- Yong, Q.; Nian, F.; Liao, B.; Pang, H.; Huang, L.; Wang, L. Synthesis and Characterization of Solvent-Free Waterborne Polyurethane Dispersion with Both Sulfonic and Carboxylic Hydrophilic Chain-Extending Agents for Matt Coating Applications. RSC Adv. 2015, 5, 107413–107420. [Google Scholar] [CrossRef]
- Grablowitz, H.G.; Thomas, F. Process for the Production of Polyurethane-Urea Dispersions; World Intellectual Property Organization: Eisenach, Germany, 2012. [Google Scholar]
- Li, J.; Zheng, W.; Zeng, W.; Zhang, D.; Peng, X. Structure, Properties and Application of a Novel Low-Glossed Waterborne Polyurethane. Appl. Surf. Sci. 2014, 307, 255–262. [Google Scholar] [CrossRef]
- Cao, X.; Ge, X.; Chen, H.; Li, W. Effects of Trimethylol Propane and AAS Salt on Properties of Waterborne Polyurethane with Low Gloss. Prog. Org. Coat. 2017, 107, 5–13. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A. The Influence of the Ionic Concentration, Concentration of the Polymer, Degree of Neutralization and Chain Extension on Aqueous Polyurethane Dispersions Prepared by the Acetone Process. Polymer 2006, 47, 1805–1811. [Google Scholar] [CrossRef]
- Barikani, M.; Ebrahimi, M.V.; Mohaghegh, S.M.S. Preparation and Characterization of Aqueous Polyurethane Dispersions Containing Ionic Centers. J. Appl. Polym. Sci. 2007, 104, 3931–3937. [Google Scholar] [CrossRef]
- Manvi, G.N.; Jagtap, R. Effect of Dmpa Content of Polyurethane Dispersion on Coating Properties. J. Dispers. Sci. Technol. 2010, 31, 1376–1382. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, B.K. Covalent Incorporation of Starch Derivative into Waterborne Polyurethane for Biodegradability. Carbohydr. Polym. 2012, 87, 1803–1809. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D. Synthesis and Characterization of Aqueous Polyurethane Containing Ions in Soft Segment. Polyurethane Ind. 1999, 14, 27–29. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).