Abstract
The flame resistance of applied coating materials affects the safety of innovative technological solutions. Silicone-containing polymeric materials are one of the most economical solutions in the field of coatings due to the effect of the unique combination of very good thermal, resistance, and surface properties. The rich chemistry of silicon compounds, which results in their very good thermal stability, allows their use as flame-resistant coating materials or as flame retardants in polymer composites. In this review, the flame resistance of PDMS systems based on their thermal degradation data, as well as possible paths of thermal degradation depending on external conditions including the effect of additives, flame resistance of hybrid silicone-containing coating materials and most important innovative applications of these materials, are reviewed. Very good results from the use of organic silicon compounds as fire retardants in polymers obtained by many research teams are one of the promising ways of overcoming the health, safety, and availability concerns of traditional halogenated fire retardants.
1. Introduction
Modern coating materials must possess excellent properties in order to meet the requirements of the most innovative applications in the fields of electronics, vehicles, household appliances, measuring and medical equipment and others [1,2,3]. Silicone-containing coating materials are characterized by very good properties and are suitable for use in modern technologies. A very important parameter that should be taken into account is the flame resistance of the applied coating materials, because it affects the safety of innovative technological solutions. Silicones and hybrid polymeric materials containing silicones are one of the most economical solutions in the field of coatings due to the effect of the unique combination of very good thermal, resistance, and surface properties [4].
The flame resistance of materials is a complex property that is dependent on their chemical composition and structure, thermal stability, material construction, and combustion conditions. The most important factor influencing flame-retardant silicone-containing coatings materials is, of course, the thermal properties of the silicone base polymer. Polydimethylsiloxane (PDMS) and their derivatives containing organofunctional chemical groups are most commonly used for the production of silicone-containing coating materials. The rich chemistry of organosilicon polymers makes it possible to obtain the coating material appropriate to the requirements of the intended use. The high thermal and oxidative resistance of silicones results from the higher stability of Si–O and Si–C chemical bonds compared to the C–C bond in organic resins. The heat of chemical bond formation is the measure of this stability. The heat of Si–O bond formation in PDMS is 452 kJ·mol−1, compared to 318–352 kJ·mol−1 for C–C bond in organic resins [5,6]. Silicones are also characterized by excellent fire resistance, e.g., the flashpoint of pure PDMS is over 300 °C, depending on the molecular weight.
Thermal decomposition PDMS is characterized by a low rate of heat release; generally in the range of 60–150 kW·m2 [7]. It should be emphasized that the combustion of silicones is not accompanied by the release of any toxic or aggressive gases. In addition, a very small amount of smoke is emitted [8]. The main products of PDMS combustion are SiO2, CO2 and H2O. Silica (SiO2) creates dust with excellent dielectric properties and provides additional protection against further fire development [9].
In addition to the basic polymer system silicone-containing coating materials usually contain different substances, such as crosslinking systems, rheology modifiers, adhesion promoters and others. These additives can have a significant, positive or negative, impact on the flame resistance of these materials. A very good example of the positive effect of fillers on the flame resistance of silicone coating materials is the achievement of Mansouri et al. [10], who, thanks to the introduction of appropriate inorganic fillers, obtained silicone ceramized layers after combustion of silicone-based compositions at 1050 °C.
Flame resistance is also influenced by the construction of a given material, e.g., in the form of laminates or multi-layer coatings. Such coating structures can reduce the flame resistance due to formation of “pockets” between the layers. Flammable products of thermal degradation can accumulate in them, decreasing flame resistance [11].
The effect of the combustion conditions on flame resistance is evident since the results obtained for the same material by using different methods are different. This is due to distinct requirements regarding combustion conditions, depending on the intended use of the material.
The aim of this review is to present the most important information concerning flame resistant silicone-containing coating materials based on a literature review. Some patents are also included in the description. This overview is divided into three main sections, including a discussion focused on flame resistance of PDMS systems based on their thermal degradation data including the effect of additives, the flame resistance of hybrid silicone-containing coating materials, and the most important innovative applications of these materials. This review does not include the use of flame retardants, especially halogenated ones. The use of such additives is limited in European Union countries and the USA on the basis of legal provisions (e.g., REACH regulation, RoHS directive).
2. Thermal Degradation of PDMS Systems
Significant differences in siloxane bond strength and segment flexibility—(Si–O)x in the main polymer chain are the reason for the significantly different thermal decomposition temperatures of PDMS as compared to organic polymers. However, these factors have an opposite effect on the thermal properties of PDMS.
As is known, the siloxane bond has a partly ionic and partly double bond character. This is due to the formation of the dπ–pπ bond between Si and O atoms. This results in a significant increase in the dissociation energy of Si–O bond of 108 kcal/mol compared to 85.2 kcal/mol for C–C bonds or 82.6 kcal/mol for C–O bonds [12], which ensures greater stability of Si–C bond at higher temperatures, while also increasing the thermal resistance of PDMS compared to organic polymers.
The extraordinary elasticity of the siloxane chain has a positive effect on the hydrophobic properties of silicones [13] and their low temperature resistance, but unfortunately reduces the stability of these polymers at higher temperatures [5]. The flexibility of the PDMS chain enables the creation of local configurations, with the formation of transient states facilitating inter- and intramolecular redistribution reactions. In addition, these reactions are partly supported by the ionic nature of the siloxane bond, which ultimately leads to the formation of thermodynamically favored low-molecular-weight cyclosiloxanes. As a result of this mechanism, the actual thermal resistance is lower than the values that could be calculated taking into account only the strength of the bonds.
Based on numerous literature reports, it can be concluded that the thermal degradation of PDMS occurs in a differentiated manner and depends primarily on the type and number of end groups in the PDMS, the presence of catalytic amounts of impurities in the polymer and the conditions of thermal degradation (under anaerobic or thermo-oxidative conditions) [7]. The process of thermal depolymerization may consist of three different reaction mechanisms: random chain cleavage, attack of the PDMS chain end groups, and reactions externally catalyzed by impurities [14]. The process of thermal depolymerization of the PDMS depends not only on the type of functional groups and their place of attachment to the chain (end groups or side), but also on the molecular weight of the PDMS. Grassie and Macfarlane [15] showed that the replacement of end hydroxyl groups with methyl groups clearly increased thermal resistance, as compared to PDMS with hydroxyl groups. Cyclic siloxane oligomers are decomposition products in both cases. Jovanovic et al. [16] suggested that vinyl end groups changed the mechanism of thermal degradation compared to methyl groups. The products of thermal decomposition of PDMS with vinyl end groups are cyclic oligomers (hexamethylcyclototrisiloxane and octylmethylcyclotetrasiloxane) [17]. The groups attached to the PDMS chain also have an impact on thermal degradation. Grassie et al. [18] showed a correlation between the content of phenyl groups and the mass of solid residue after thermal decomposition of poly (dimethyl/diphenylsiloxane). In addition, in studies of thermal degradation of polymethylsiloxane containing methylhydrogen siloxane moieties, it was found that an increase in crosslinking density increased heat resistance [16]. This is due to the possibility of additional crosslinking by the Si–H group during the initial stage of thermal degradation.
According to Lipowitz [19] and Lipowitz and Ziemelis [20], in the initial stage of thermal decomposition of silicone rubbers, low-molecular siloxanes are released, e.g., cyclosiloxanes. At higher temperatures, the main products of thermal decomposition of silicone rubbers are SiO2, CO2, H2O.
In practical applications of PDMS as coating materials, their thermo-oxidative degradation is important. In contrast to the mildly thermal depolymerization process resulting in cyclosiloxanes, the thermo-oxidative degradation process proceeds with the release, in the initial stage, of CO, H2O, CH2O, CO2, methanol, and residual amounts of formic acid as degradation products of organic groups in the main chain [21]. In the final stage of thermo-oxidative degradation, the SiO2, CO2, and H2O are released as products; see Figure 1.
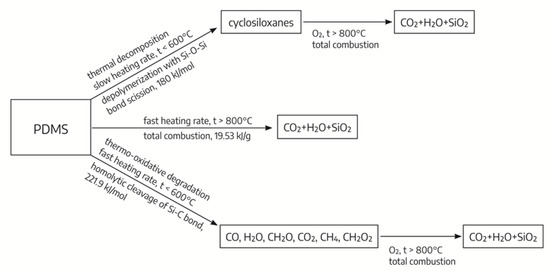
Figure 1.
The possible paths of thermal degradation of PDMS depending on external conditions.
Regardless of the path of thermal degradation of organosilicon compounds, at temperatures above 800 °C, the same final decomposition products are formed.
Based on the study of thermo-oxidative degradation processes, it can be stated that the stability of PDMS depends primarily on the nature of the organic groups that are substituents in the siloxane chain. The stability of these substituents can be ranked as follows: C6H5 > ClC6H4 > Cl3C6H2 > Cl2C6H3 > CH2 = CH > CH3 > C2H5 [22].
The second factor influencing the thermal stability of PDMS is their molecular weight. Based on the examination of thermal degradation of PDMS, Grassie et al. [18] showed that the amount of solid residue resulting from the thermal degradation is proportional to the molecular weight of the PDMS. Meanwhile, based on cone calorimetry tests, it was found that as the length of siloxane chain increased, the ignition time decreased due to the greater amount of flammable hydrocarbons formed as a result of chain degradation [23]. Detailed data were provided for hydroxy-terminated polyorganosiloxanes HODnOH (n = 5, 21, 55), where D was [Si(CH3)2O]. The times to ignition determined for these PDMS were 570, 267 and 33 s, respectively. At the same time, an increase in heat release rate was found with the values of 38.7, 34.4 and 69.8 (kW m2, 3 min), respectively.
As is known, PDMS retain their properties very well at temperatures up to 200 °C and even 220 °C, which allows their long-term use in this temperature range. However, at higher temperatures, thermal degradation can occur, which is accompanied by deterioration of properties, especially flexibility. Therefore, the determination of the temperature usefulness of the coating material should be done on the basis of aging tests at the assumed application temperature in combination with the precise definition of application parameters after the aging process [14].
3. The Effect of Crosslinking Systems on Flame Resistance
Transition Metals and Their Compounds
Platinum, iridium and rhodium complexes used as catalysts for PDMS addition crosslinking reactions have a clear effect in terms of improving flame resistance. They are usually used in quantities below 1 wt. %. During thermo-oxidation, these metals catalyze the formation of methylene bridges both inside and between molecules, which causes an increase in crosslinking density and thus a reduction in thermo-oxidation susceptibility [24]. Platinum or its compounds were used as a flame-retardant additive of polyorganosiloxane foams used as protective coatings or fillings [25]. The platinum catalyst was added in an amount of 5–200 ppm based on the platinum content of the polyorganosiloxane composition. The fire retardancy was determined in accordance with UL94 Standard [26], and it was found that a sample of polyorganosiloxane foam after a 60 s flame exposure extinguished in less than 2 s and had a burn distance of less than 1.27 cm. The effect of platinum, rhodium and iridium content on the flammability of PDMS composites was also investigated [11]. It was found that the addition of a 1 wt. % platinum catalyst to the silicone rubber reduced the flammability determined by the FAR 25.853 method (a vertical Bunsen burner test designed by the Federal Aviation Administration for cabin and compartment materials): after flame time by 78% and char length by 68%. Method FAR 25.853 is based on the same measurement principle as the UL94 method. In the UL94 method, a sample of the tested material is ignited twice. The addition of iridium or rhodium complexes in an amount of 0.25 phr reduced the after-flame time by 57%. The sample was determined by the FAR method 25.853.
Based on the results presented in numerous publications, it can be stated that transition metal oxides such as Fe, Ce, Ti, Zr, Zn have a positive effect on high-temperature resistance, and thus flame resistance [27]. It was found that mixtures of FeO, Fe2O3, CeO and TiO2 oxides introduced into PDMS applied as coatings for airbag improve their temperature resistance. However, a clearly adverse effect was also observed for moisture on the properties of the coatings for such materials as polyamide, polyester fibers or polyurethane boards. A similar phenomenon was observed for silicone rubber composites containing a mixture of platinum and titanium oxides [28]. The solution to this problem was the use of transition metal oxides treated with an organosilane, which made it possible not only to obtain a lasting improvement in the flame resistance of the tested materials, but also to reduce the amount of metal oxides introduced into the PDMS composite without deteriorating their properties. Optimizing the content of metal oxides, especially TiO2, is important, because too high TiO2 content deteriorates the mechanical properties of the composite and hinders its processing. A mixture of ZnO and TiO2 with aluminum trihydrate was used to obtain optimal fire resistance and smoke evolution properties of coating materials manufactured by spraying siloxane-free polydimethylsiloxane emulsion [29]. Synergy was also found between Ce(OH)2, TiO2, a mixture of iron oxides (FeO and Fe2O3) with platinum, making it possible to obtain silicone composites with increased fire resistance, defined as V0 based on the UL94 method [30,31].
4. The Effect of Additives on Flame Resistance
The use of carefully selected additives enables a significant increase in the flame resistance of PDMS composites. The role and mechanism of action of the additives depends on many factors, and above all on their morphology, the presence of functional groups, and their thermal resistance. Classification of the additives by their morphologies identifies them as including one-dimensional (including nanotube, rods, and nanofibers), two-dimensional (including nanosheets and plates), and three-dimensional (such as spheres, cubes, and clusters). The morphology of these additives has a diverse effect on the flame resistance of PDMS; see Figure 2.
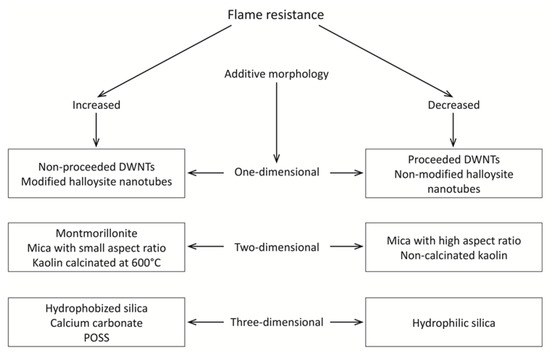
Figure 2.
The effect of the additive morphology on the flame resistance of PDMS composites.
It is also important to properly modify these additives in order to better incorporate them into the polymer matrix, which will be discussed for each type of additive.
Most additives used in plastics technology are mineral fillers, which can generally be divided into inactive fillers used to reduce the raw material cost of polymer composites and active fillers to improve the properties of such composites [32]. The effect of fillers on the properties of polymer composites depends on many factors, including their chemical composition, particle size and shape, surface properties, and the presence of functional groups. In general, fillers used in plastic technology can be classified into three groups in terms of their impact on the thermal properties of polymer composites: non-hydrated, water-emitting, and functionalized. Non-hydrated fillers improve the thermal resistance of silicone composites, while fillers that release water from crystallization processes or condensation of hydroxyl groups reduce the thermal properties of these composites [8]. The presence of moisture or hydroxyl groups can reduce the temperature of thermal degradation of PDMS by surface-catalyzed attack of hydroxyl groups [33]. An important aspect is also the shape and size of filler particles. Fillers with lamellar or fibrous particles may be oriented during processing, positively affecting the properties of polymer composites [34]. The presence of functional groups on the surface of filler particles is also of great importance, as it may enable interaction with the polymer matrix, which can also positively affect the properties of the composite [35]. The impact of each type of filler will be discussed below.
4.1. Carbon and Halloysite Nanotubes
A silicone composite containing carbon nanotubes designed as a coating material for aeronautical applications has been developed [36]. The advantage of this material is the use of double walled nanotubes DWNTs (double-wall carbon nanotubes), with a small addition of carbon nanotubes in the amount of 0.25–0.5 wt. %. Particularly good results were obtained in terms of flame-retardant properties for DWNTs not processed after synthesis, which showed better affinity to the silicone matrix. The flame retardancy of such silicone composites is explained by the dissipation of heat through the carbon nanotubes. The use of halloysite nanotubes can also improve the thermal stability of PDMS. However, in this case, due to their structural formula of Al2(OH)4Si2O5·nH2O, modification is necessary to obtain good miscibility with the polymer matrix. The universal modification method is the use of carbofunctional silanes, e.g., (3−aminopropyl) triethoxysilane. As with carbon nanotubes, composites containing halloysite nanotubes show a significant increase in flame resistance. This effect is associated with the formation of a thermal barrier and the accumulation of degradation product of PDMS in the halloysite lumen [32].
4.2. Calcium Carbonate
Calcium carbonate derived from natural deposits as chalk or obtained by precipitation is a widely used filler for polymer composites. Its application is associated with the stability of the properties and low price enabling the reduction of the raw material cost of polymer composites. Hermanssen et al. [37] found that a positive effect on the thermal resistance of polymer composites was related to the fact that calcium carbonate degradation begins at a temperature of about 500 °C and progresses steadily to a temperature of about 900 °C with the release of calcium oxide as a solid residue with 56 wt. % and carbon dioxide as a gas product. The resulting calcium oxide reacts with silica to form calcium silicate, whose crystal structure depends on the amount of calcium carbonate and silica formed in the process of PDMS degradation. For this reason, calcium carbonate is an essential component of PDMS composites capable of producing ceramized layers or intumescent coatings, providing a flame retardant barrier effect. The formation of calcium silicate with a fibrous structure of wollastonite is particularly advantageous, as it promotes the formation of protective layers with good mechanical properties. Furthermore, the addition of calcium carbonate to PDMS allows neutralization of acidic residues from the polymerization process. According to Hermansson et al. [37], PDMS degrades at around 300 °C, and the addition of calcium carbonate increases this temperature to 500 °C.
4.3. Silica
Silica is a silicone rubber compound filler that is commonly used because its addition improves the mechanical properties of silicone rubber by creating hydrogen bonds between Si–O–Si chains and silanol groups present on the surface of silica particles [36,38]. However, the formation of hydrogen bonds between oxygen from the organosilicon polymer chain and silica silanol groups can also lead to undesirable phenomena that hinder the processing of the polymer composite. To avoid the above difficulties, it is recommended to use hydrophobized fillers with blocked hydroxyl groups on their surface [39].
The technique of introducing silica into the composite has the greatest impact on flame retardancy, as along with particle size and type of silica. Kashiwagi et al. [40] proved that the mechanism of increasing flame retardancy due to the addition of silica was associated with the physical processes occurring in the solid phase, and not with chemical reactions. Therefore, good dispersion of silica in the polymer matrix has a significant impact on obtaining the desired properties, including flame retardancy. Nodera and Kanai [41] studied the effect of nanosilica particle size added to the PC/PDMS (Polycarbonate/Polydimethylsiloxane) composite in an amount of 0.5 wt. % on flame retardancy and found that nanosilica with a particle size of 20 nm had a significantly greater effect on improving flame retardancy compared to nanosilica with a particle size of 50 nm.
In recent years, application of functionalized silicas and nanosilicates obtained by modification of silica with molecules of compounds or oligomers and polymers attached through silanol groups have been increasing. A PDMS/silica hybrid obtained by grafting monoglycidylether-terminated polydimethylsiloxane on nanosilica particles showed increased flame retardancy as compared to pure PDMS [42]. Alongi et al. [43] found synergy between phosphorus and silica in flammability and cone calorimetry tests of cotton protected with a hybrid phosphorous–silica coating.
There has also been a significant intensification of research on polysilsesquioxanes (POSS), which have a very well-defined chemical composition and structure [44]. Numerous literature reports have described the use of POSS as nanofillers for polymer composites, including silicone [45]. An important advantage of POSS is the possibility of introducing various functional groups into their structure, ensuring their chemical binding with the polymer matrix, which ensures very good stability of the properties of the obtained polymer composites. Furthermore, synergistic effects may occur between POSS and silica, making it possible to obtain polymer composites with very good properties [46]. However, a major barrier to the widespread use of POSS as nanofillers is their price [44].
4.4. Layered Fillers
The group of layered fillers includes mica, montmorillonite, kaolin, and talc, which differ in their chemical composition, but due to the similarity of their structures, they can have a similar effect on the thermal and flame resistance properties of PDMS composites. The basic condition for the positive effect of these fillers on the composite properties is their good dispersion in the polymer matrix without damaging the layered structure, which can cause processing difficulties and increase the cost of the composite.
Based on the results of research carried out by different research groups, it can be concluded that the mechanism of flame retardance of these fillers is associated with the formation of a sintered layer of a silica–carbon composite in the combustion PDMS [47,48,49]. Sinter formation is a complex process associated with several possible actions that increase flame resistance, see Table 1.

Table 1.
Actions increasing flame resistance in polymer/montmorillonite composites.
Mica is a layered aluminosilicate, and is usually used as a filler in two crystallographic forms, muscovite and phlogopite, which are commercially available. It was found that the influence of mica on the thermal properties of polydimethylsiloxane composite and the formation of the sintered layer depends to a large extent on the particle size and the amount of filler introduced. Osman et al. [56] found that mica with a high aspect ratio increased the thermal degradation of the PDMS composite, while mica with a small aspect ratio caused an increase in thermal stability. It was found that mica with a particle size of 20 μm increases the amount of combustion residue to 60%, while the addition of mica with a particle size of 110 μm increases the residue to 63%, compared to 54% obtained after the thermal degradation of pure PDMS [48]. It should be emphasized, however, that larger mica particles create a ceramic layer containing many cracks and weakly bonded fragments. This is due to the incomplete wettability of large mica particles by the eutectic formed during firing at 1100 °C. Smaller mica particles are better wetted for steric reasons, which makes it possible to obtain a ceramized layer with a more homogeneous structure.
Kaolin is another filler from the group of layered silicates. Only kaolin calcined at 600 °C has a slight positive effect on the thermal stability of PDMS composites, because the kaolin found in the natural environment is hydrated. However, the main advantage of adding kaolin to PDMS composites is the creation of a sintered layer with fewer cracks [57].
5. Flame Resistance of Hybrid Silicone-Containing Coating Materials
In recent years, there has been a noticeable increase in the intensity of research on the use of PDMS as flame retardants modifying the properties of organic polymers due to limitations in the use of flame retardants, especially halogenated ones, in accordance with the REACH regulations and the RoHS directive. The basic methods of introducing PDMS into the matrix of organic polymers include direct mixing with the polymer, deposition of PDMS on the filler particles and synthesis of block or graft copolymers [58]. The choice of method depends on the properties of polymers and, above all, their thermodynamic miscibility [59]. For immiscible systems, or those with very limited miscibility, the degree of dispersion of the PDMS in the polymer matrix may be insufficient to achieve the desired flame retardant effect. The effect of PDMS structure on the oxygen index value of the polycarbonate/silicone composite was examined, taking into account the degree of chain branching, the type of substituents, and the end groups in the siloxane chain [60]. Methyl/phenyl branched PDMS showed the superior flame-retardant effect as compared to linear polydimethylsiloxane due to excellent dispersion in a polycarbonate matrix. Very good dispersion of the PDMS in the polymer matrix can be obtained using powdered silicone additives for plastics [61]. The main component of these powders is polydimethylsiloxane and fumed silica. It has been found that the addition of such powder in an amount of 1–5 wt. % to various polymers, e.g., polystyrene, polyolefin, polycarbonate or polyoxyphenylene clearly improves their fireproofing properties such as heat release rate, level of CO generation and smoke evolution determined by cone calorimeter measurement [62]. So far, several silicone-based flame retardants containing boron, aluminum and/or titanium [63,64,65] have been patented. However, the compositions described in the patents had only limited flame-retardant performance because their antidripping effect in UL-94 was unsatisfactory. Very good results improving scratch resistance and flame-retardant properties were obtained for silicone resins comprising metallosiloxane with Si–O–Metal bonds or borosiloxane containing Si–O–B bonds and potentially Si–O–Si and/or B–O–B bonds [66]. The best results were obtained with the simultaneous use of boron, aluminum, and phosphorus.
It is also important to choose the right silicone resin, which should be characterized by a significant degree of branching, expressed as a content of tri- and tetrafunctional groups in the branched silicone resin of at least 75%. It was demonstrated in the examples of patent description that new phosphorylated resin showed good flame-retardant synergies with other flame retardant additives, such as magnesium silicates (e.g., talc). The combination of 5 wt. % talc with the developed silicone resin added to polycarbonate matrix was able to reach the UL-94 V-0 rating [66]. This type of silicone-based flame retardant can also be used to increase the flame resistance of other general-purpose polymers.
For polymers with limited thermodynamic miscibility with PDMS, such as polyolefins, an effective solution may be to produce block or graft copolymers using compatibilizers. For this purpose, the use of ethylene-methyl acrylate copolymer (EMA) as a chemical compatibilizer in a low-density polyethylene/polydimethylsiloxane (LDPE-PDMS) mixture was investigated [67]. During melt mixing at 180 °C, EMA reacts with PDMS rubber, leading to the formation of EMA-grafted PDMS rubber containing C–C bonds. In addition, EMA grafting increases compound stability by creating virtual bridges between the two polymeric phases (linear low-density polyethylene LLDPE continuous and PDMS rubber dispersed) [68]. It was confirmed that the optimum concentration of EMA in a blend of 50:50 (LLDPE: PDMS) is 12 wt. %. With the increase in the content of EMA, the phase morphology changes from an unstable phase morphology to a discrete domain size, which was observed with the optimum EMA concentration. However, in order to achieve increased thermal stability, the optimal EMA content was 2 wt. % [68].
6. Selected Applications for Silicone-Containing Coating Materials
Silicone ceramizable composites for electrical cables and silicone-based intumescent paints are undoubtedly the most widely known applications for silicone-based flame-resistant coatings [10,69,70]. In both types of these coatings, the fire protective effect is associated with the formation of a protective layer during the combustion of the coating. In this process, fillers have a significant impact, as discussed in this article. Detailed examples of these applications will not be provided, because the broad range of this issue lies beyond the scope of this paper. However, examples of flame-resistant silicone-based coating materials in which the effect of thermal stability is obtained thanks to modifications of the chemical structure of PDMS will be given. Selected examples of applications are shown in Table 2.

Table 2.
The examples of the flame-resistant silicone-based coating.
Particularly noteworthy are the results of testing the thermal resistance of glass fiber coatings obtained using PDMS with different structures: polysilazane, methyl silicone resin, phenylmethyl PDMS [73]. Thermal stability of polysilazane and methyl silicone resin-coated samples is higher when compared to phenylmethyl PDMS-coated samples. At the same time, it was found that the mechanical properties of fiberglass with a polysilazane coating are the worst. Overall, it can be concluded that coatings with methyl silicone resin showed improvements in thermo-mechanical properties compared to polysilazane coating. This PDMS would be a highly promising polymer for continuous glass fiber coatings for a wide range of composites in the architecture market.
The effect of the branching PDMS structure on the increase in thermal resistance is also confirmed by the results of tests obtained for bismaleimide/cyanate ester composites modified with hyperbranched PDMS with high content of phenyl groups (HPPSi) [74]. The high content of silanol groups in HBPSi allows the formation of a strongly cross-linked structure with bismaleimide/cyanate ester, which has a significant impact on increasing the thermal stability of the composite. The introduction of HPPSi resulted in a decrease in the mass loss rate and an increase in char yield at 800 °C under an air atmosphere. Based on the kinetic study of the composite degradation process, it was found that the reactions between HBPSi and BCE resin change the thermo-oxidative degradation mechanism of the first step in the thermo-oxidative degradation. The research results discussed above justify the statement that a properly designed chemical structure of PDMS makes it possible to obtain high-performance polymer composites that possess high flame retardancy.
It can be concluded that PDMS can be a good base to explore structures with clearly better thermal stability. The rich chemistry of silicon compounds enables the use of various catalytic processes, such as metathesis, silylative coupling, and others [75,76], including frequently used hydrosilylation [77]. Polydimethylsiloxanes functionalized with biphenyl phosphates were synthesized using 1,1,3,3-tetramethyldivinyldisiloxane in the hydrosilylation process with further crosslinking in the presence of platinum Kartsedt’s catalyst [72]. Based on thermogravimetric studies of phosphate derivatives bearing allyl (ABP) or phenyl (PBP) groups, it was found that silicone rubbers functionalized with PBP showed significantly better thermal stability compared to rubbers containing ABP. In addition, it was also found that thermal stability also increased with the crosslink density of phosphorous-containing silicone rubber.
The research results discussed above justify the statement that a properly designed chemical structure of PDMS makes it possible to obtain high-performance polymer composites that possess high flame retardancy.
When creating new technological and recipe solutions of coating materials based on silicone -containing systems cost vs performance relationship should take into account. Very important factor are also environmental restrictions which also affect the price. These include new environmental regulations which have led to the shut down and/or closure of many production sites, affecting global supply [78]. In the field of high-temperature silicone coatings, which are mostly solvent-based, there is a large space for further innovative solutions. Currently, commercial water-based products often do not meet expectations, not only because of their incorrect recipes, e.g. too high content of co-solvents causing poor drying performance, but may also result from the wrong selection of base polymers. This review discusses the wide possibilities of using polysiloxanes in flame resistant applications, which will certainly be developed in the future to provide materials with the properties necessary for constantly emerging new technological solutions. The potential of polysiloxanes and their derivatives resulting from their chemistry will certainly be used.
7. Conclusions
In conclusion, it should be noted that the rich chemistry of silicon compounds, which result in their very good thermal stability, enables their use as flame-resistant coating materials or as flame retardants in polymer composites. Depending on the chemical structure and the conditions of thermal degradation of organic silicon, compounds can proceed according to different mechanisms. This has a direct effect on the composition of the degradation products, especially the intermediate products released during thermal degradation occurring in the temperature range 200–400 °C. It should be emphasized that the final decomposition products of organic silicon compounds−SiO2, CO2, and H2O−are non-toxic. Fillers, especially, with a fibrous or layered structure, support the formation of flame-resistant protective coatings in the form of sintered layer such as ceramized or intumescent. Very good results obtained by many research teams for the use of organic silicon compounds as fire retardants in polymers are one of the promising ways to overcome the health, safety, and availability concerns surrounding traditional halogenated fire retardants. For this purpose, both PDMS with different degrees of branching, polysilsesquioxanes can be used, as well as structures containing heteroatoms. The effectiveness of such flame retardants depends not only on their chemical structure, but also on their miscibility with the base polymer. It should be summarized that a properly designed chemical structure of organosilicon compounds makes it possible to obtain high flame retardancy in silicone-containing coating materials.
Funding
This work was supported by a subsidy for the research potential of the Scientific and Research Center for Fire Protection National Research Institute, project 057/BS/MNiSW/2020 Research methods of fire vehicles and tools and firefighting equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uhlmann, P.; Frenzel, R.; Voit, B.; Mock, U.; Szyszka, B.; Schmidt, B.; Ondratschek, D.; Gochermann, J.; Roths, K. Research agenda surface technology: Future demands for research in the field of coatings materials. Prog. Org. Coat. 2007, 58, 122–126. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Balabanava, N.; Wierzbicki, R.; Zielecka, M.; Rymuza, Z. Effect of roughness on adhesion of polymeric coatings used for microgrippers. Microelectron. Eng. 2007, 84, 1227–1230. [Google Scholar] [CrossRef]
- Bayer, I.S. On the Durability and Wear Resistance of Transparent Superhydrophobic Coatings. Coatings 2017, 7, 12. [Google Scholar] [CrossRef]
- Dvornic, P.R. Thermal properties of polysiloxanes in Silicon-Containing Polymers The Science and Technology of Their Synthesis and Applications; Jones, R.G., Ando., W., Chojnowski, J., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 185–213. [Google Scholar]
- Dobkowski, Z.; Zielecka, M. Thermal analysis of the poly(siloxane)-poly(tetrafluoroethylene) coating system. J. Therm. Anal. Calorim. 2002, 68, 147–158. [Google Scholar] [CrossRef]
- Buch, R.R. Rates of heat release and related fire parameters for silicones. Fire Safety J. 1991, 17, 1–12. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stabil. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Hshieh, F.Y. Shielding effects of silica-ash layer on the combustion of silicones and their possible applications on the fire retardancy of organic polymers. Fire Mater. 1998, 22, 69–76. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B.; Hanu, L. Formation of strong ceramified ash from silicone-based compositions. J. Mater. Sci. 2005, 40, 5741–5749. [Google Scholar] [CrossRef]
- David, C.T., Jr. Silicone Rubber Flame Resistance. In Proceedings of the Hose Manufacturer Conference, Cleveland, OH, USA, 11–12 June 2007; Rubber and Plastics News: The Hague, The Netherlands, 2007. [Google Scholar]
- Dvornic, P.R.; Lenz, R.W. High Temperature Siloxane Elastomers; Hϋthig and Wept: Heidelberg, Germany; New York, NY, USA, 1990. [Google Scholar]
- Zielecka, M.; Bujnowska, E. Silicone-containing polymer matrices as protective coatings. Properties and applications. Prog. Org. Coat. 2006, 55, 160–167. [Google Scholar] [CrossRef]
- Dvornic, P.R. High Temperature Stability of Polysiloxanes in Silicon Compounds: Silanes and Silicones, Gelest Catalog; Gelest, Inc.: Morrisville, PA, USA, 2004; pp. 419–432. [Google Scholar]
- Grassie, N.; Macfarlane, I.G. The thermal degradation of polysiloxanes—I. Poly(dimethylsiloxane). Eur. Polym. J. 1978, 14, 875–884. [Google Scholar] [CrossRef]
- Jovanovic, J.D.; Govedarica, M.N.; Dvornic, P.R.; Popovic, I.G. The thermogravimetric analysis of some polysiloxanes. Polym. Degrad. Stabil. 1998, 61, 87–93. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. The effect of phenyl content on the degradation of poly(dimethyl diphenyl) siloxane copolymers. Polym. Degrad. Stabil. 2001, 74, 363–370. [Google Scholar] [CrossRef]
- Grassie, N.; Francey, K.F.; Macfarlane, I.G. The thermal degradation of polysiloxanes—Part 4: Poly(dimethyl/diphenyl siloxane). Polym. Degrad. Stabil. 1980, 2, 67–83. [Google Scholar] [CrossRef]
- Lipowitz, J. Flammability of poly(dimethylsiloxanes). 1. A model for combustion. J. Fire Flammabl. 1976, 7, 482–503. [Google Scholar]
- Lipowitz, J.; Ziemelis, M.J. Flammability of poly(dimethylsiloxanes). 2. Flammability and fire hazard properties. J. Fire Flammabl. 1976, 7, 504–529. [Google Scholar]
- Andrianov, K.A. Metalorganic Polymers; Interscience: New York, NY, USA, 1965; p. 50. [Google Scholar]
- Sobolevski, M.V.; Skorokhodov, N.I.; Ditsent, V.E.; Sobolevskaya, L.V.; Vovshin, E.I.; Blekh, L.M. Study of Thermal Transformations of Oligomethylphenylsiloxanes. Vysokomol. Soedin. 1974, 16, 729–734. [Google Scholar]
- Connell, J.E.; Metcalfe, E.; Thomas, M.J.K. Silicate–siloxane fire retardant composites. Polym. Int. 2000, 49, 1092–1094. [Google Scholar] [CrossRef]
- Hadayashida, K.; Tsuge, S.; Ohtani, H. Flame retardant mechanism of polydimethysiloxane material containing platinum compound studied by analytical pyrolysis technique and alkaline gas chromatography. Polymer 2003, 44, 5611–5616. [Google Scholar] [CrossRef]
- Dow Corning Corporation. Method of Preparing Fire Retardant Siloxane Foams and Foams Prepared Therefrom. U.S. Patent 3,923, 2 December 1975. [Google Scholar]
- UL 94 Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances. Edition No.6, Edition Date: 2013-03-28. Available online: https://standardscatalog.ul.com/standards/en/standard_94_6 (accessed on 14 May 2020).
- Shin-Etsu Chemical Co. Silicone Coated Base Material and Air Bag Base Material. European Patent Application 0,669,419, 30 August 1995. [Google Scholar]
- Toshiba Silicone Kabushiki Kaisha. Flame Retardant Silicone Rubber Compositions. U.S. Patent 3,862,082, 21 January 1975. [Google Scholar]
- Dow Corning Corporation. Sprayable Silicone Emulsions Which Form Elastomers Having Smoke and Fire Resistant Properties. European Patent Application 0,839,853, 6 May 1998. [Google Scholar]
- Socieété des Usines Chimiques Rhône-Poulenc. Compositions Organopolysiloxaniques. French Patent 2,166,313, 7 January 1972. [Google Scholar]
- Rhodia Chimie. Use of Mixtures with Base of Pt and of Transition Metal Compounds other than Pt Improving the Resistance to Arc Tracking and to Arc Erosion of Silicone Elastomers. World Patent 98,029,488, 9 July 1998. [Google Scholar]
- Xanthos, M. (Ed.) Functional Fillers for Plastics, 2nd ed.; Updated and Enlarged; Wiley-VCH: Weinheim, Germany, 2010; pp. 15–21. [Google Scholar]
- Hornsby, P.R.; Rothon, R.N. Fire Retardant Fillers for Polymers in Fire Retardancy of Polymers: New Applications of Mineral Fillers; Royal Society of Chemistry: Cambridge, UK, 2005; pp. 19–41. [Google Scholar]
- Osman, M.A.; Atallah, A.; Kahr, G.; Suter, U.W. Reinforcement of poly(dimethylsiloxane) networks by montmorillonite platelets. J. Appl. Polym. Sci. 2002, 83, 2175–2183. [Google Scholar] [CrossRef]
- Wypych, G. Functional Fillers: Chemical Composition, Morphology, Performance, Applications; ChemTech Publishing: Toronto, ON, Canada, 2018; pp. 10–221. [Google Scholar]
- Nanocyl, S.A. Fireproof composition. World Patent 2007. [Google Scholar]
- Hermansson, A.; Hjertberg, T.; Sultan, B.A. The flame retardant mechanism of polyolefins modified with chalk and silicone elastomer. Fire Mater. 2003, 27, 51–70. [Google Scholar] [CrossRef]
- Demir, M.M.; Menceloglu, Y.Z.; Erman, B. Effect of filler amount on thermoelastic properties of poly(dimethylsiloxane) networks. Polymer 2005, 46, 4127–4134. [Google Scholar] [CrossRef][Green Version]
- Chimie, R.; Shin-Etsu Chemical Co., Ltd. Use of a Pretreated Precipitated Silica as a Reinforcing Filler for Silicon Elastomer and the Curable Silicone Elastomer Compositions thus Obtained by Cold Mixing. U.S. Patent 8,907,001 B2, 9 December 2014. [Google Scholar]
- Kashiwagi, T.; Gilman, J.W.; Buter, K.M.; Harris, R.H.; Shields, J.R.; Asano, A. Flame retardant mechanism of silica gel/silicas. Fire Mater. 2000, 24, 277–289. [Google Scholar] [CrossRef]
- Nodera, A.; Kanai, T. Flame retardancy of polycarbonate–polydimethylsiloxane block copolymer/silica nanocomposites. J. Appl. Polym. Sci. 2006, 101, 3862–3868. [Google Scholar] [CrossRef]
- Liu, Y.L.; Li, S.H. Poly(dimethylsiloxane) star polymers having nanosized silica cores. Macromol. Rapid. Commun. 2004, 25, 1392–1395. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Phosphorus- and nitrogen-doped silica coatings for enhancing the flame retardancy of cotton: Synergisms or additive effects? Polym. Degrad. Stab. 2013, 98, 579–589. [Google Scholar] [CrossRef]
- Maciejewski, H.; Dutkiewicz, M.; Byczynski, L.; Marciniec, B. Silsesquioxanes as nanofillers. Part I. Silicone matrix nanocomposites. Polimery 2012, 57, 535–544. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Vatani, A.; Mohammadi, T. Synergistic interactions between POSS and fumed silica and their effect on the properties of crosslinked PDMS nanocomposite membranes. RSC Adv. 2015, 5, 82460–82470. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Richard, H., Jr. Flammability properties of polymer-layered-silicate nanocomposites: Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Hanu, L.G.; Simon, G.P.; Mansouri, J.; Burford, R.P.; Cheng, Y.B. Development of polymer–ceramic composites for improved fire resistance. J. Mater. Process. Tech. 2004, 153, 401–407. [Google Scholar] [CrossRef]
- Leszczyńska, A.; Njuguna, J.; Pielichowski, K.; Banerjee, J.R. Polymer/ montmorillonite nanocomposites with improved thermal properties Part I. Factors influencing thermal stability and mechanisms of thermal stability improvement. Thermochim. Acta. 2007, 453, 75–96. [Google Scholar] [CrossRef]
- Gilman, J.W. Flammability and Thermal Stability Studies of Polymer Layered-Silicate (Clay) Nanocomposites. Appl. Clay Sci. 1999, 15, 31–49. [Google Scholar] [CrossRef]
- Gilman, J.; Kashiwagi, T.; Brown, J.; Lomakin, S.; Giannelis, E. Flammability studies of polymer layered silicate nanocomposites. In Proceedings of the 43rd International SAMPE Symposium, Anaheim, CA, USA, 31 May–4 June 1998; Kliger, H.S., Rasmussen, B.M., Pilato, L.A., Tolle, T.B., Eds.; Society for the Advancement of Material and Process Engineering SAMPE: Covina, CA, USA, 1998; pp. 1053–1066. [Google Scholar]
- Chang, J.H.; Kim, S.J.; Joo, Y.L.; Im, S. Poly(ethylene terephthalate) nanocomposites by in situ interlayer polymerization: The thermo-mechanical properties and morphology of the hybrid fibers. Polymer 2004, 45, 919–926. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A. Synthesis and Properties of Polyimide-Clay Hybrid Films. J. Polym. Sci. A Polym. Chem. 1997, 35, 2289–2294. [Google Scholar] [CrossRef]
- Blumstein, A. Polymerization of adsorbed monolayers. II Polymerization of adsorbed monolayers. II. Thermal degradation of the inserted polymer. J. Polym. Sci. Part A General Papers 1965, 3, 2665–2672. [Google Scholar] [CrossRef]
- Costache, M.C.; Wang, D.; Heidecker, M.J.; Manias, E.; Wilkie, C.A. The thermal degradation of poly(methyl methacrylate) nanocomposites with montmorillonite layered double hydroxides and carbon nanotubes. Polym. Adv. Technol. 2006, 17, 272–280. [Google Scholar] [CrossRef]
- Osman, M.A.; Atallah, A.; Müller, M.; Suter, U.W. Reinforcement of poly(dimethylsiloxane) networks by mica flakes. Polymer 2001, 42, 6545–6556. [Google Scholar] [CrossRef]
- Genovese, A.; Shanks, R.A. Fire performance of poly(dimethyl siloxane) composites evaluated by cone calorimetry. Compos. Part A Appl. Sci. Manuf. 2008, 39, 398–405. [Google Scholar] [CrossRef]
- Serra, A.; Ramis, X.; Fernández-Francos, X. Epoxy Sol-Gel Hybrid Thermosets. Coatings 2016, 6, 8. [Google Scholar] [CrossRef]
- Stary, Z. Thermodynamics and Morphology and Compatibilization of Polymer Blends in Characterisation of Polymer Blends; Thomas, S., Grohens, Y., Jyotishkumar, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 1, pp. 93–132. [Google Scholar]
- Iji, M.; Serizawa, S. Silicone derivatives as new flame retardants for aromatic thermoplastics used in electronic devices. Polym. Adv. Technol. 1998, 9, 593–600. [Google Scholar] [CrossRef]
- Innes, J.; Innes, A. Plastic Flame Retardants: Technology and Current Developments; Rapra review reports 168; iSmithers Rapra Publishing: Shawbury, UK, 2003. [Google Scholar]
- Pape, P.G.; Romenesko, D.J. The role of silicone powders in reducing the heat release rate and evolution of smoke in flame retardant thermoplastics. J. Vinyl. Addit. Technol. 1997, 3, 225–232. [Google Scholar] [CrossRef]
- Kaneka Corporation. Flame Retardant and Flame Retardant Resin Composition Containing the Same. U.S. Patent 6716952, 4 June 2004. [Google Scholar]
- Dow Corning Corporation, Co. Borosiloxane Composition, Borosiloxane Adhesive, Coated and Laminated Substrates. U.S. Patent 2010/0316876, 16 December 2010. [Google Scholar]
- Albemarle Corporation. Synthetic Inorganic Flame Retardants, Methods for Their Preparation, and Their Use as Flame Retardants. U.S. Patent 2011/0213065, 1 September 2011. [Google Scholar]
- Dow Corning Corporation; Dow Corning Toray Co., Ltd. Silicone Resins and Their Use in Polymers. WO 2013/095812, 27 June 2013. [Google Scholar]
- Jana, R.N.; Mukunda, P.G.; Nando, G.B. Thermogravimetric analysis of compatibilized blends of low density polyethylene and poly(dimethyl siloxane) rubber. Polym. Degrad. Stabil. 2003, 80, 75–82. [Google Scholar] [CrossRef]
- Giri, R.; Naskar, K.; Nando, G.B. In-Situ Compatibilization of Linear Low-Density Polyethylene and Polydimethyl Siloxane Rubber Through Reactive Blending. Mater. Express 2012, 2, 37–50. [Google Scholar] [CrossRef]
- Gardelle, B.; Duquesne, S.; Vandereecken, P.; Bellayer, S.; Bourbigot, S. Resistance to fire of intumescent silicone based coating: The role of organoclay. Prog. Org. Coat. 2013, 76, 1633–1641. [Google Scholar] [CrossRef]
- Gardelle, B.; Duquesne, S.; Rerate, V.; Bourbigot, S. Thermal degradation and fire performance of intumescent silicone-based coatings. Polym. Adv. Technol. 2013, 24, 62–69. [Google Scholar] [CrossRef]
- Przybylak, M.; Maciejewski, H.; Dutkiewicz, A.; Wesołek, D.; Władyka-Przybylak, M. Multifunctional, strongly hydrophobic and flame-retarded cotton fabrics modified with flame retardant agents and silicon compounds. Polym. Degrad. Stabil. 2016, 128, 55–64. [Google Scholar] [CrossRef]
- Januszewski, R.; Dutkiewicz, M.; Maciejewski, H.; Marciniec, B. Synthesis and characterization of phosphorus-containing, silicone rubber based flame retardant coatings. React. Funct. Polym. 2018, 123, 1–9. [Google Scholar] [CrossRef]
- Shayed, M.A.; Hund, R.D.; Cherif, C. Effect of thermal-resistant polymeric coatings on thermomechanical and topographical properties of glass fiber. J. Ind. Text. 2015, 44, 682–698. [Google Scholar] [CrossRef]
- Zhuo, D.; Gu, A.; Liang, G.; Hu, J.T.; Cao, L.; Li Yuan, L. Flame retardancy and flame retarding mechanism of high performance hyperbranched PDMS modified bismaleimide/cyanate ester resin. Polym. Degrad. Stabil. 2011, 96, 505–514. [Google Scholar] [CrossRef]
- Dudziec, B.; Żak, P.; Dutkiewicz, M.; Franczyk, A.; Marciniec, B. Synthesis of Functionalized Silsesquioxanes as Molecular Templates for Hybrid Materials in Efficient Methods for Preparing Silicon Compounds, 1st ed.; Roesky, H.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 10; pp. 143–159. [Google Scholar]
- Pietraszuk, C.; Pawluć, P.; Marciniec, B. Metathesis of Silicon-Containing Olefins. In Handbook of Metathesis, 2nd ed.; Grubbs, R.H., O’Leary, D.J., Eds.; VCH–Wiley: Weinheim, Germany, 2015; Volume 2, Chapter 9; pp. 583–631. [Google Scholar]
- Marciniec, B. Hydrosilylation: A Comprehensive Review on Recent Advances; Springer: Berlin, Germany, 2009; pp. 159–189. [Google Scholar]
- Expanding Applications for Silicone Chemistry. Available online: https://www.paint.org/coatingstech-magazine/articles/expanding-applications-silicone-chemistry/ (accessed on 14 May 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
