Effects of Al Sputtering Film on the Oxidation Behavior of NiCrAlY Bondcoat
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Al Sputtering Process
2.3. Oxidation
2.4. Sample Characterization
3. Results
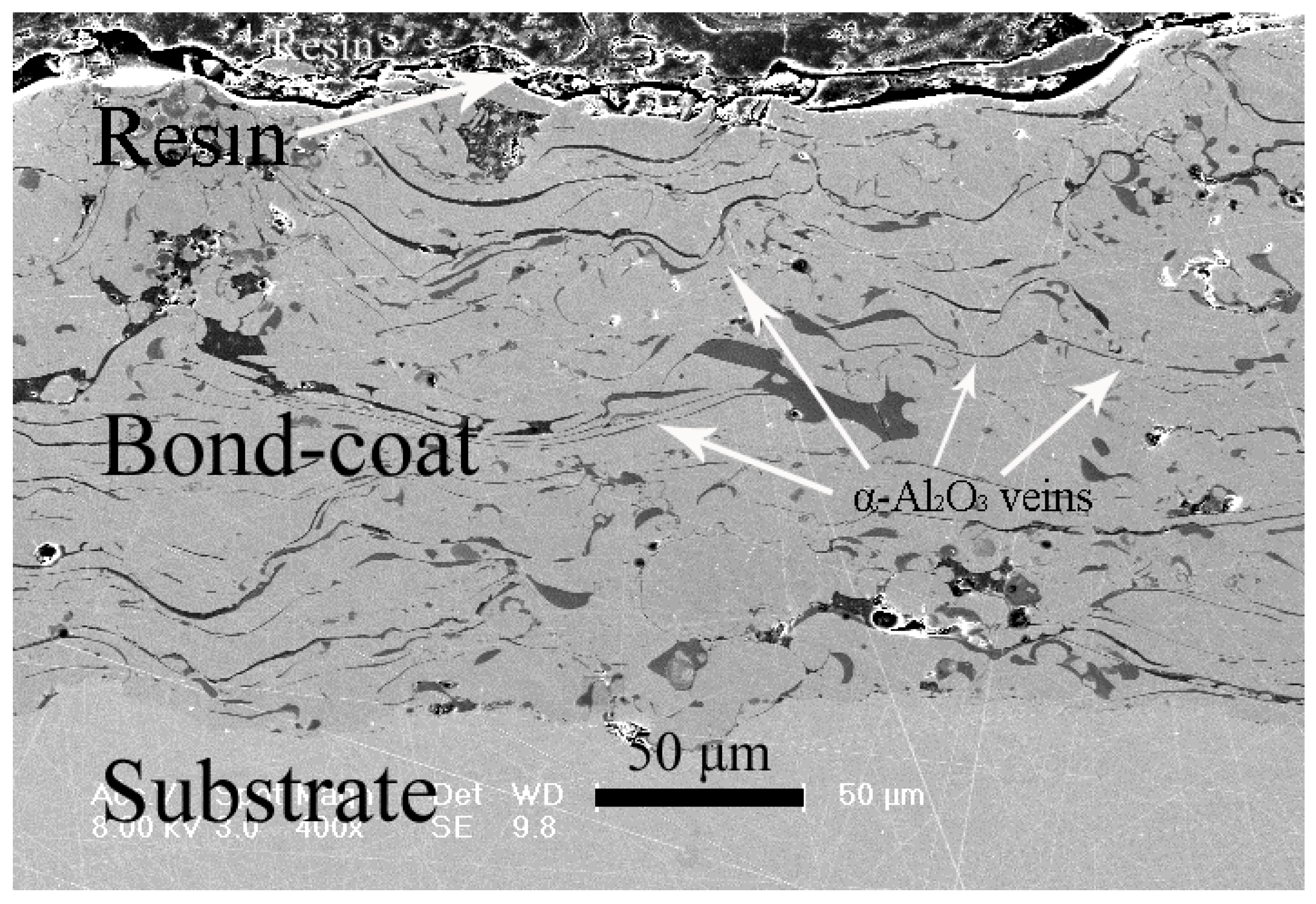
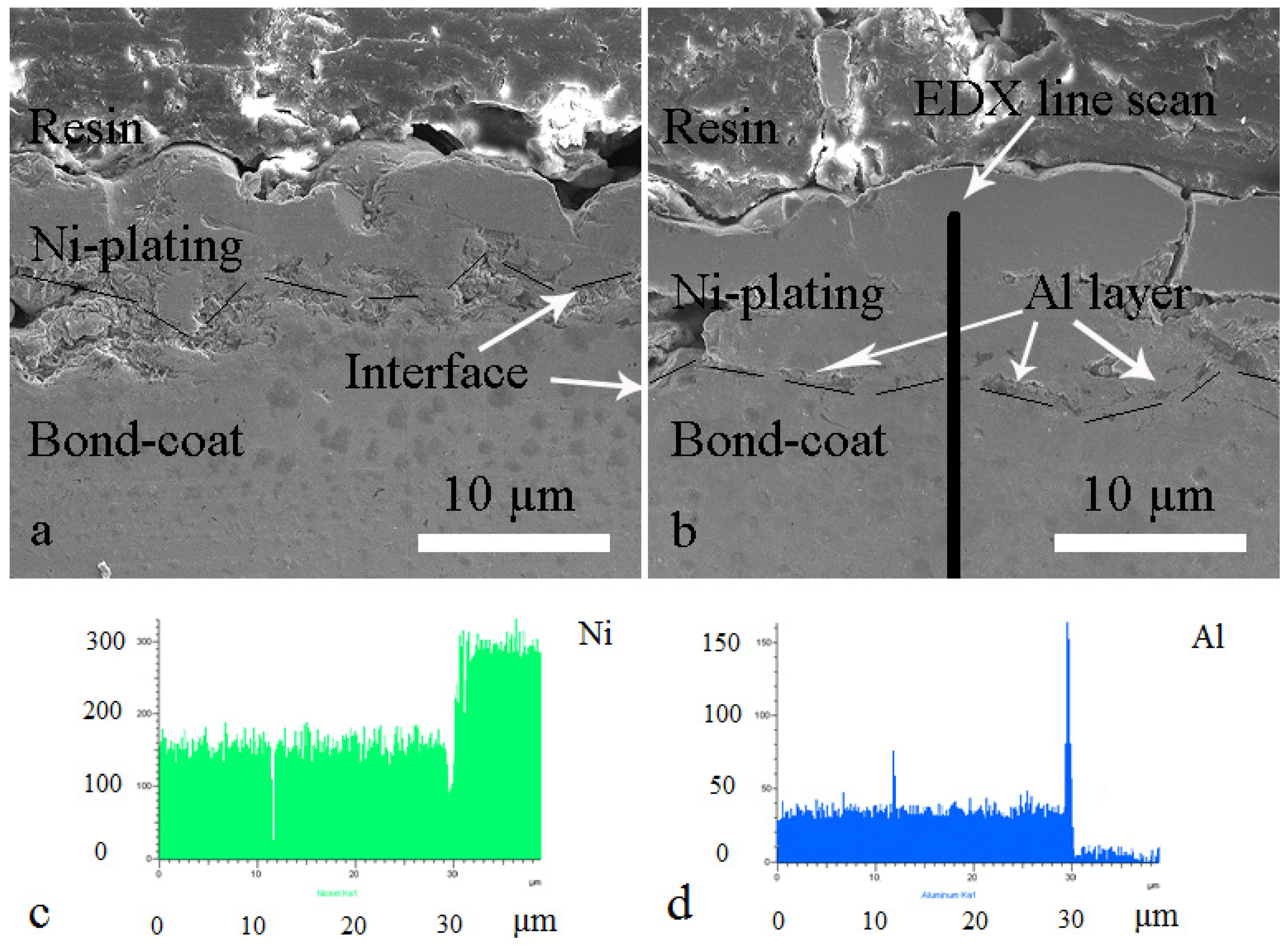
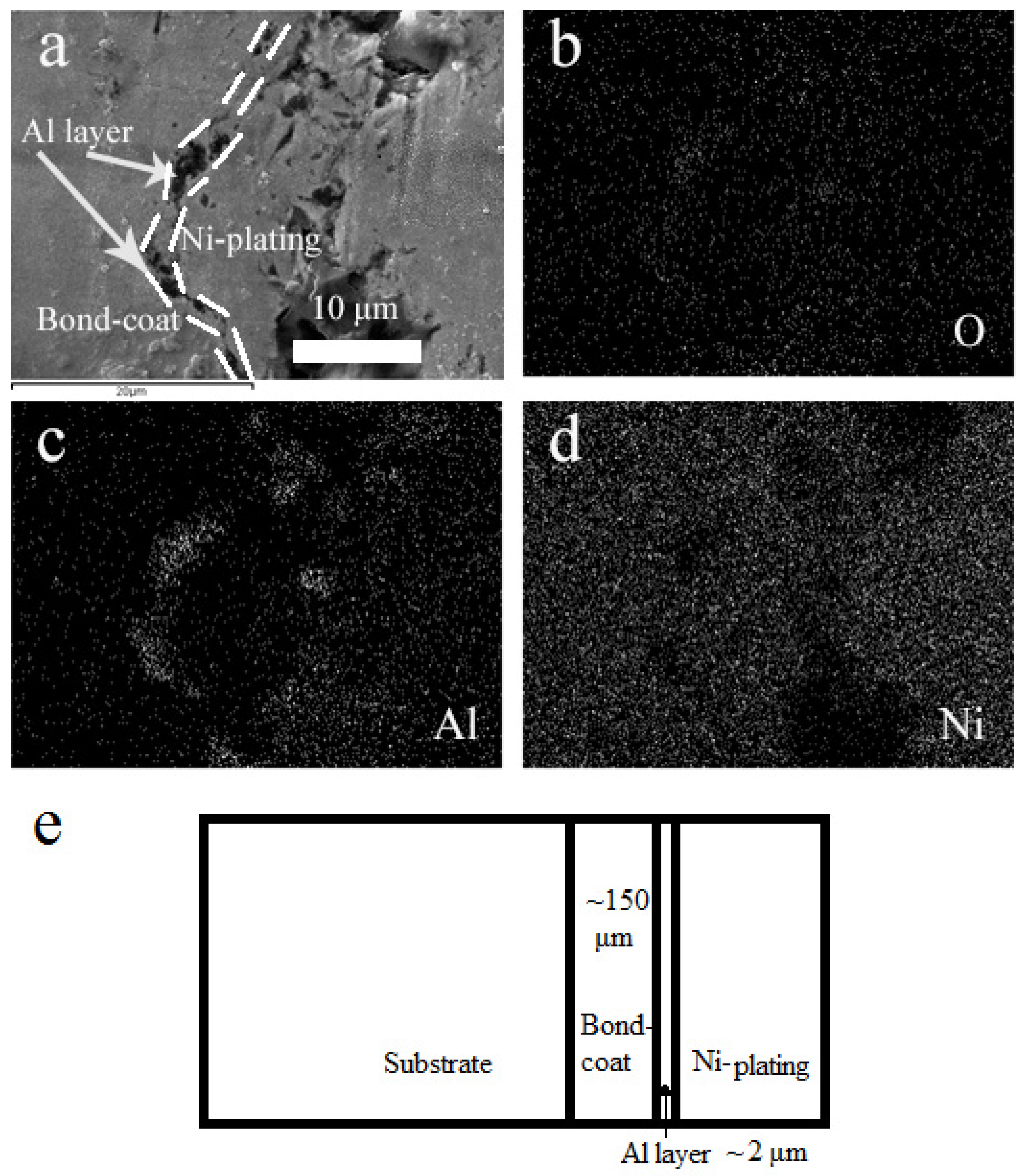
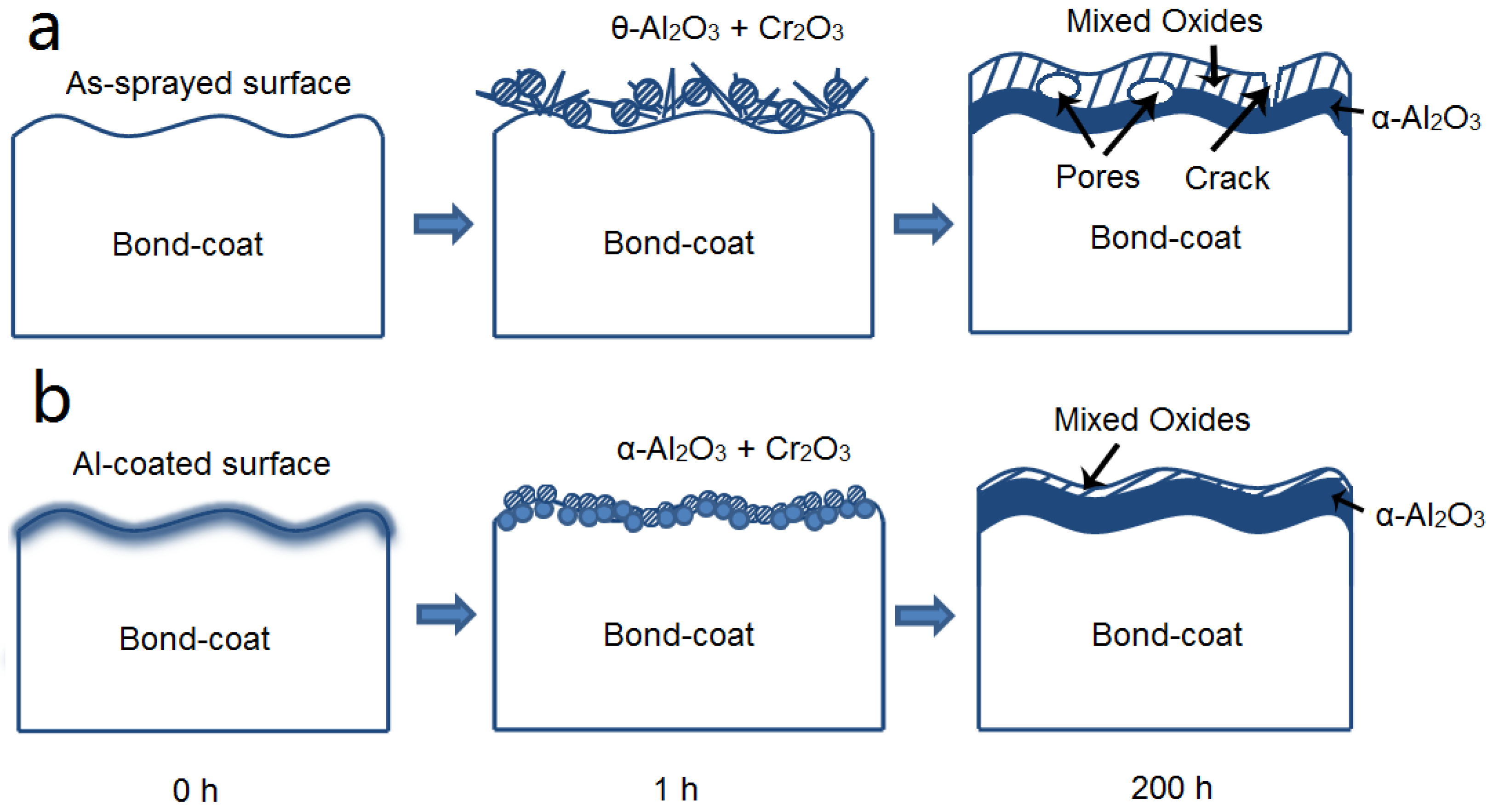
3.1. Microstructure of as-Sprayed and Al Coated NiCrAlY Bondcoat Samples
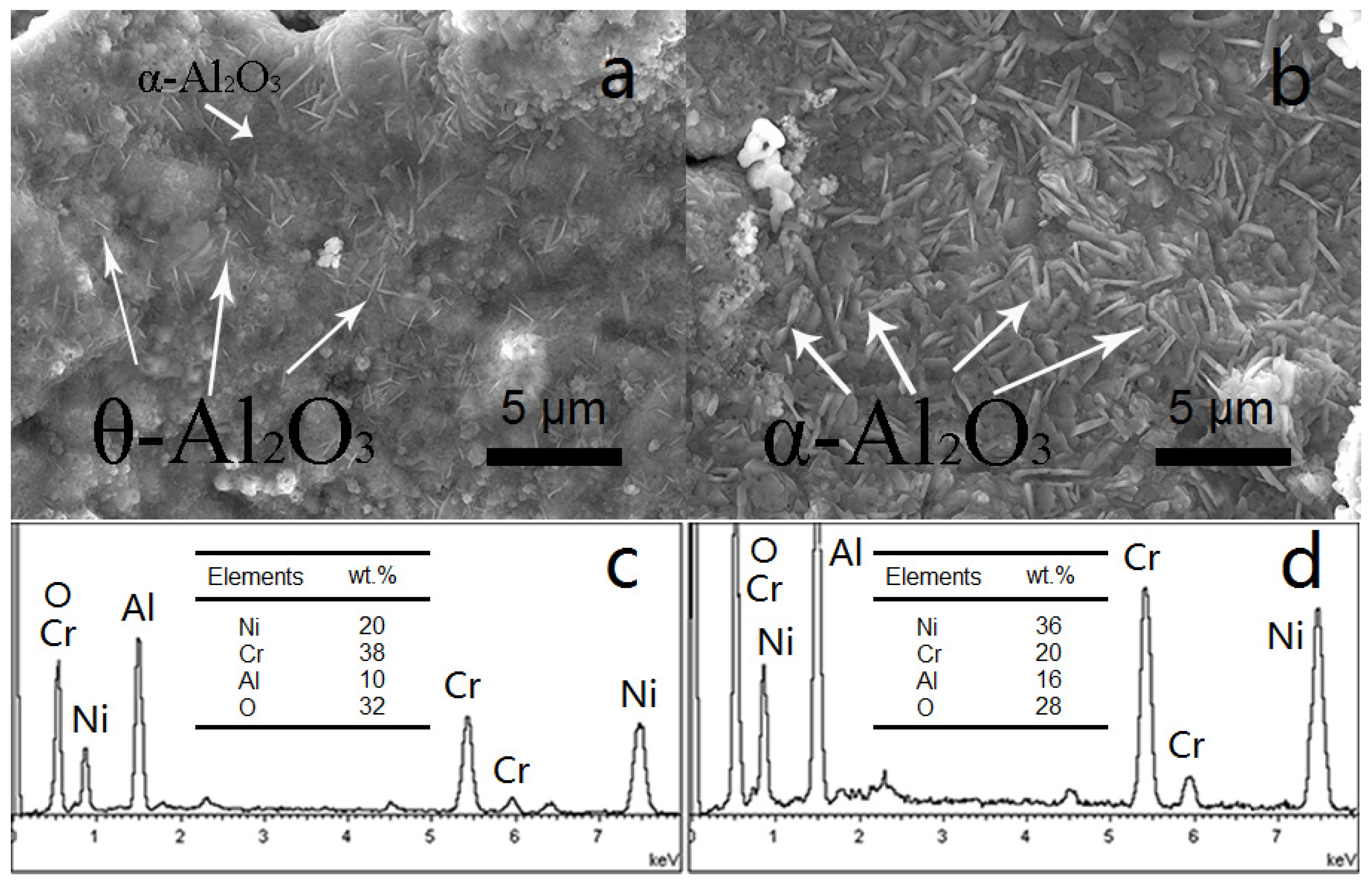
3.2. NiCrAlY Bondcoat Samples after Isothermal Oxidation for 1 h at 1200 °C
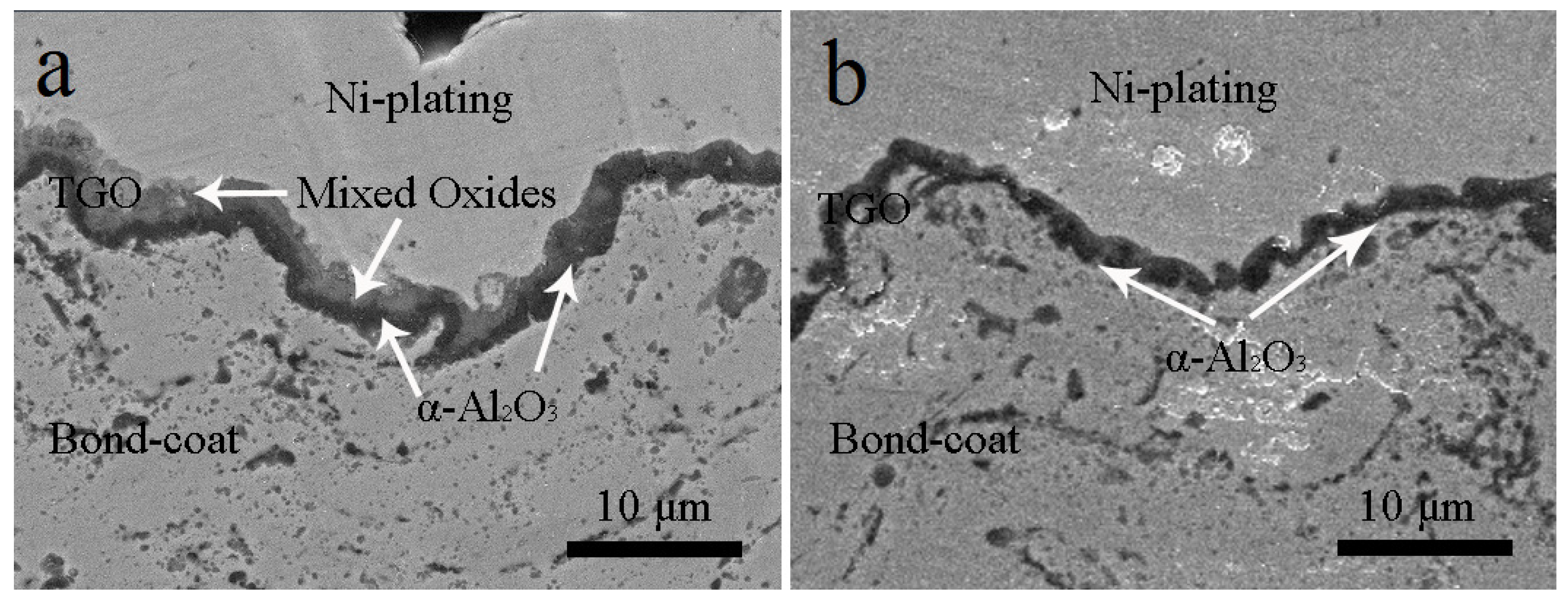
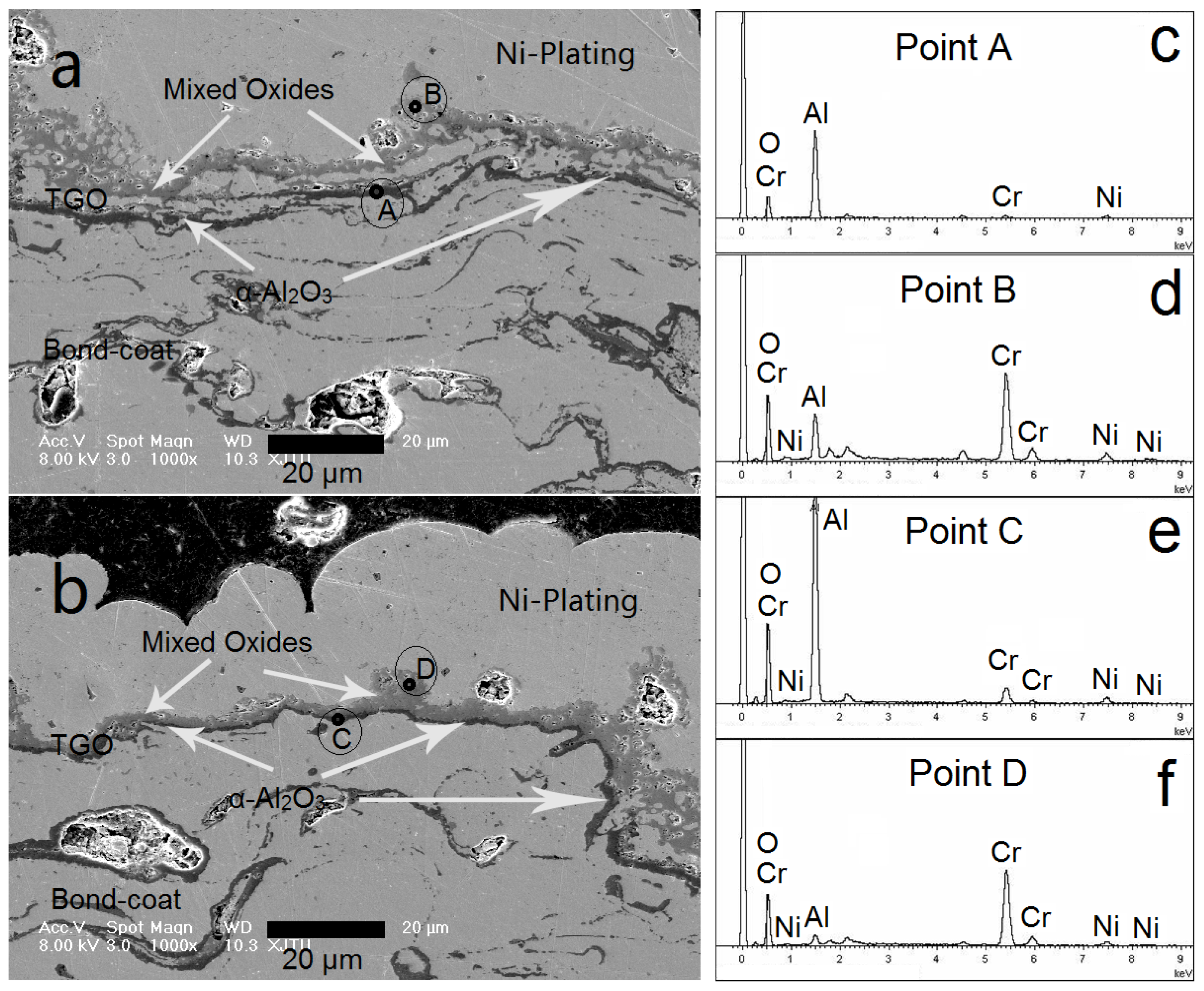
3.3. NiCrAlY Bondcoat Samples after Isothermal Oxidation at 1200 °C
3.4. Thermal Cycling Oxidation Behavior of NiCrAlY Bondcoat Samples
4. Discussion
5. Conclusions
- The isothermal oxidation of Al coated bondcoat samples under 1200 °C for 1 h led to the formation of α-Al2O3 and Cr2O3. Under the same oxidation conditions, θ-Al2O3 and Cr2O3 formed on the surface of as-sprayed bondcoat samples. This indicated that enhanced Al content suppressed the formation of metastable aluminas.
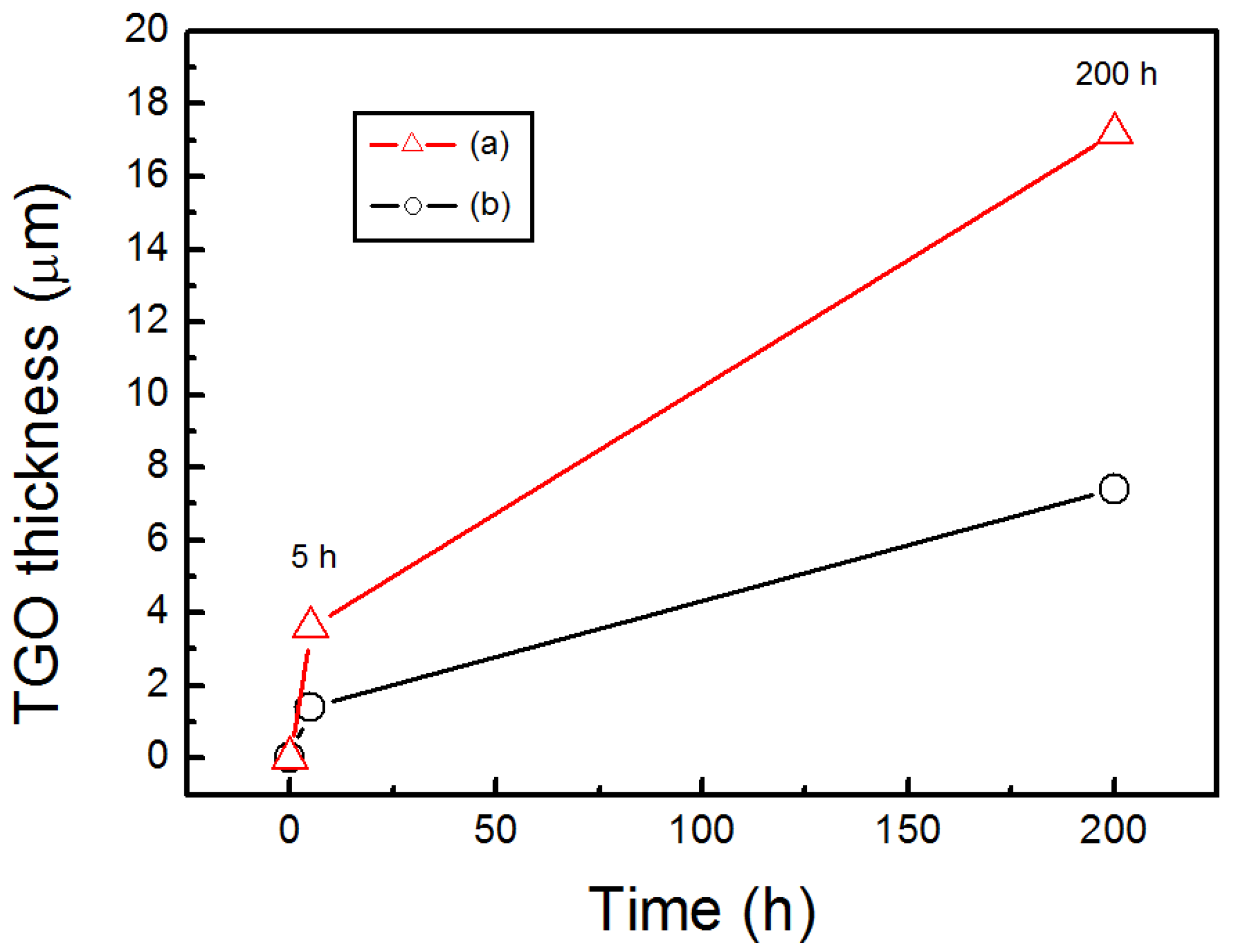
- After isothermally oxidized at 1200 °C for 200 h, the TGO layers formed on the surface of Al coated and as-sprayed bondcoat samples. TGOs consisted of a bright outer mixed oxides layer and a dark inner α-Al2O3 layer. The average thickness of the TGO of as-sprayed samples was ~17.2 μm; while that of Al coated samples was ~7.4 μm. The average thickness of α-Al2O3 layer in as-sprayed samples was <2.0 μm, while that of Al coated samples was ~3.3 μm. This indicated that a finer TGO formed after increasing the Al content on the surface of bondcoat.
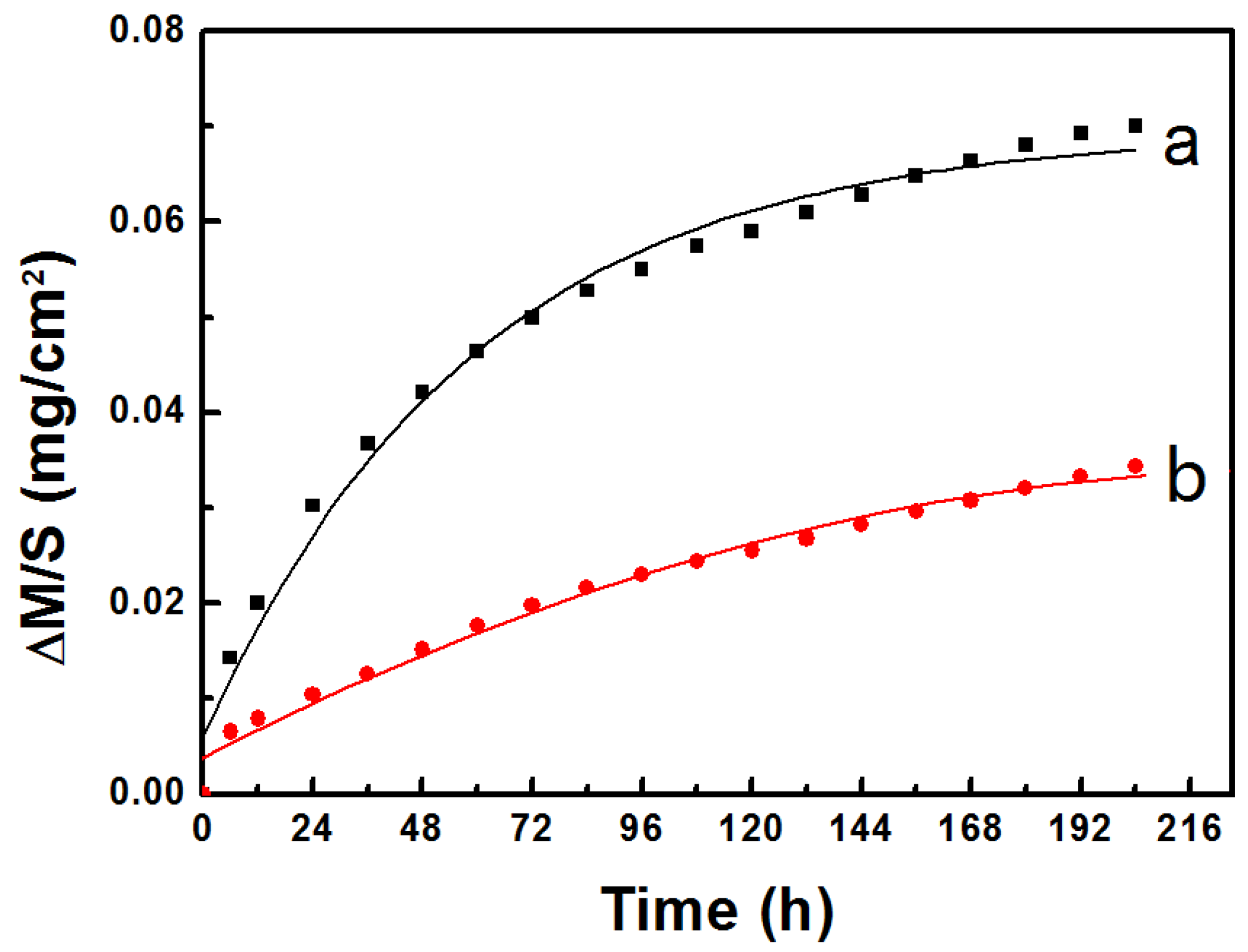
- Cyclic oxidation was performed at 1050 °C for 204 h. Results showed that the weight gain per unit area of Al coated bondcoat samples was smaller than that of as-sprayed bondcoat samples. Thus, better oxidation resistance was achieved by Al sputtering.
Author Contributions
Funding
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials design for the next generation thermal barrier coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, F.; Zhang, Z.; Li, H.; Wang, Y.; Ren, L.; Liu, M. Effects of selective laser modification and Al deposition on the hot corrosion resistance of ceria and yttria-stabilized zirconia thermal barrier coatings. Coatings 2019, 9, 353. [Google Scholar] [CrossRef]
- Song, D.; Song, T.; Paik, U.; Lyu, G.; Jung, Y.G.; Choi, B.G.; Kim, I.S.; Zhang, J. Crack-growth behavior in thermal barrier coatings with cyclic thermal exposure. Coatings 2019, 9, 365. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stover, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Vassen, R.; Stuke, A.; Stover, D. Recent developments in the field of thermal barrier coatings. J. Therm. Spray Technol. 2009, 18, 181–186. [Google Scholar] [CrossRef]
- Bumgardner, C.; Croom, B.; Li, X. High-temperature delamination mechanisms of thermal barrier coatings. Acta Mater. 2017, 128, 54–63. [Google Scholar] [CrossRef]
- Meng, G.H.; Zhang, B.Y.; Sun, X.G.; Pan, Z.Y.; He, G.Q.; Zhou, Y.; Wu, P.L. Vacuum heat treatment mechanisms promoting the adhesion strength of thermally sprayed metallic coatings. Surf. Coat. Technol. 2018, 344, 102–110. [Google Scholar] [CrossRef]
- Mahalingam, S.; Yunus, S.M.; Li, F.; Manap, A.; Afandi, N.M.; Zainuddin, R.A.; Kadir, N.F. Crack propagation and effect of mixed oxides on TGO growth in thick La–Gd–YSZ thermal barrier coating. Coatings 2019, 9, 719. [Google Scholar] [CrossRef]
- Feng, J.; Ren, X.; Wang, X.; Zhou, R.; Pan, W. Thermal conductivity of ytterbia-stabilized zirconia. Scripta Mater. 2012, 66, 41–44. [Google Scholar] [CrossRef]
- Cordier, A.; El Khal, H.; Siebert, E.; Steil, M.C. On the role of the pore morphology on the electrical conductivity of porous yttria-stabilized zirconia. J. Eur. Ceram. Soc. 2019, 39, 2518–2525. [Google Scholar] [CrossRef]
- Ozgurluk, Y.; Doleker, K.M.; Ozkan, D.; Ahlatci, H.; Karaoglanli, A.C. Cyclic hot corrosion failure behaviors of EB-PVD TBC systems in the presence of sulfate and vanadate molten salts. Coatings 2019, 9, 166. [Google Scholar] [CrossRef]
- Lima, R.S.; Guerreiro, B.M.H.; Aghasibeig, M. Microstructural Characterization and Room-Temperature Erosion Behavior of As-Deposited SPS, EB-PVD and APS YSZ-Based TBCs. J. Therm. Spray Technol. 2019, 28, 223–232. [Google Scholar] [CrossRef]
- Saldaña, J.M.; Schulz, U.; Rodríguez, G.C.M.; Cacerse-Diaz, L.A.; Lau, H. Microstructure and lifetime of Hf or Zr doped sputtered NiAlCr bond coat/7YSZ EB-PVD TBC systems. Surf. Coat. Technol. 2018, 335, 41–51. [Google Scholar] [CrossRef]
- Meng, G.H.; Liu, H.; Liu, M.J.; Xu, T.; Yang, G.J.; Li, C.X.; Li, C.J. Highly oxidation resistant MCrAlY bond coats prepared by heat treatment under low oxygen content. Surf. Coat. Technol. 2019, 368, 192–201. [Google Scholar] [CrossRef]
- Meng, G.H.; Zhang, B.Y.; Liu, H.; Yang, G.J.; Xu, T.; Li, C.X.; Li, C.J. Highly oxidation resistant and cost effective MCrAlY bond coats prepared by controlled atmosphere heat treatment. Surf. Coat. Technol. 2018, 347, 54–65. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, P. Thermal barrier coatings on nickel superalloy substrates. Sci. Forum 2009, 606, 1–26. [Google Scholar] [CrossRef]
- Zhu, C.; Li, P.; Javed, A.; Liang, G.Y.; Xiao, P. An investigation on the microstructure and oxidation behavior of laser remelted air plasma sprayed thermal barrier coatings. Surf. Coat. Technol. 2012, 206, 3739–3746. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Surface rumpling of a (Ni, Pt) Al bond coat induced by cyclic oxidation. Acta Mater. 2000, 48, 3283–3293. [Google Scholar] [CrossRef]
- Parlikar, C.; Alam, M.Z.; Chatterjee, D.; Das, D.K. Oxidation and concomitant effects on the microstructure and high temperature tensile properties of a DS Ni-base superalloy applied with different thicknesses of Pt-aluminide (PtAl) bond coat. Surf. Coat. Technol. 2019, 373, 25–37. [Google Scholar] [CrossRef]
- Lu, Z.; Lyu, G.; Gulhane, A.; Park, H.M.; Kim, J.S.; Jung, Y.J.; Zhang, J. Experimental and modeling studies of bond coat species dffect on microstructure evolution in EB-PVD thermal barrier coatings in cyclic thermal environments. Coatings 2019, 9, 626. [Google Scholar] [CrossRef]
- Zhu, C.; Javed, A.; Li, P.; Yang, F.; Liang, G.Y.; Xiao, P. A study of the microstructure and oxidation behavior of alumina/yttria-stabilized zirconia (Al2O3/YSZ) thermal barrier coatings. Surf. Coat. Technol. 2012, 206, 214–222. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Meng, G.H.; Yang, G.J.; Li, C.X.; Li, C.J. Dependence of scale thickness on the breaking behavior of the initial oxide on plasma spray bond coat surface during vacuum pre-treatment. Appl. Surf. Sci. 2017, 397, 125–132. [Google Scholar] [CrossRef]
- Xu, S.Q.; Zhu, C.; Zhang, Y. Effects of laser remelting and oxidation on NiCrAlY/8Y2O3-ZrO2 thermal barrier coatings. J. Therm. Spray Technol. 2018, 27, 412–422. [Google Scholar] [CrossRef]
- An, T.F.; Guan, H.R.; Sun, X.F.; Hu, Z.Q. Effect of the θ–α-Al2O3 transformation in scales on the oxidation behavior of a nickel-base superalloy with an aluminide diffusion coating. Oxid. Met. 2000, 54, 301–316. [Google Scholar] [CrossRef]
- Puetz, P.; Huang, X.; Yang, Q.; Tang, Z. Transient oxide formation on APS NiCrAlY after oxidation heat treatment. J. Therm. Spray Technol. 2011, 20, 621–629. [Google Scholar] [CrossRef]
- Zhu, C.; Li, P.; Wu, X.Y. A study of the diffusion and pre-oxidation treatment on the formation of Al2O3 ceramic scale on NiCrAlY bond-coat during initial oxidation process. Ceram. Int. 2016, 42, 7708–7716. [Google Scholar] [CrossRef]
- Niranatlumpong, P.; Ponton, C.B.; Evans, H.E. The failure of protective oxides on plasma-sprayed NiCrAlY overlay coatings. Oxid. Met. 2000, 53, 241–258. [Google Scholar] [CrossRef]
- Swadźba, R. Interfacial phenomena and evolution of modified aluminide bondcoatings in thermal barrier coatings. Appl. Surf. Sci. 2018, 445, 133–144. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Shi, J.; Yang, G.J.; Li, C.X.; Li, C.J. Healing of the interface between splashed particles and underlying bulk coating and its influence on isothermal oxidation behavior of LPPS MCrAlY bond coat. Therm. Spray Technol. 2015, 24, 611–621. [Google Scholar] [CrossRef]
- Goward, G.W. Progress in coatings for gas turbine airfoils. Surf. Coat. Technol. 1998, 108–109, 73–79. [Google Scholar] [CrossRef]
- Brossard, J.M.; Balmain, J.; Sanchette, F.; Bonnet, G. High-temperature oxidation of an aluminized NiCr alloy formed by a magnetron-sputtered Al diffusion coating. Oxid. Met. 2005, 64, 43–61. [Google Scholar] [CrossRef]
- Zhu, C.; Javed, A.; Li, P.; Liang, G.Y.; Xiao, P. Study of the effect of laser treatment on the initial oxidation behaviour of Al-coated NiCrAlY bond-coat. Surf. Interface Anal. 2013, 45, 1680–1689. [Google Scholar] [CrossRef]
- Zhang, P.P.; Li, F.H.; Zhang, X.F.; Zhang, Z.H.; Zhou, F.F.; Ren, L.Q.; Liu, M. Thermal shock resistance of thermal barrier coatings with different surface shapes modified by laser remelting. J. Therm. Spray Technol. 2019, 28, 417–432. [Google Scholar] [CrossRef]
- Haynes, J.A.; Unocic, K.A.; Pint, B.A. Effect of water vapor on the 1100°C oxidation behavior of plasma-sprayed TBCs with HVOF NiCoCrAlX bond coatings. Surf. Coat. Technol. 2013, 215, 39–45. [Google Scholar] [CrossRef]
- Strawbridge, A.; Evans, H.E.; Ponton, C.B. Spallation of oxide scales from NiCrAlY overlay coatings. Mater. Sci. Forum. 2009, 251–254, 365–372. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Jeurgens, L.P.H.; Sloof, W.G. Promoting exclusive α-Al2O3 growth upon high-temperature oxidation of NiCrAl alloys: Experiment versus model predictions. Acta Mater. 2005, 53, 1643–1653. [Google Scholar] [CrossRef]
- Chen, W.R.; Archer, R.; Huang, X.; Marple, B.R. TGO growth and crack propagation in a thermal barrier coating. J. Therm. Spray Technol. 2008, 17, 858–864. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Sloof, W.G. Modelling of composition and phase changes in multiphase alloys due to growth of an oxide layer. Acta Mater. 2008, 56, 4972–4983. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Dudzinski, X. Influence of thermal cycle frequency on the TGO growth and cracking behaviors of an APS-TBC. J. Therm. Spray Technol. 2012, 21, 1294–1299. [Google Scholar] [CrossRef]
- Chen, W.R. Degradation of a TBC with HVOF-CoNiCrAlY bond coat. J. Therm. Spray Technol. 2014, 23, 876–884. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Marijnissen, G.H.; Vergeldt, E.; Kloosterman, A.B.; Sloof, W.G. Development of a pre-oxidation treatment to improve the adhesion between thermal barrier coatings and NiCoCrAlY bond coatings. Oxid. Met. 2006, 66, 269–294. [Google Scholar] [CrossRef]
- Nijdam, T.J.; Sloof, W.G. Combined pre-annealing and pre-oxidation treatment for the processing of thermal barrier coatings on NiCoCrAlY bond coatings. Surf. Coat. Technol. 2006, 201, 3894–3900. [Google Scholar] [CrossRef]
- Evans, A.G.; Clarke, D.R.; Levi, C.G. The influence of oxides on the performance of advanced gas turbines. J. Eur. Ceram. Soc. 2008, 28, 1405–1419. [Google Scholar] [CrossRef]
- Yang, G.J.; Xiang, X.D.; Xing, L.K.; Li, D.J.; Li, C.X.; Li, C.J. Isothermal oxidation behavior of NiCoCrAlTaY coating deposited by high velocity air-fuel spraying. J. Therm. Spray Technol. 2012, 21, 391–399. [Google Scholar] [CrossRef]
- Haynes, J.A.; Pint, B.A.; Porter, W.D.; Wright, I.G. Comparison of thermal expansion and oxidation behavior of various high-temperature coating materials and superalloys. Mater. High Temp. 2004, 21, 87–94. [Google Scholar] [CrossRef]
- Weeks, M.D.; Subramanian, R.; Vaidya, A.; Mumm, D.R. Defining optimal morphology of the bond coat–thermal barrier coating interface of air-plasma sprayed thermal barrier coating systems. Surf. Coat. Technol. 2015, 273, 50–59. [Google Scholar] [CrossRef]
- Lance, M.J.; Haynes, J.A.; Pint, B.A. The effects of temperature and substrate curvature on TBC lifetime and residual stress in alumina scales beneath APS YSZ. Surf. Coat. Technol. 2016, 308, 19–23. [Google Scholar] [CrossRef]
- Gildersleeve, V.E.; Viswanathan, V.; Lance, M.J.; Haynes, J.A.; Pint, B.A.; Sampath, S. Role of bond coat processing methods on the durability of plasma sprayed thermal barrier systems. Surf. Coat. Technol. 2019, 375, 782–792. [Google Scholar] [CrossRef]
- Kane, K.A.; Lance, M.J.; Sweet, M.; Pint, B.A. The effect of bond coating surface modification on the performance of atmospheric plasma spray thermal barrier coatings. Surf. Coat. Technol. 2019, 378, 125042. [Google Scholar] [CrossRef]

| Powder | Nominal Particle Size Distributions D (0.1)–D (0.9) | Average Particle Sizes D (0.5) | Compositions (wt.%) |
|---|---|---|---|
| NiCrAlY powder | 10.0–40.0 μm | 25.0 μm | 69Ni–20Cr–10Al–1Y |
| Parameter | Unit | NiCrAlY Feedstocks |
|---|---|---|
| Gun nozzle inner diameter | mm | 6 |
| Arc current | A | 600 |
| Arc voltage | V | 70 |
| Primary gas flow rate (Ar) | L/min | 80 |
| Secondary gas flow rate (H2) | L/min | 6 |
| Carrier gas flow rate (Ar) | L/min | 10 |
| Gun traverse speed | mm/s | 800 |
| Powder feed rate | g/min | 40 |
| Spray distance | mm | 100 |
| Substrate | Unit | NiCrAlY Bondcoat |
|---|---|---|
| Target | / | Al (99.9%) |
| Target size | mm3 | 320 × 200 × 6 |
| Substrate to target distance | mm | 50 |
| Pre-sputtering time | min | 20 |
| Working pressure | Pa | 0.16 |
| DC power | W | 25 |
| Ar flow rate | mL/min | 23 |
| Deposition time | min | 20 |
| Al coating thickness | μm | ~2 |
| Composition (wt.%) | As-Sprayed Sample | Al Coated Sample | ||
|---|---|---|---|---|
| Site A | Site B | Site C | Site D | |
| Al | 80 | 28 | 85 | 18 |
| Cr | 14 | 60 | 10 | 72 |
| Ni | 6 | 12 | 5 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, G.; Yang, Q.; Cao, W.; Pu, J.; Zhu, C. Effects of Al Sputtering Film on the Oxidation Behavior of NiCrAlY Bondcoat. Coatings 2020, 10, 376. https://doi.org/10.3390/coatings10040376
Zhang Y, Zhang G, Yang Q, Cao W, Pu J, Zhu C. Effects of Al Sputtering Film on the Oxidation Behavior of NiCrAlY Bondcoat. Coatings. 2020; 10(4):376. https://doi.org/10.3390/coatings10040376
Chicago/Turabian StyleZhang, Yong, Gengfei Zhang, Qiang Yang, Weicheng Cao, Jian Pu, and Chao Zhu. 2020. "Effects of Al Sputtering Film on the Oxidation Behavior of NiCrAlY Bondcoat" Coatings 10, no. 4: 376. https://doi.org/10.3390/coatings10040376
APA StyleZhang, Y., Zhang, G., Yang, Q., Cao, W., Pu, J., & Zhu, C. (2020). Effects of Al Sputtering Film on the Oxidation Behavior of NiCrAlY Bondcoat. Coatings, 10(4), 376. https://doi.org/10.3390/coatings10040376





