Electrical Resistivity of Ni–Fe Wires Coated with Sn Using low-Pressure Chemical Vapor Deposition
Abstract
1. Introduction
2. Materials and Methods
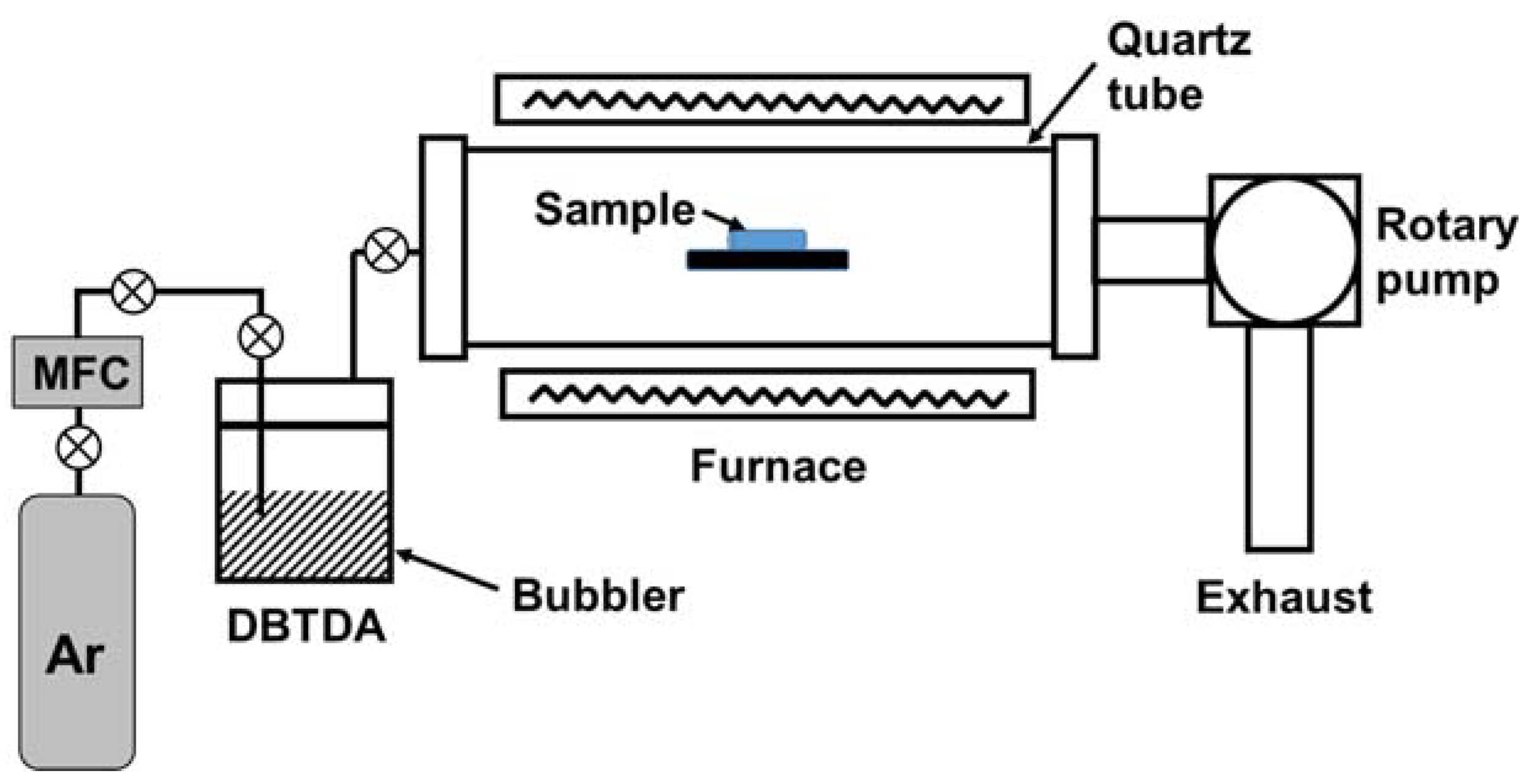
2.1. Deposition Method
2.2. Materials Characterization
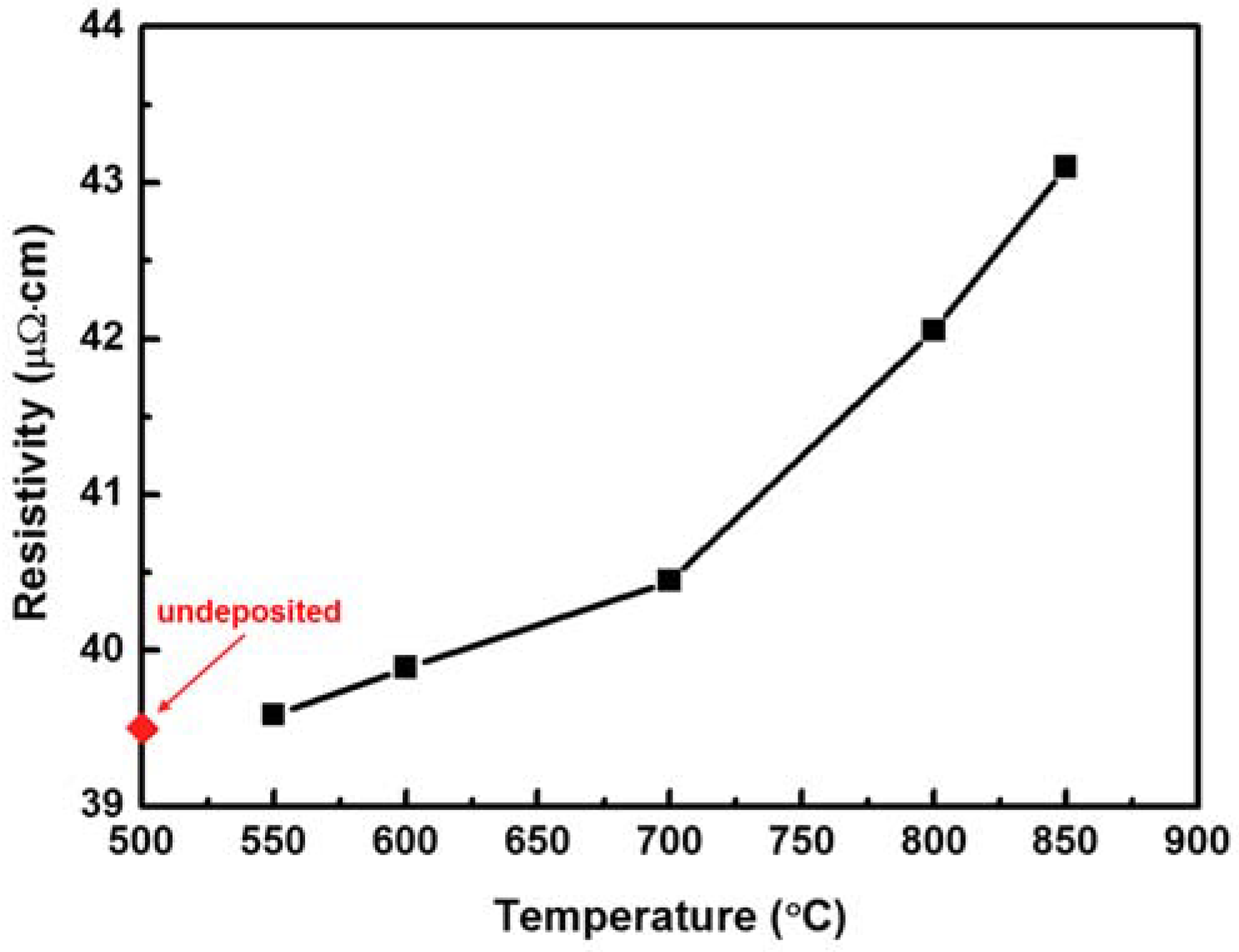
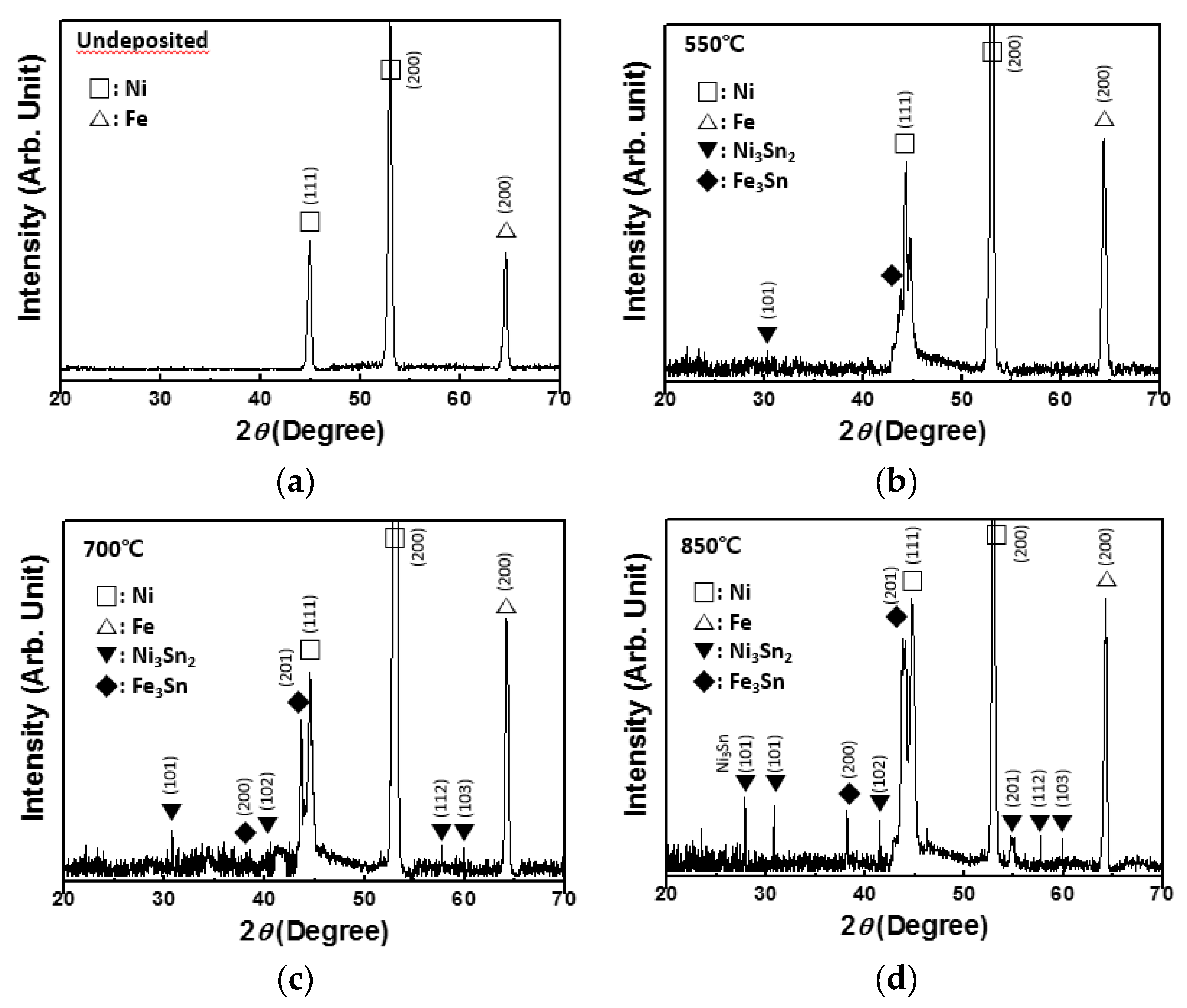
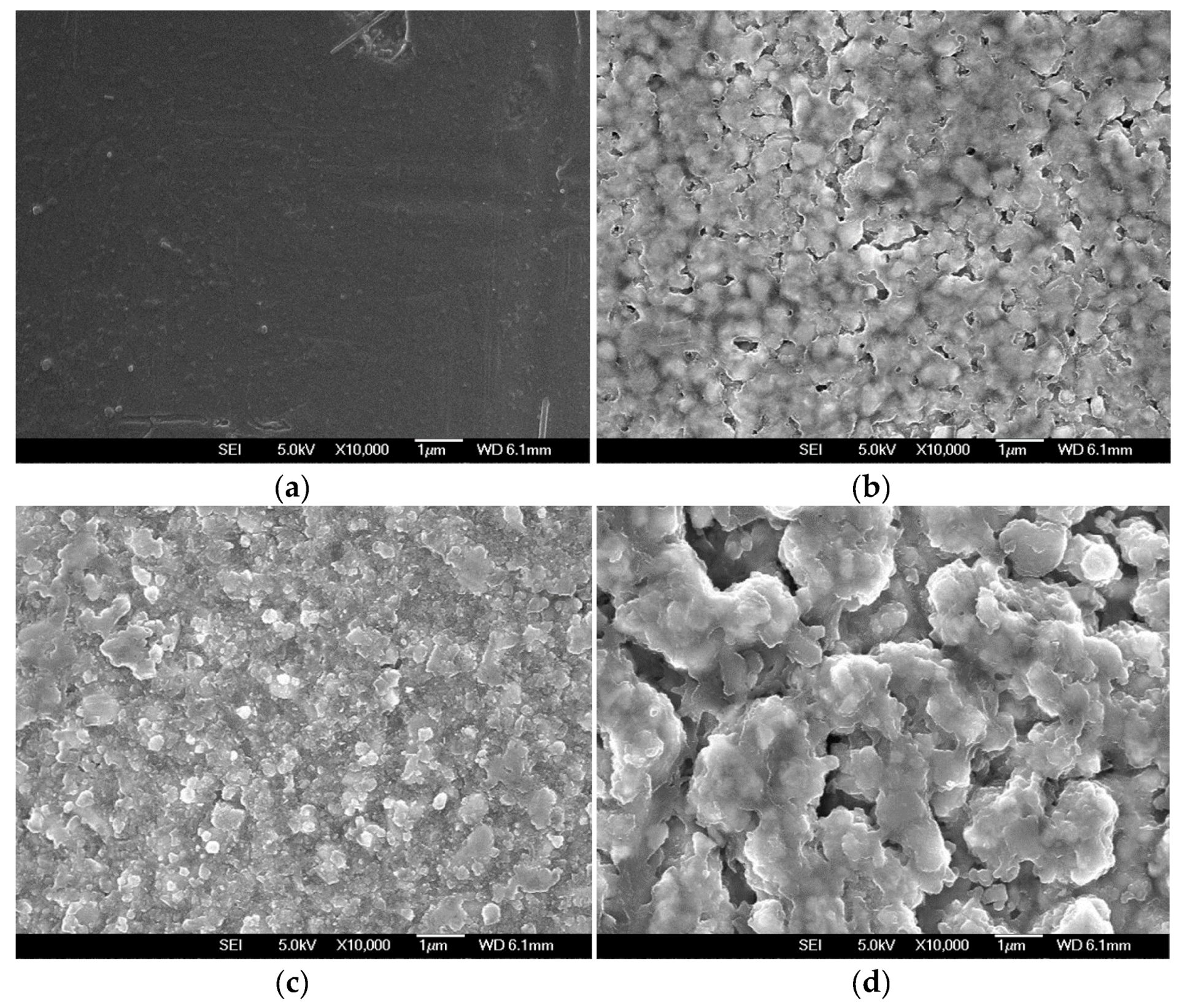
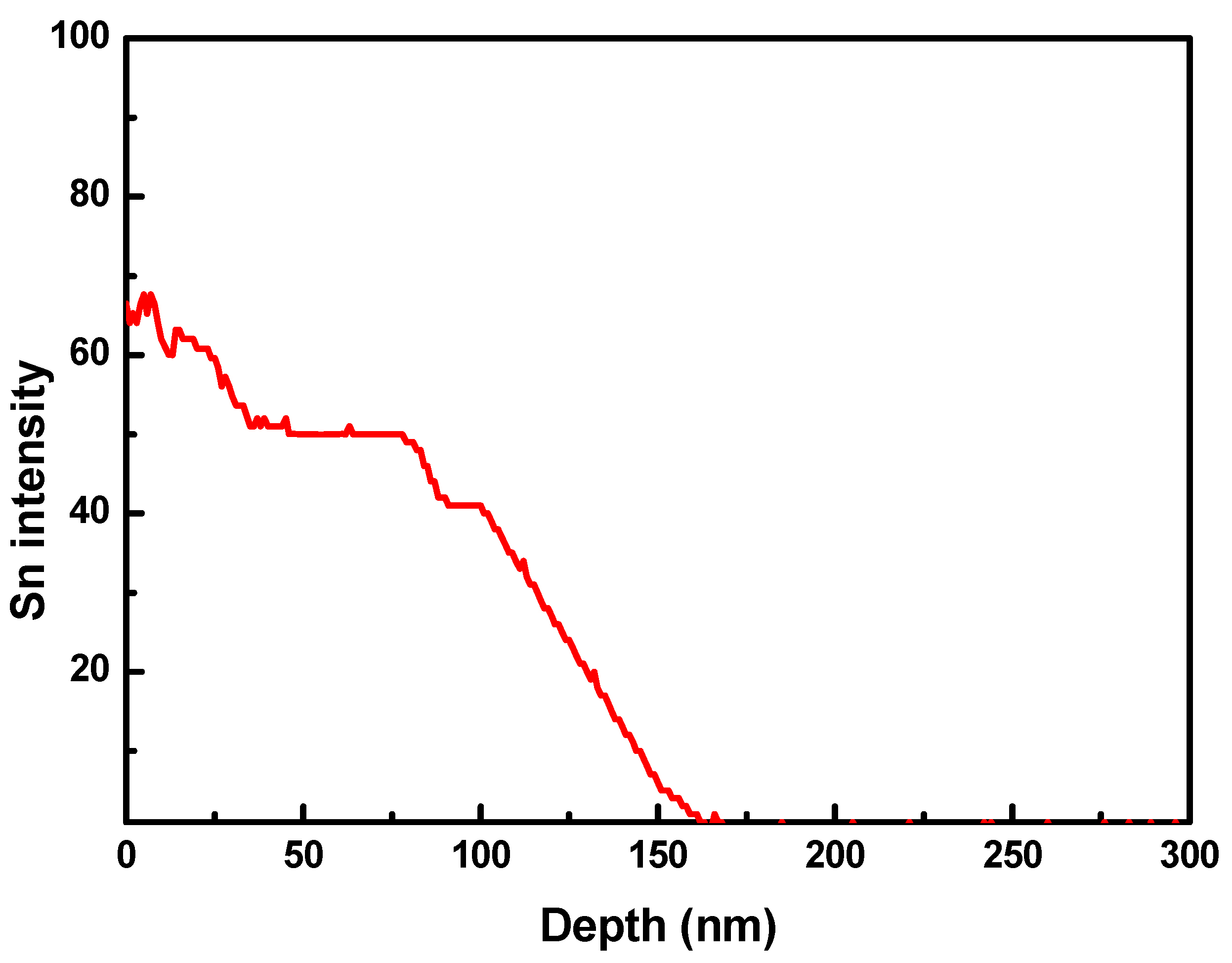
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, S.; Xu, Z.; Chen, S.; Xu, Y.; Wu, G. The design principle and application of power resistor with over temperature and over current protection function. Adv. Mat. Res. 2012, 562, 1501–1504. [Google Scholar] [CrossRef]
- Schroeder, K. Fail-safe wirewound resistors for robust applications. Power Electron. Technol. 2010, 36, 41–43. [Google Scholar]
- Kurosawa, H.; Tomimuro, H. Hybrid integrated surge protector for digital switching system. IEEE Trans. Compon. HybridsManuf. Technol. 1985, 8, 259–263. [Google Scholar] [CrossRef]
- Yao, Y.D.; Chen, Y.Y.; Tzeng, S.J.; Chuang, T.H. Electrical resistivity, magnetization, and grain boundary precipitates in Ni-Sn alloys. Phys. Status Solidi A Appl. Mat. 1990, 121, 213–218. [Google Scholar] [CrossRef]
- Geny, J.F.; Marchal, G.; Mangin, P.; Janot, C.H.R.; Piecuch, M. Structure, forming ability, and electrical-transport properties of isotypical amorphous alloys MxSn1−x (M = Fe, Co, Ni). Phys. Rev. B 1982, 25, 7449–7466. [Google Scholar] [CrossRef]
- Bailey, W.; Kabos, P.; Mancoff, F.; Russek, S. Control of magnetization dynamics in Ni81Fe19 thin films through the use of rare-earth dopants. IEEE Trans. Magn. 2001, 37, 1749–1754. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, S.W.; Kang, D.W.; Lee, K.M.; Baek, C.Y.; Lee, H.M.; Kim, C.K. Electrical, structural, and morphological characteristics of dopantless tin oxide films prepared by low pressure chemical vapor deposition. Sci. Adv. Mater. 2016, 8, 117–121. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.M.; Kang, D.W.; Lee, K.M.; Kim, C.K. Effect of oxygen flow rate on the electrical and optical characteristics of dopantless tin oxide films fabricated by LPCVD. Korean J. Chem. Eng. 2016, 33, 2711–2715. [Google Scholar] [CrossRef]
- Vernardou, D.; Psifis, K.; Louloudakis, D.; Papadimitropoulos, G.; Davazoglou, D.; Katsarakis, N.; Koudoumas, E. Low Pressure CVD of Electrochromic WO3 at 400 °C. J. Electrochem. Soc. 2015, 162, H579–H582. [Google Scholar] [CrossRef]
- Kim, H.T.; Lee, S.Y.; Lee, H.R.; Park, C.H. Effects of growth temperature on titanium carbide (TiC) film formation using low-frequency (60 Hz) plasma-enhanced chemical vapor deposition. Korean J. Chem. Eng. 2018, 35, 246–250. [Google Scholar] [CrossRef]
- Li, J.; Qin, H.; Liu, Y.; Ye, F.; Li, Z.; Cheng, L.; Zhang, L. Effect of the SiCl4 flow rate on SiBN deposition kinetics in SiCl4-BCl3-NH3-H2-Ar environment. Materials 2017, 10, 627. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, M.; Liu, Y.; Chai, N.; Ye, F.; Qin, H.; Cheng, L.; Zhang, L. Microstructure and dielectric properties of LPCVD/CVI-SiBCN ceramics annealed at different temperatures. Materials 2017, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, G.; Liu, B.; Yan, G.; Guan, M.; Zhang, Y.; Zhang, F.; Chen, Y.; Dong, L.; Zheng, L.; et al. Growth of hexagonal columnar nanograin structured SiC thin films on silicon substrates with graphene-graphitic carbon nanoflakes templates from solid carbon sources. Materials 2013, 6, 1543–1553. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Xin, H.; Zhang, L.; Yang, J.; Jiang, G. Controllable preparation of SiC coating protecting carbon fiber from oxidation damage during sintering process and SiC coated carbon fiber reinforced hydroxyapatite composites. Appl. Surf. Sci. 2018, 450, 265–273. [Google Scholar] [CrossRef]
- Yang, M.; Sasaki, S.; Suzuki, K.; Miura, H. Control of the nucleation and quality of graphene grown by low-pressure chemical vapor deposition with acetylene. Appl. Surf. Sci. 2016, 366, 219–226. [Google Scholar] [CrossRef]
- Cheng, C.; Zheng, F.; Zhang, C.; Du, C.; Fang, Z.; Zhang, Z.; Chen, W. High-efficiency bifunctional electrocatalyst based on 3D freestanding Cu foam in situ armored CoNi alloy nanosheet arrays for overall water splitting. J. Power Sources 2019, 427, 184–193. [Google Scholar] [CrossRef]
- Ni, W.; Wang, B.; Cheng, J.; Li, X.; Guan, Q.; Guab, G.; Huang, L. Hierarchical foam of exposed ultrathin nickel nanosheets supported on chainlike Ni-nanowires and the derivative chalcogenide for enhanced pseudocapacitance. Nanoscale 2014, 6, 2618–2623. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Jia, D. Facile solid-state synthesis of Fe/FeOOH hierarchical nanostructures assembled from ultrathin nanosheets and their application in water treatment. Cryst. Eng. Comm. 2016, 18, 8465–8471. [Google Scholar] [CrossRef]
- Astuti, M.D.; Hara, T.; Ichikuni, N.; Shimazu, S. One-pot selective conversion of C5-furan into 1,4-pentanediol over bulk Ni-Sn alloy catalysts in an ethanol/H2O solvent mixture. Green Chem. 2019, 21, 2307–2315. [Google Scholar]
- Kim, D.; Lee, D.; Kim, J.; Moon, J. Electrospun Ni-added SnO2–carbon nanofiber composite anode for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 5408–5415. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.M.; Abdelkareem, M.A.; Abdelghani, E.A.M. Influence of Sn content, nanostructural morphology, and synthesis temperature on the electrochemical active area of Ni-Sn/C nanocomposite: Verification of methanol and urea electrooxidation. Catalysts 2019, 9, 330. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, A.; Zhang, X.; Yin, H.; Wang, S.; Tang, Y.; Zhou, Y.; Wu, P. Pyrolysis of cyano-bridged hetero-metallic aerogels: A general route to immobilize Sn–M (M = Fe, Ni) alloys within a carbon matrix for stable and fast lithium storage. Nanoscale 2018, 10, 4962–4968. [Google Scholar] [CrossRef] [PubMed]
- Sales, B.C.; Saparov, B.; McGuire, M.A.; Singh, D.J.; Parker, D.S. Ferromagnetism of Fe3Sn and alloys. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vitina, I.; Pelece, I.; Rubene, V.; Belmane, V.; Lubane, M.; Krumina, A.; Zarina, Z. Formation of intermediate intermetallic layers on interaction of electrodeposited Sn, Ni-Fe, Ni-B coatings with different metallic substrate. J. Adhes. Sci. Technol. 1997, 11, 835–860. [Google Scholar] [CrossRef]
- Choe, G.; Steinback, M. Surface roughness effects on magnetoresistive and magnetic properties of NiFe thin films. J. Appl. Phys. 1999, 85, 5777–5779. [Google Scholar] [CrossRef]
- Tang, W.; Xu, K.; Wang, P.; Li, X. Surface roughness and resistivity of Au films on Si-(111) substrate. Microelectron. Eng. 2003, 66, 445–450. [Google Scholar] [CrossRef]
- Onda, A.; Komatsu, T.; Yashima, T. Preparation and catalytic properties of single-phase Ni–Sn intermetallic compound particles by CVD of Sn(CH3)4 onto Ni/Silica. J. Catal. 2001, 201, 13–21. [Google Scholar] [CrossRef]
- Trumpy, G.; Both, E.; Djega-Mariadassou, C.; Lecocq, P. Mossbauer-effect studies of iron-tin alloys. Phys. Rev. B 1970, 2, 3477–3490. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Bak, J.G.; Kim, C.-K. Electrical Resistivity of Ni–Fe Wires Coated with Sn Using low-Pressure Chemical Vapor Deposition. Coatings 2020, 10, 317. https://doi.org/10.3390/coatings10040317
Kim J-H, Bak JG, Kim C-K. Electrical Resistivity of Ni–Fe Wires Coated with Sn Using low-Pressure Chemical Vapor Deposition. Coatings. 2020; 10(4):317. https://doi.org/10.3390/coatings10040317
Chicago/Turabian StyleKim, Jun-Hyun, Jeong Geun Bak, and Chang-Koo Kim. 2020. "Electrical Resistivity of Ni–Fe Wires Coated with Sn Using low-Pressure Chemical Vapor Deposition" Coatings 10, no. 4: 317. https://doi.org/10.3390/coatings10040317
APA StyleKim, J.-H., Bak, J. G., & Kim, C.-K. (2020). Electrical Resistivity of Ni–Fe Wires Coated with Sn Using low-Pressure Chemical Vapor Deposition. Coatings, 10(4), 317. https://doi.org/10.3390/coatings10040317





