Abstract
Cr–Al alloys are attracting much attention as heat- and corrosion-resistant coating materials due to their excellent high-temperature properties. In order to investigate the effect of aluminum content on the microstructure and oxidation resistance of Cr–Al–Si alloys, cast specimens were prepared by using a vacuum-arc melting furnace, and high-temperature oxidation tests were conducted with the specimens, for 1 h, at 1100 °C, in air. In the case of cast microstructure of Cr–Al–Si alloys, it consists mainly of Cr single phase, up to 5 at.% Al, and AlCr phases were additionally formed in alloys containing 10% Al or more. In the specimen with 20% Al added, CrSi phase was also found in addition to the AlCr phase. The weight change of the specimens heated for 1 h, at 1100 °C, indicated that all had excellent oxidation resistance. However, when the Al content was less than 10%, the weight gain tended to be a little lower than that of 10% or more.
1. Introduction
The demand for alloys that surpass the high-temperature properties of Ni-based superalloys is steadily increasing as the operation temperature of high-temperature components increases. It is a refractory-based alloy having a melting point higher than 1900 °C that is promising as an alternative metallic material [1]. Especially since Cr and Cr–Al alloys are excellent in high temperature oxidation resistance, they are suitable as materials for high-temperature components or oxidation-resistant coating, and related research has been conducted [2,3,4,5,6,7,8]. For instance, some research has been carried out to apply Cr or Cr–Al powders to the surface coating of the nuclear fuel cladding by using a laser surface-melting method [8,9]. Recently, a study was conducted that focused on Cr–Al alloys containing Si for the purpose of enhancing the high-temperature hardness and strength of the Cr–Al alloys [10]. Thermal stability of Cr3Si phase and the possibility of improvement of high-temperature strength due to formation of it have already been reported; however, only a few research studies have been done on the formation of Cr3Si phase in Cr–Al–Si alloys [11,12]. In addition, although the oxidation resistance due to the formation of Cr and Al oxides in Cr–Al–Si alloys has been studied to some extent [13,14], the microstructure changes due to the addition of Si are still insufficient. For example, it has been reported that, when a certain amount of silicon is added, a Cr3Si phase is formed, but the critical Si content is related to the amount of Al added, which requires further investigation [10,15].
Various properties, such as oxidation resistance and high-temperature strength, of Cr–Al–Si alloys seem to be closely related to the chemical composition and microstructure. Thermodynamically, SiO2 is more stable than Cr2O3, but Cr oxide can be formed predominately due to a higher activity and faster diffusion in Cr phase. The addition of Si to Cr has been known to improve the oxidation resistance [1]. The formation of Al2O3 on the surface of Cr alloys through the addition of aluminum may be also desirable depending on the conditions of use of the material. Although Al and Cr are oxidized simultaneously during oxidation of Al-added Cr alloys, the replacement of Cr2O3 with Al2O3 can occur, since the free energy for Al2O3 formation is much more negative than that for Cr2O3 at high temperatures [3]. However, for most cases, a sufficient amount of Al should be added for continuous surface Al2O3, but it is obviously undesirable to form relatively low melting point AlCr phases in terms of heat resistance. Therefore, knowledge about the microstructure of Cr–Al–Si alloys is the prerequisite for the actual practical use of them. In this study, Cr–5% Si (at.%) alloy was selected as the basic composition, and the difference of the microstructure after casting and high-temperature isothermal heating, according to the amount of Al added, was investigated.
2. Materials and Methods
Cr–5% Si (at.%) with different Al contents (0, 5, 10, and 20 at.%), which are candidates for high-temperature coating materials, were vacuum-arc melted (KHAM-500, Misung S & I, Cheonan, Korea) and cast to produce 35 g of button-shaped ingots. Commercially pure Cr, Al, and Si materials (99.9 wt.%, NeoDM, Daejeon, Korea) were used for the vacuum melting. Then, 10 × 10 × 5 mm plate specimens were prepared by electric-discharge machining the ingots and used for microstructure investigation and a high-temperature oxidation test. Two cast ingots were fabricated for the same composition, and four plate specimens were prepared for each ingot. Cross sections of the plate specimens were mechanically polished by using sandpaper and diamond suspension, with a diameter of 1 μm, to a mirror surface.
In the case of the high-temperature oxidation test, a method of examining and comparing the weight change of the specimen during isothermal heating up to 1 h in an air atmosphere of 1100 °C was applied. Thermogravimetric analysis (TGA, Mettler-Toledo, Zurich, Sweden), with about 200 mg of alloy specimens, was used for the test, and the weight change was measured every 4 s, after reaching the target temperature of 1100 °C.
The alloy microstructure before and after the high-temperature heating was investigated by using a scanning electron microscope (JEOL, Tokyo, Japan) equipped with an energy-dispersive X-ray spectrometer (EDS, JEOL, Tokyo, Japan), and the oxides on the surface of the alloys after the oxidation test were analyzed by using an X-ray diffractometer (XRD, Rigaku, Tokyo, Japan). Cu Kα radiation was used for XRD. Micro-hardness of the specimen was measured by using a micro-Vickers tester (Duramin-40, Struers, Cleveland, OH, USA). The applied load condition was 0.2 N for 10 s, and the representative hardness values were determined by randomly measuring 10 times of various specimen parts and averaging them.
3. Results and Discussions
3.1. Microstructure of As-Cast Cr–Al–Si Alloys
Figure 1 shows typical SEM micrographs of as-cast Cr–xAl–5%Si alloys. According to the Cr–Si binary diagram, about 5% Si is expected to be completely dissolved in the Cr matrix, at a high temperature, above 1000 °C. However, as the temperature decreases, the solid solubility decreases, and thus, a Cr3Si phase may be formed at room temperature [15,16]. On the other hand, the solubility of Al element in Cr matrix is relatively high, and it is expected to be completely dissolved in matrix up to 10% Al, even at room temperature [16]. Virtually, single Cr-phase is observed in the case of 0% and 5% Al-added alloys. Due to the small size of the cast specimens produced by vacuum-arc melting, the solidification rate is comparatively high, and the alloying elements are considered to be slightly supersaturated. In the case of alloys containing 10% or 20% Al, dark phases were formed on the Cr base, showing a different microstructure from that of the low Al alloy.
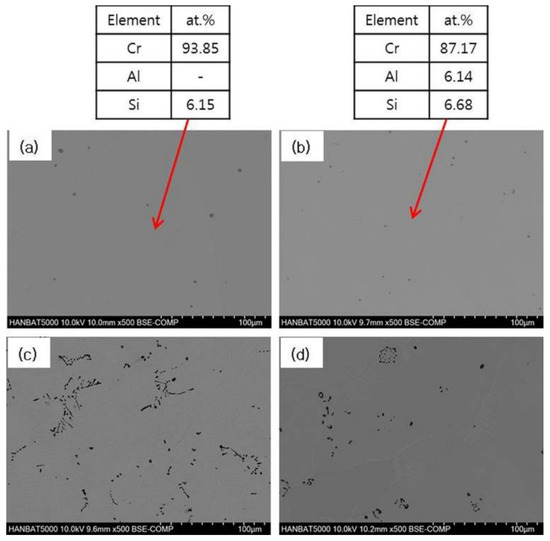
Figure 1.
SEM-EDS analyses of as-cast Cr–xAl–5%Si alloys (500X): (a) 0% Al; (b) 5% Al; (c) 10% Al; and (d) 20% Al.
The microstructure of Cr–Al–Si alloys containing 10% or 20% of Al was observed and analyzed at higher magnification, as shown in Figure 2. Considering the literature and the EDS analysis results, the dark phase is assumed to be Al8Cr5 or Al9Cr4 [17]. Al9Cr4 phase is sometimes treated as an extension of the Al8Cr5 phase [15,18]. The maximum amount of Si dissolved in Al8Cr5 phase was known to be 5.6%, but an amount smaller than that, about 2.3%, was measured in this study [18]. Meanwhile, it was observed that a large amount of CrSi phase was formed, in addition to some AlCr phase in the alloy containing 20% Al, and the CrSi phase is considered to be Cr3Si, based on the literature and EDS analysis results [16]. It is interesting to note that, although Al content is further increased, the amount of AlCr phase is not increased and instead a large amount of CrSi phase is formed. This phenomenon is presumed to have occurred because the element of Si dissolved in the Cr matrix was released from the base due to the precipitation of the CrSi phase when the Al content was increased above a certain amount.
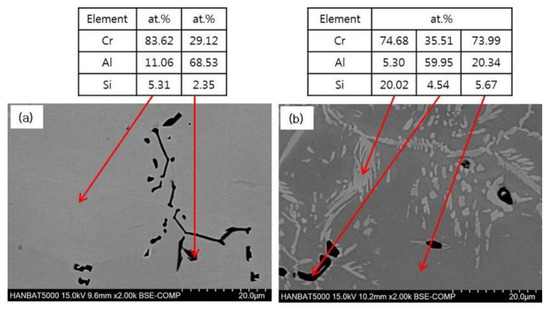
Figure 2.
SEM-EDS analyses of as-cast Cr–xAl–5%Si alloys (2000X): (a) 10%Al; (b) 20%Al.
Figure 3 indicates that the micro-hardness of Cr–Al–Si alloys increases in proportion to the aluminum content. Since aluminum and silicon added as alloying elements are completely dissolved in the Cr–xAl–5%Si alloys’ matrix up to 5% Al, solid solution hardening seems to be the main reason for the increase in hardness of the 5% Al-added alloy. In the case of 10% Al-added alloy, the increase in hardness due to the formation of the AlCr phase may be added. A more significant increase in hardness was observed at 20% Al, which seems to be associated with the formation of Cr3Si phase. Cr3Si is a very hard phase, and the hardness of Cr3Si phase on VHN basis has been reported to be more than eight times that of Cr phase [19]. Since the Cr3Si is stable at high temperatures [11,12], formation of the Cr3Si phase is expected to improve not only the wear resistance but also the strength of the Cr–Al–Si alloys at high temperatures.
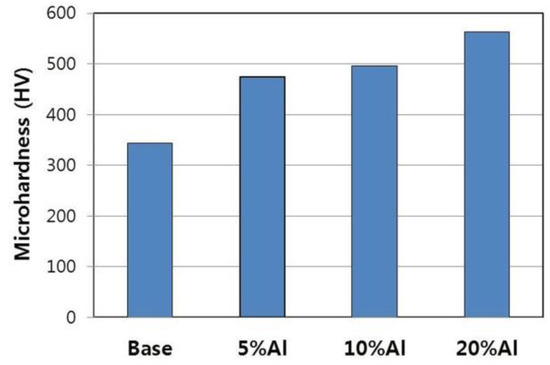
Figure 3.
Micro-hardness of as-cast Cr–xAl–5%Si alloys.
3.2. Microstructural Change after the High-Temperature Oxidation Test
The microstructure of Cr–xAl–5%Si alloys with 5% and 10% Al after 1 h in air at 1100 °C is shown in Figure 4. The isothermally heated microstructure of 0% Al-added alloy is composed of virtually single Cr-phase structure, as in the cast state. In the case of 5% Al-added alloy, a few AlCr phases were formed after high-temperature heating, unlike the casting state, and it is presumed that this phase was mainly formed during the cooling process. In the case of 10% Al-added alloy, more AlCr phases were formed than in 5% Al-added alloy, and any CrSi phases were not observed in both alloys.
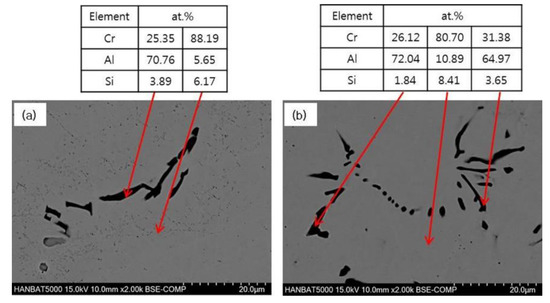
Figure 4.
SEM-EDS analyses of isothermally heated Cr–xAl–5%Si alloys (2000X): (a) 5% Al, (b) 10% Al.
Figure 5 shows the microstructure after heating 20% Al-added alloy at 1100 °C, and it can be seen that a large amount of Cr3Si phase, which is seen as a bright color, was formed. Occasionally, Al-rich phases were observed in some regions, but the Al8Cr5 (or Al9Cr4) phases, which were observed in the cast microstructure, could no longer be found. Due to solidification segregation, there can be regions with high Al concentration in the cast specimen, where small liquid pools may be formed during the high-temperature heating. It is estimated that a few Al-rich phases were formed during the solidification of the liquid parts.
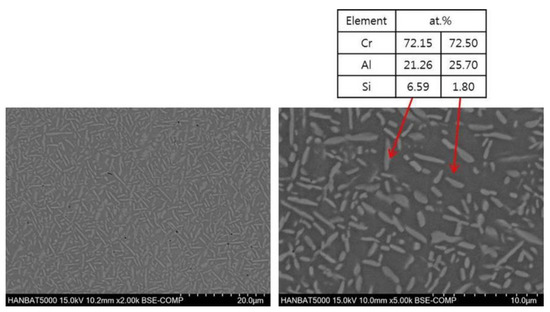
Figure 5.
SEM–EDS analyses of isothermally heated Cr-20%Al-5%Si alloys (2000X).
3.3. Oxidation Resistance of Cr–Al–Si Alloys
Even after 1 h heating at 1100 °C in air, the surface of the specimens was relatively good, as shown in Figure 6, and the excellent oxidation resistance of these alloys was confirmed. However, a slight spalling occurred in some parts of the specimen, and this may affect the weight of the specimens a little. This spalling is thought to occur mainly during cooling because of the large difference in coefficient of thermal expansion between surface oxide and alloy substrate.
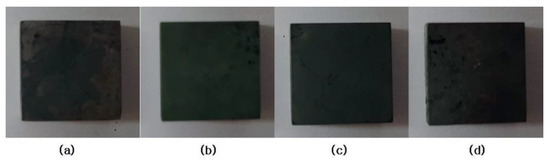
Figure 6.
Appearance of Cr–xAl–5%Si alloy surfaces after the isothermal heating: (a) 0% Al; (b) 5% Al; (c) 10% Al; and (d) 20% Al.
As indicated in Figure 7, when the oxidation resistance was evaluated based on the weight change, the increase of Al addition did not increase the oxidation resistance of the Cr–Al–Si alloys, but rather decreased slightly. The weight increase of 5% Al alloy was slightly lower than that of base alloy (0% Al), and those of other high Al alloys was apparently larger. When spalling occurs in the oxidation process, it is not accurate to evaluate oxidation resistance only by weight increase. On the other hand, the reason why the weight gain is higher in the high-Al alloys is believed to be partly due to the formation of alumina in addition to Cr oxide.
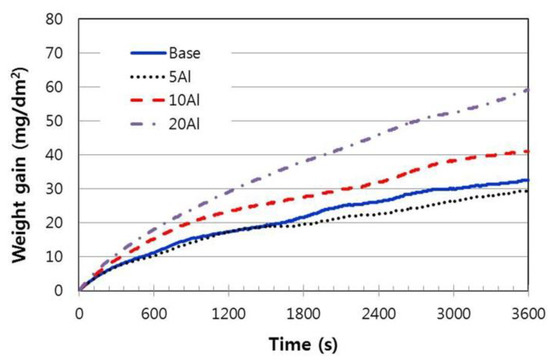
Figure 7.
Oxidation behavior of Cr–xAl–5%Si alloys during the isothermal heating at 1100 °C.
The largest difference between the surface oxides of high- and low-Al alloys is the presence or absence of alumina, as shown in Figure 8. In alloys with less than 10% Al, most of the oxides were Cr2O3, while in more than 10% Al-added alloys, additional Al2O3 was observed. In particular, a significant amount of Al2O3 was formed on the surface of the 20% Al-added alloy. Both Cr2O3 and Al2O3 are generally stable at high temperatures, but Al is known to have more oxidation tendency and to be more stable. Thus, if the Al content and the heating time are sufficient, Al2O3 may form a continuous external layer over most of the specimen’s surface [8,20]. Under the oxidation condition of the current study, 20% Al of the experiment alloy was considered to be insufficient to give a complete external scale of Al2O3. However, if exposed to a high temperature for a long time, it is expected that the corrosion resistance of the high-Al alloy can be superior to those of low-Al alloys.
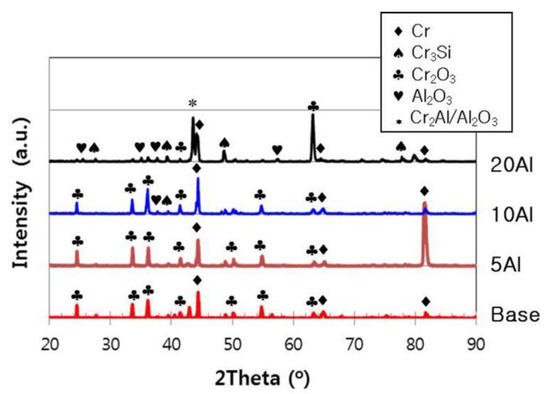
Figure 8.
XRD analysis results for the surfaces of Cr–xAl–5%Si alloys after the isothermal heating.
4. Conclusions
It was observed that the as-cast microstructure of Cr–xAl–5%Si alloys was mainly composed of Cr single phase, up to 5% Al, and Al8Cr5 phase was additionally formed in the case of 10% or more of Al. When 20% Al was added, Cr3Si phase were also found. Meanwhile, the weight change of the specimens heated for 1 h at 1100 °C in air showed that all experiment alloys had excellent oxidation resistance. Although the microstructure change of the most alloys after the isothermal heating was not remarkable, it is noteworthy that Al8Cr5 phase disappeared and a large amount of Cr3Si phase was formed in the 20% Al-added alloy.
The results of XRD analysis showed that the Al2O3 oxide film was formed with Cr2O3 in the high Al alloys, whereas only the Cr2O3 oxide film was mainly formed on the surfaces of the lower Al containing alloy specimens. It is expected that high Al containing Cr–Al–Si alloys, such as 20% Al, can be made into powders and utilized as high-oxidation and wear-resistant coating materials for thermal spraying or laser coating.
Author Contributions
Conceptualization, J.-M.K.; methodology, J.-M.K.; investigation, J.-M.K. and C.-Y.K.; writing—original draft preparation, J.-M.K.; writing—review and editing, J.-M.K.; funding acquisition, J.-M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the research fund of Hanbat National University, Korea, in 2019.
Acknowledgments
The authors wish to thank W. Yang for help in vacuum arc melting.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ulrich, A.S.; Pfizenmaier, P.; Solimani, A.; Glatzel, U.; Galetz, M.C. Improving the oxidation resistance of Cr-Si-based alloys by ternary alloying. Corros. Sci. 2020, 165, 108376. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, P.; Xia, H.; Yang, Z.; Konovalov, S.; Chen, X.; Guan, Q. The microstructure and properties of nanostructured Cr-Al alloying layer fabricated by high-current pulsed electron beam. Vacuum 2019, 167, 263–270. [Google Scholar] [CrossRef]
- Chen, C.; Feng, X.; Shen, Y. Effects of annealing treatment and pre-refinement of raw material on microstructures and properties of mechanically alloyed Cr-Al composite coatings on Ti-6Al-4V alloy. Mater. Charact. 2016, 120, 97–108. [Google Scholar] [CrossRef]
- Fukumoto, M.; Matsumura, Y.; Hayashi, S.; Narita, T.; Sakamoto, K.; Kasama, A.; Tanaka, R. Coatings of Nb-based alloy by Cr and/or Al pack cementations and its oxidation behavior in air at 1273-1473 K. Mater. Trans. 2003, 44, 731–735. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Duan, C.; Feng, X.; Shen, Y. Investigation of Cr-Al composite coatings fabricated on pure Ti substrate via mechanical alloying method: Effects of Cr-Al ratio and milling time on coating, and oxidation behavior of coating. J. Alloy. Compd. 2016, 660, 208–219. [Google Scholar] [CrossRef]
- Zhong, W.; Mouche, P.A.; Heuser, B.J. Response of Cr and Cr-Al coatings on Zircaloy-2 to high temperature steam. J. Nucl. Mater. 2018, 498, 137–148. [Google Scholar] [CrossRef]
- Kuprin, A.S.; Belous, V.A.; Voyevodin, V.N.; Bryk, V.V.; Vasilenko, R.L.; Ovcharenko, V.D.; Reshetnyak, E.N.; Tolmachova, G.N.; V’yugov, P.N. Vacuum-arc chromium-based coatings for protection of zirconium alloys from the high-temperature oxidation in air. J. Nucl. Mater. 2015, 465, 400–406. [Google Scholar] [CrossRef]
- Kim, J.M.; Ha, T.H.; Kim, I.H.; Kim, H.G. Microstructure and oxidation behavior of Cr-Al laser-coated Zircaloy-4 alloy. Metals 2017, 7, 59. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, I.H.; Jung, Y.I.; Park, D.J.; Park, J.Y.; Koo, Y.H. Adhesion property and high-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, I.H.; Kim, H.G. Effects of Si addition on the microstructure and properties of Cr-Al alloy for high temperature coating. Korean J. Mater. Res. 2019, 29, 7–10. [Google Scholar] [CrossRef]
- Gali, A.; Bei, H.; George, E.P. Thermal stability of Cr-Cr3Si eutectic microstructures. Acta Metall. 2009, 57, 3823–3829. [Google Scholar] [CrossRef]
- Ulrich, A.S.; Pfizenmaier, P.; Solimani, A.; Glatzel, U.; Galetz, M.C. Strengthened Cr-Si-base alloys for high temperature applications. Int. J. Refrect. Met. Hard Mater. 2018, 76, 72–81. [Google Scholar] [CrossRef]
- Dong, Y.; Ge, F.; Meng, F.; Zhao, G.; Zhou, J.; Deng, Z.; Huang, Q.; Huang, F. Improved oxidation resistance of zirconium at high-temperature steam by magnetron sputtered Cr-Al-Si ternary coatings. Surf. Coat. Technol. 2018, 350, 841–847. [Google Scholar] [CrossRef]
- Yang, R.; Wu, Q.; Li, S.; Gong, S. Effects of Cr-Al-Si and Cr-Al coatings on the high temperature oxidation resistance of a Ni3Al-Mo based single crystal alloy. Procedia Eng. 2012, 27, 976–982. [Google Scholar] [CrossRef]
- Cui, S.; Jung, I.H. Thermodynamic assessments of the Cr-Si and Al-Cr-Si systems. J. Alloy. Compd. 2017, 708, 887–902. [Google Scholar] [CrossRef]
- Solimani, A.; Galetz, M.C. Oxidation and nitridation behavior of Cr-Si alloys in air at 1473K. Oxid. Met. 2015, 84, 73–90. [Google Scholar] [CrossRef]
- Cui, S.; Jung, I.H.; Kim, J.; Xin, J. A coupled experimental and thermodynamic study of the Al-Cr and Al-Cr-Mg systems. J. Alloy. Compd. 2017, 698, 1038–1057. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, C.; Li, C.; Du, Z. A thermodynamic description of the Al-Cr-Si system. JPEDAV 2009, 30, 462–479. [Google Scholar] [CrossRef]
- Newkirk, J.W.; Hawk, J.A. Abrasive wear properties of Cr-Cr3Si composites. Wear 2001, 251, 1361–1371. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High-temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; pp. 115–122. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).