Effect of Boron Doping on Diamond Film and Electrochemical Properties of BDD According to Thickness and Morphology
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liou, Y.; Inspektor, A.; Weimer, R.; Knight, D.; Messier, R. The Effect of Oxygen in Diamond Deposition by Microwave Plasma Enhanced Chemical Vapor-Deposition. J. Mater. Res. 1990, 5, 2305–2312. [Google Scholar] [CrossRef]
- Matsuda, J.; Fukunaga, K.; Ito, K. Noble-Gas Studies in Vapor-Growth Diamonds—Comparison with Shock-Produced Diamonds and the Origin of Diamonds in Ureilites. Geochim. Cosmochim. Ac. 1991, 55, 2011–2023. [Google Scholar] [CrossRef]
- Deshpande, S.V.; Dupuie, J.L.; Gulari, E. Hot Filament Assisted Deposition of Silicon-Nitride Thin-Films. Appl. Phys. Lett. 1992, 61, 1420–1422. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Ihara, M.; Komiyama, H. Temperature-Dependence of Growth-Rate for Diamonds Grown Using a Hot-Filament Assisted Chemical-Vapor-Deposition Method at Low Substrate Temperatures. Appl. Phys. Lett. 1994, 64, 1306–1308. [Google Scholar] [CrossRef]
- Scardi, P.; Veneri, S.; Leoni, M.; Polini, R.; Traversa, E. Lattice disorder and texture in diamond coatings deposited by HFCVD on Co-cemented tungsten carbide. Thin Solid Films 1996, 290, 136–142. [Google Scholar] [CrossRef]
- Gomez-Ruiz, B.; Gomez-Lavin, S.; Diban, N.; Boiteux, V.; Colin, A.; Dauchy, X.; Urtiaga, A. Boron doped diamond electrooxidation of 6:2 fluorotelomers and perfluorocarboxylic acids. Application to industrial wastewaters treatment. J. Electroanal. Chem. 2017, 798, 51–57. [Google Scholar] [CrossRef]
- Li, H.; Yu, Q.N.; Yang, B.; Li, Z.J.; Lei, L.C. Electrochemical treatment of artificial humidity condensate by large-scale boron doped diamond electrode. Sep. Purif. Technol. 2014, 138, 13–20. [Google Scholar] [CrossRef]
- Arevalo, E.; Calmano, W. Studies on electrochemical treatment of wastewater contaminated with organotin compounds. J. Hazard. Mater. 2007, 146, 540–545. [Google Scholar] [CrossRef]
- Du, L.L.; Sun, J.R.; Cui, H.; Li, H.D.; Cu, T.; Lin, H.B. Synthesis and Temperature-dependent Electrochemical Properties of Boron-doped Diamond Electrodes on Titanium. Chem. Res. Chinese U. 2012, 28, 507–510. [Google Scholar]
- Schmalz, V.; Dittmar, T.; Haaken, D.; Worch, E. Electrochemical disinfection of biologically treated wastewater from small treatment systems by using boron-doped diamond (BDD) electrodes - Contribution for direct reuse of domestic wastewater. Water Res. 2009, 43, 5260–5266. [Google Scholar] [CrossRef]
- Stolte, S.; Abdulkarim, S.; Arning, J.; Blomeyer-Nienstedt, A.K.; Bottin-Weber, U.; Matzke, M.; Ranke, J.; Jastorff, B.; Thoming, J. Primary biodegradation of ionic liquid cations, identification of degradation products of 1-methyl-3-octylimidazolium chloride and electrochemical wastewater treatment of poorly biodegradable compounds. Green Chem. 2008, 10, 214–224. [Google Scholar] [CrossRef]
- Song, C.W.; Lee, Y.H.; Choi, S.; Hwang, N.M.; Kim, K.H. Temperature Simulation and Diamond Deposition Behavior with Distance Between Filament and Susceptor During Hot-Filament Chemical Vapor Deposition. Nanosci. Nanotech. Let. 2018, 10, 761–766. [Google Scholar] [CrossRef]
- Song, C.W.; Lee, Y.H.; Heo, S.Y.; Hwang, N.M.; Choi, S.; Kim, K.H. Computer Simulation of Temperature Parameter for Diamond Formation by Using Hot-Filament Chemical Vapor Deposition. Coatings 2018, 8, 15. [Google Scholar] [CrossRef]
- Ogi, H.; Kai, S.; Ledbetter, H.; Tarumi, R.; Hirao, M.; Takashima, K. Titanium’s high-temperature elastic constants through the hcp-bcc phase transformation. Acta. Mater. 2004, 52, 2075–2080. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, Y. The Effect of Deposition Parameters on the Growth Rate of Microcrystalline Diamond Powders Synthesized by HFCVD Method. Coatings 2017, 7, 95. [Google Scholar] [CrossRef]
- Amaral, M.; Silva, D.J.; Fernandes, A.J.S.; Costa, F.M.; Oliveira, F.J.; Silva, R.F. Surface activation pre-treatments for NCD films grown by HFCVD. Vacuum 2009, 83, 1228–1232. [Google Scholar] [CrossRef]
- Yang, T.M.; Wei, Q.P.; Qi, Y.; Yu, Z.M. The diffusion behavior of carbon in sputtered tungsten film and sintered tungsten block and its effect on diamond nucleation and growth. Diam. Relat. Mater. 2015, 52, 49–58. [Google Scholar] [CrossRef]
- Yaws, C.L. The Yaws Handbook of Vapor Pressure: Antoine Coefficients; Gulf Professional Publishing: Waltham, MA, USA, 2015. [Google Scholar]
- Chen, Z.C.; Subhash, G.; Tulenko, J.S. Raman spectroscopic investigation of graphitization of diamond during spark plasma sintering of UO2-diamond composite nuclear fuel. J. Nucl. Mater. 2016, 475, 1–5. [Google Scholar] [CrossRef]
- De Feudis, M.; Caricato, A.P.; Taurino, A.; Ossi, P.M.; Castiglioni, C.; Brambilla, L.; Maruccio, G.; Monteduro, A.G.; Broitman, E.; Chiodini, G.; et al. Diamond graphitization by laser-writing for all-carbon detector applications. Diam. Relat. Mater. 2017, 75, 25–33. [Google Scholar] [CrossRef]
- Uzansaguy, C.; Cytermann, C.; Brener, R.; Richter, V.; Shaanan, M.; Kalish, R. Damage Threshold for Ion-Beam-Induced Graphitization of Diamond. Appl. Phys. Lett. 1995, 67, 1194–1196. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Sun, F.H.; Shen, B.; Zhang, Z.M. The effect of boron doping on the morphology and growth rate of micron diamond powders synthesized by HFCVD method. Diam. Relat. Mater. 2013, 40, 82–88. [Google Scholar] [CrossRef]
- Ushizawa, K.; Watanabe, K.; Ando, T.; Sakaguchi, I.; Nishitani-Gamo, M.; Sato, Y.; Kanda, H. Boron concentration dependence of Raman spectra on {100} and {111} facets of B-doped CVD diamond. Diam. Relat. Mater. 1998, 7, 1719–1722. [Google Scholar] [CrossRef]
- Tang, C.J.; Fernandes, A.J.S.; Costa, F.; Pinto, J.L. Effect of microwave power and nitrogen addition on the formation of {100} faceted diamond from microcrystalline to nanocrystalline. Vacuum 2011, 85, 1130–1134. [Google Scholar] [CrossRef]
- Hwang, N.M. Crystal growth by charged cluster focused on CVD diamond process. J. Cryst. Growth. 1999, 198, 945–950. [Google Scholar] [CrossRef]
- Hwang, N.M.; Kim, D.Y. Charged clusters in thin film growth. Int. Mater. Rev. 2004, 49, 171–190. [Google Scholar] [CrossRef]
- Bou, P.; Vandenbulcke, L. Raman Investigations on Diamond Films and Crystals Deposited by Plasma-Assisted Cvd. J. Electrochem. Soc. 1991, 138, 2991–3000. [Google Scholar] [CrossRef]
- Kanetkar, S.M.; Matera, G.; Chen, X.K.; Pramanick, S.; Tiwari, P.; Narayan, J.; Pfeiffer, G.; Paesler, M. Growth of Diamond Films on Si(100) with and without Boron-Nitride Buffer Layer. J. Electron. Mater. 1991, 20, 141–149. [Google Scholar] [CrossRef]
- Mortet, V.; Taylor, A.; Zivcova, Z.V.; Machon, D.; Frank, O.; Hubik, P.; Tremouilles, D.; Kavan, L. Analysis of heavily boron-doped diamond Raman spectrum. Diam. Relat. Mater. 2018, 88, 163–166. [Google Scholar] [CrossRef]
- Long, H.Y.; Luo, H.; Luo, J.Q.; Xie, Y.N.; Deng, Z.J.; Zhang, X.W.; Wang, Y.J.; Wei, Q.P.; Yu, Z.M. The concentration gradient of boron along the growth direction in boron doped chemical vapor deposited diamond. Mater. Lett. 2015, 157, 34–37. [Google Scholar] [CrossRef]
- Mortet, V.; Zivcova, Z.V.; Taylor, A.; Frank, O.; Hubik, P.; Tremouilles, D.; Jomard, F.; Barjon, J.; Kavan, L. Insight into boron-doped diamond Raman spectra characteristic features. Carbon 2017, 115, 279–284. [Google Scholar] [CrossRef]
- Liu, Z.L.; Li, H.J.; Li, M.J.; Li, C.P.; Qian, L.R.; Su, L.; Yang, B.H. Preparation of polycrystalline BDD/Ta electrodes for electrochemical oxidation of organic matter. Electrochim. Acta. 2018, 290, 109–117. [Google Scholar] [CrossRef]
- Song, C.W.; Jin, R.; Hwang, N.M.; Kim, K.H. Deposition Behavior of Boron-Doped Diamond with Varying Amount of Acetone by Hot Filament Chemical Vapor Deposition. Electron. Mater. Lett. 2019, 15, 630–638. [Google Scholar] [CrossRef]
- Son, M.J.; Zhang, T.F.; Jo, Y.J.; Kim, K.H. Enhanced electrochemical properties of the DLC films with an arc interlayer, nitrogen doping and annealing. Surf. Coat. Tech. 2017, 329, 77–85. [Google Scholar] [CrossRef]
- Jo, Y.J.; Zhang, T.F.; Son, M.J.; Kim, K.H. Synthesis and electrochemical properties of Ti-doped DLC films by a hybrid PVD/PECVD process. Appl. Surf. Sci. 2018, 433, 1184–1191. [Google Scholar] [CrossRef]
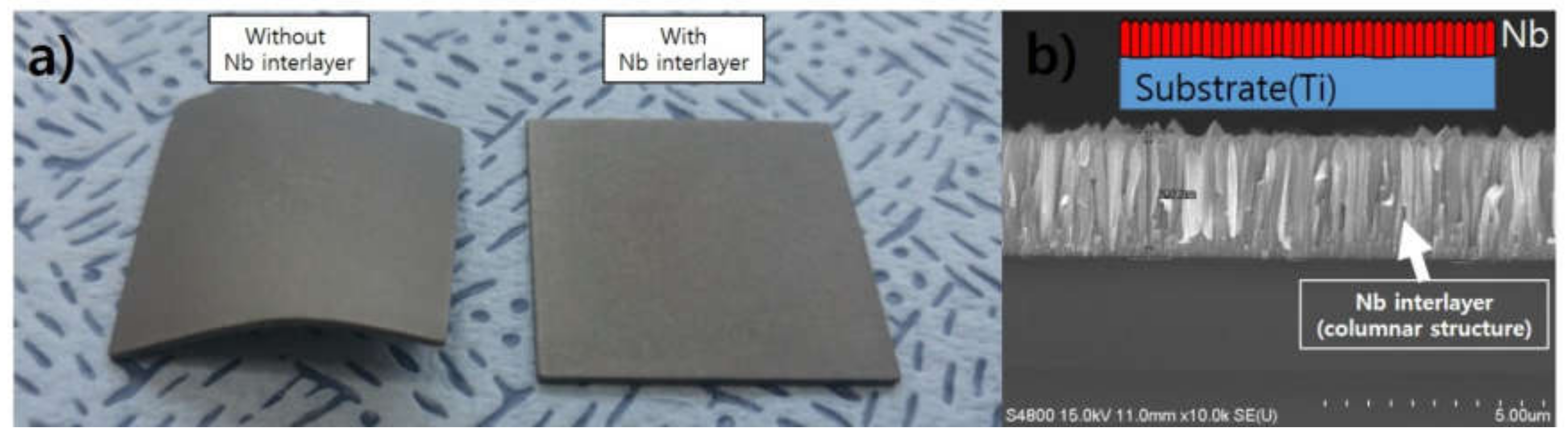
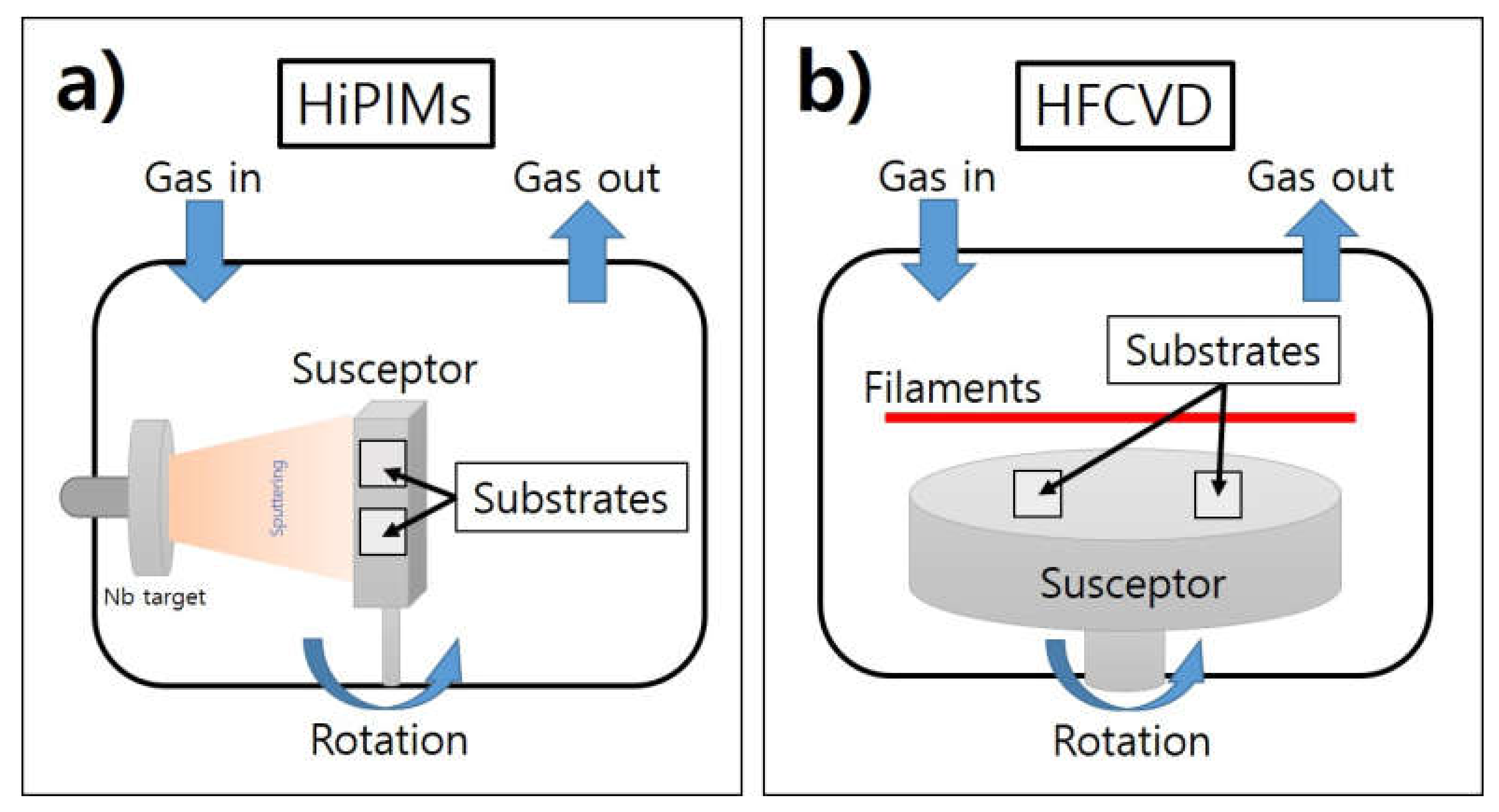

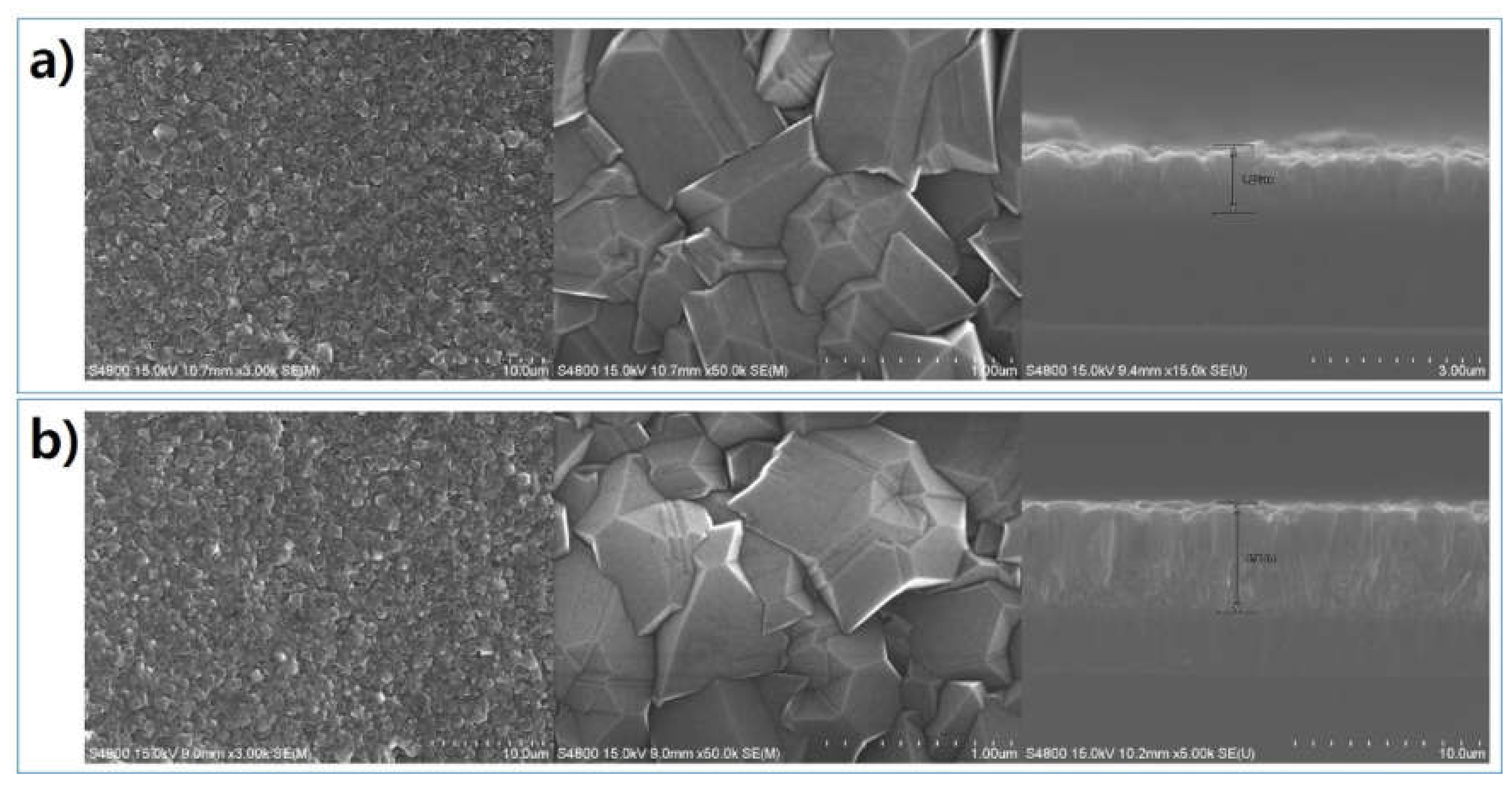

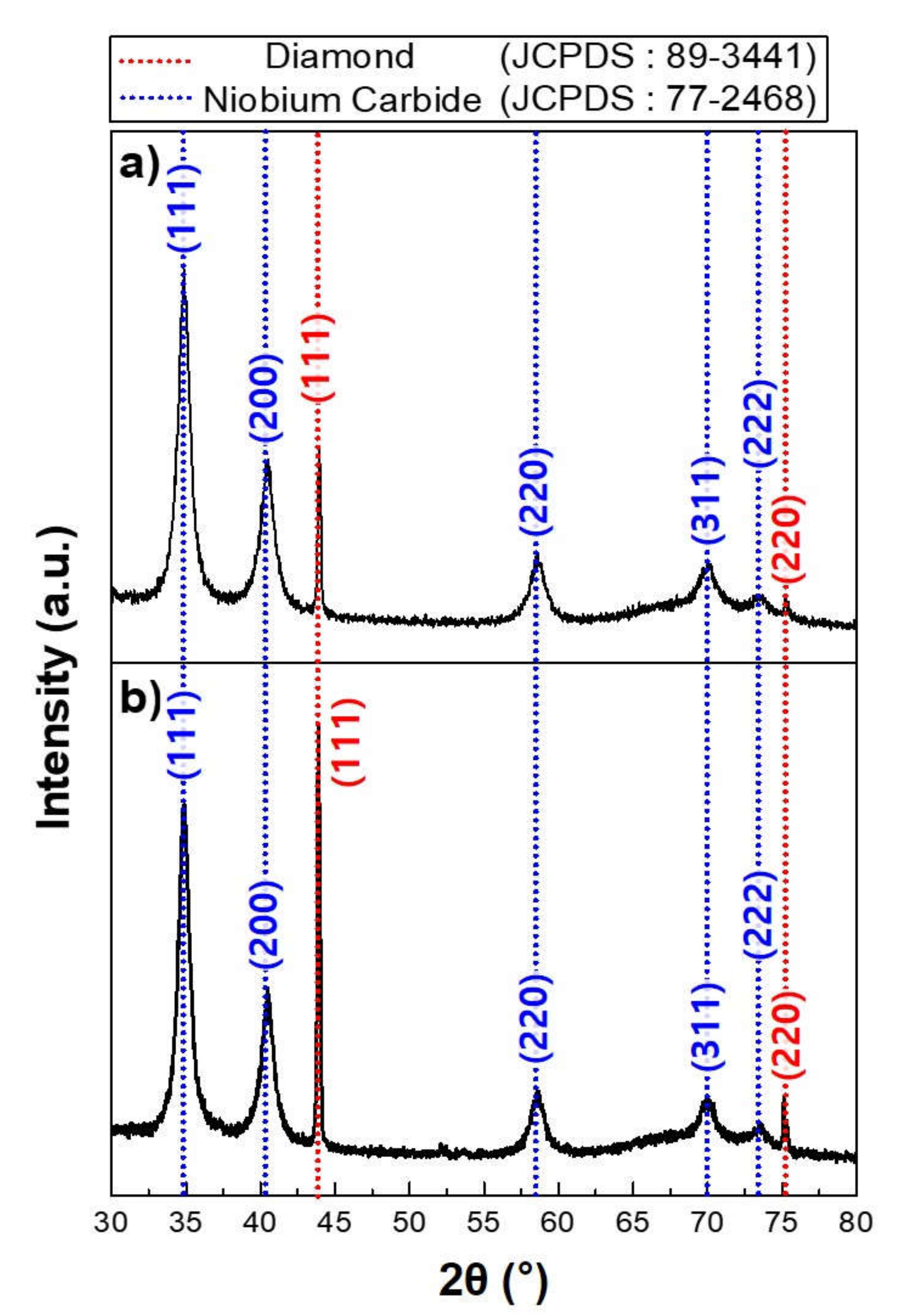
| Parameter | Detail |
|---|---|
| Base pressure | 5.0e−5 torr |
| Working pressure | 8.0e−3 torr |
| Temperature | 100 °C |
| Gas uses | Ar (85 sccm) |
| Deposition Time | 1 h |
| Power source | Nb sputter target 1.0 A |
| Parameter | Value |
|---|---|
| Filament power (kW) | 16 |
| Number of filaments | 12 |
| Length of each filament (cm) | 32 |
| Pressure (Pa) | 4000 |
| Distance between filament and susceptor (mm) | 10 |
| Distance between filaments (mm) | 20 |
| Flux of Acetone (sccm) | 90 |
| Flux of trimethyl borate (TMB, sccm) | 6 |
| Flux of Hydrogen (sccm) | 400 |
| Deposition time (h) | 12 and 60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.W.; Cho, D.S.; Lee, J.M.; Song, P.K. Effect of Boron Doping on Diamond Film and Electrochemical Properties of BDD According to Thickness and Morphology. Coatings 2020, 10, 331. https://doi.org/10.3390/coatings10040331
Song CW, Cho DS, Lee JM, Song PK. Effect of Boron Doping on Diamond Film and Electrochemical Properties of BDD According to Thickness and Morphology. Coatings. 2020; 10(4):331. https://doi.org/10.3390/coatings10040331
Chicago/Turabian StyleSong, Chang Weon, Dae Seung Cho, Jae Myung Lee, and Pung Keun Song. 2020. "Effect of Boron Doping on Diamond Film and Electrochemical Properties of BDD According to Thickness and Morphology" Coatings 10, no. 4: 331. https://doi.org/10.3390/coatings10040331
APA StyleSong, C. W., Cho, D. S., Lee, J. M., & Song, P. K. (2020). Effect of Boron Doping on Diamond Film and Electrochemical Properties of BDD According to Thickness and Morphology. Coatings, 10(4), 331. https://doi.org/10.3390/coatings10040331





