One-Step Synthesis of Graphene, Copper and Zinc Oxide Graphene Hybrids via Arc Discharge: Experiments and Modeling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
2.3. Methods
3. Results
4. Modeling
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Iakunkov, A.; Skrypnychuk, V.; Nordenström, A.; Shilayev, E.A.; Korobov, M.; Prodana, M.; Enachescu, M.; Larsson, S.H.; Talyzin, A.V. Activated graphene as a material for supercapacitor electrodes: Effects of surface area, pore size distribution and hydrophilicity. Phys. Chem. Chem. Phys. 2019, 21, 17901–17912. [Google Scholar] [CrossRef] [PubMed]
- Banszerus, L.; Sohier, T.; Epping, A.; Winkler, F.; Libisch, F.; Haupt, F.; Watanabe, K.; Taniguchi, T.; Müller-Caspary, K.; Marzari, N.; et al. Extraordinary high room-temperature carrier mobility in graphene-WSe2 heterostructures. arXiv 2019, arXiv:1909.09523. [Google Scholar]
- Kim, S.H.; Song, W.; Jung, M.W.; Kang, M.-A.; Kim, K.; Chang, S.-J.; Lee, S.S.; Lim, J.; Hwang, J.; Myung, S.; et al. Carbon Nanotube and graphene hybrid thin film for transparent electrodes and field effect transistors. Adv. Mater. 2014, 26, 4247–4252. [Google Scholar] [CrossRef]
- Badhulika, S.; Thakoor, T.T.; Villarreal, C.; Mulchandani, A. Graphene hybrids: Synthesis strategies and applications in sensors and sensitized solar cells. Front. Chem. 2015, 3, 38. [Google Scholar] [CrossRef]
- Hidalgo-Manrique, P.; Lei, X.; Xu, R.; Zhou, M.; Kinloch, I.A.; Young, R.J. Copper/graphene composites: A review. J. Mater. Sci. 2019, 54, 12236–12289. [Google Scholar] [CrossRef]
- Xiao, Q.; Yi, X.; Jiang, B.; Qin, Z.; Hu, J.; Jiang, Y.; Liu, H.; Wang, B.; Yi, D. In-situ synthesis of graphene on surface of copper powder by rotary CVD and its application in fabrication of reinforced Cu-matrix composites. Adv. Mater. Sci. 2017, 2, 5–6. [Google Scholar] [CrossRef]
- Cseri, L.; Baugh, J.; Alabi, A.; AlHajaj, A.; Zou, L.; Dryfe, R.A.W.; Budd, P.M.; Szekely, G. Graphene oxide-polybenzimidazolium nanocomposite anion exchange membranes for electrodialysis. J. Mater. Chem. A 2018, 6, 24728. [Google Scholar] [CrossRef]
- Kweon, H.; Lin, C.W.; Hasan, M.M.F.; Kaner, R.; Sant, G.N. Highly permeable polyaniline-graphene oxide nanocomposite membranes for CO2 separations. ACS Appl. Polym. Mater. 2019, 1, 12. [Google Scholar] [CrossRef]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust covalently crosslinked polybenzimidazole/graphene oxide membranes for high-flux organic solvent nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Vallés, C.; Zhang, X.; Cao, J.; Lin, F.; Young, R.J.; Lombardo, A.; Ferrari, A.C.; Burk, L.; Rolf Mülhaupt, R.; Kinloch, I.A. Graphene/polyelectrolyte layer-by-layer coatings for electromagnetic interference shielding. ACS Appl. Nano Mater. 2019, 2, 5272–5281. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Yun, X.; Zhou, W.; Xi, L.; Li, N.; Hu, Z. Hydrothermal synthesis of nanoflakes-assembled (Ni0.5Co0.5)0.85Se microspheres as cathode and reduced graphene oxide/porous Fe2O3 nanospheres composite as anode for novel alkaline aqueous batteries. ACS Sustain. Chem. Eng. 2020, 8, 561–572. [Google Scholar] [CrossRef]
- Kong, W.; Liu, F.; Liu, Y. Design of nitrogen-doped graphitized 2D hierarchical porous carbons as efficient solid base catalysts for transesterification to biodiesel. Green Chem. 2020, 22, 903–912. [Google Scholar] [CrossRef]
- Prasad, K.P.; Chen, Y.; Chen, P. Three-dimensional graphene-carbon nanotube hybrid for high-performance enzymatic biofuel cells. ACS Appl. Mater. Interfaces 2014, 6, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, X.; Wang, L.; Song, H.; Zhang, H.; Huang, W.; Chen, P. 3D Graphene foam as a monolithic and macroporous carbon electrode for electrochemical sensing. ACS Appl. Mater. Interfaces 2012, 4, 3129–3133. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Subramani, B. Synthesis of zinc oxide nanoparticles on graphene-carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef]
- Li, Y.F.; Dong, F.X.; Chen, Y.; Zhang, X.L.; Wang, L.; Bi, Y.G.; Tian, Z.N.; Liu, Y.F.; Feng, J.; Sun, H.B. As-grown graphene/copper nanoparticles hybrid nanostructures for enhanced intensity and stability of surface plasmon resonance. Sci. Rep. 2016, 6, 37190. [Google Scholar] [CrossRef]
- Cho, B.; Yoonb, J.; Hahm, M.G.; Kim, D.H.; Kim, A.R.; Kahng, Y.H.; Park, S.W.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; et al. Graphene-based gas sensor: Metal decoration effect and application to a flexible device. J. Mater. Chem. C2 2014, 2, 5280–5285. [Google Scholar] [CrossRef]
- Echtermeyer, T.J.; Britnell, L.; Jasnos, P.K.; Lombardo, A.; Gorbachev, R.V.; Grigorenko, A.N.; Geim, A.K.; Ferrari, A.C.; Novoselov, K.S. Strong plasmonic enhancement of photovoltage in graphene. Nat. Commun. 2011, 2, 458. [Google Scholar] [CrossRef]
- Emani, N.K.; Chung, T.F.; Ni, X.; Kildishev, A.V.; Chen, Y.P.; Boltasseva, A. Electrically tunable damping of plasmonic resonances with graphene. Nano Lett. 2012, 12, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.; Steiner, M.; Lombardo, A.; Ferrari, A.C.; Löhneysen, H.V.; Avouris, P.; Krupke, R. Light-matter interaction in a microcavity-controlled graphene transistor. Nat. Commun. 2012, 3, 906. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Choy, W.C.H.; Ren, X.G.; Zhang, D.; Lu, H.F. Highly intensity surface enhanced raman scattering by usinig monolayer graphene as the nanospacer of metal film-metal nanoparticle coupling system. Adv. Funct. Mater. 2014, 24, 3114–3122. [Google Scholar] [CrossRef]
- Reckinger, N.; Vlad, A.; Melinte, S.; Colomer, J.F.; Sarrazin, M. Graphene-coated holey metal films: Tunable molecular sensing by surface plasmon resonance. Appl. Phys. Lett. 2013, 102, 211108. [Google Scholar] [CrossRef]
- Xu, M.; Feng, J.; Liu, Y.S.; Jin, Y.; Wang, H.Y.; Sun, H.B. Effective and tunable light trapping in bulk heterojunction organic solar cells by employing Au-Ag alloy nanoparticles. Appl. Phys. Lett. 2014, 105, 153303. [Google Scholar] [CrossRef]
- Leem, J.; Wang, M.C.; Kang, P.; Nam, S. Mechanically self-assembled, three-dimensional graphene-gold hybrid nanostructures for advanced nanoplasmonic sensors. Nano Lett. 2015, 15, 7684–7690. [Google Scholar] [CrossRef]
- Xie, H.; Lee, H.Y.; Youn, W.; Choi, M. Nanofluids containing multiwalled carbon nanotubes and their enhanced thermal conductivities. J. Appl. Phys. 2003, 94, 4967–4971. [Google Scholar] [CrossRef]
- Green, M.; Chen, X. Recent progress of nanomaterials for microwave absorption. JMAT 2019, 5, 503–541. [Google Scholar] [CrossRef]
- Hosni, M.; Kusumawati, Y.; Farhat, S.; Jouini, N.; Pauporté, T. Effects of oxide nanoparticle size and shape on electronic structure, charge transport, and recombination in dye-sensitized solar cell photoelectrodes. J. Phys. Chem. C 2013, 118, 16791–16798. [Google Scholar] [CrossRef]
- Liu, J.W.; Wu, J.; Ahmad, M.Z.; Wlodarski, W. Hybrid aligned zinc oxide nanowires array on CVD graphene for hydrogen sensing. In Proceedings of the 2013 Transducers & Eurosensors XXVII: 17th International Conference on Solid State Sensors, Actuators and Microsystems (Transducers & Eurosensors XXVII), Barcelona, Spain, 16–20 June 2013; pp. 194–197. [Google Scholar]
- Yi, J.; Lee, J.M.; Park, W. Vertically aligned ZnO nanorods and graphene hybrid architectures for high-sensitive flexible gas sensors. Sens. Actuators B 2011, 155, 264–269. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.H.; Chung, J.S.; Shin, E.W.; Yoo, D.H.; Hahn, S.H.; Huh, J.S.; Rue, G.H.; Kim, E.J.; Hur, S.H.; et al. Solution-processed ZnO-chemically converted graphene gas sensor. Mater. Lett. 2010, 64, 2479–2482. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Fabrication of WO3 nanorods on graphene nanosheets for improved visible light-induced photocapacitive and photocatalytic performance. RSC Adv. 2016, 6, 20824–20833. [Google Scholar] [CrossRef]
- Mohammada, A.; Khan, M.E.; Karima, M.R.; Choa, M.H. Synergistically effective and highly visible light responsive SnO2-g-C3N4 nanostructures for improved photocatalytic and photoelectrochemical performance. Appl. Surf. Sci. 2019, 495, 143432. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, J.R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Defected graphene nano-platelets for enhanced hydrophilic nature and visible light-induced photoelectrochemical performances. J. Phys. Chem. Solids 2017, 104, 233–242. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Kim, E.; An, H.; Jang, H.; Cho, W.J.; Lee, N.; Lee, W.G.; Jung, J. Growth of few-layer graphene on a thin cobalt film on a Si/SiO2 substrate. Chem. Vap. Depos. 2011, 17, 9–14. [Google Scholar] [CrossRef]
- Coraux, J.; N’Diaye, T.; Engler, M.; Busse, C.; Wall, D.; Buckanie, N.; Heringdorf, F.J.M.Z.; Gaste, R.V.; Poelsema, B.; Michely, T. Growth of graphene on Ir(111). New J. Phys. 2009, 11, 023006. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Won, S.Y.; Ciobanu, C.V.; Petrova, V.; Shenoy, V.B.; Bareno, J.; Gambin, V.; Petroy, I.; Kodambaka, S. Growth of semiconducting graphene on palladium. Nano Lett. 2009, 9, 3985–3990. [Google Scholar] [CrossRef]
- Fujita, T.; Kobayashi, W.; Oshima, C. Novel structures of carbon layers on a Pt(111) surface. Surf. Interface Anal. 2005, 37, 120–123. [Google Scholar] [CrossRef]
- Mehedi, H.A.; Baudrillart, B.; Alloyeau, D.; Mouhoub, O.; Ricolleau, C.; Pham, V.D.; Chacon, C.; Gicquel, A.; Lagoute, J.; Farhat, S. Synthesis of graphene by cobalt-catalyzed decomposition of methane in plasma-enhanced CVD: Optimization of experimental parameters with Taguchi method. J. Appl. Phys. 2016, 120, 065304. [Google Scholar] [CrossRef]
- Pashova, K.; Hinkov, I.; Aubert, X.; Prasanna, S.; Bénédic, F.; Farhat, S. Graphene synthesis by microwave plasma chemical vapor deposition: Analysis of the emission spectra and modeling. Plasma Sources Sci. Technol. 2019, 28, 045001. [Google Scholar] [CrossRef]
- Bacon, R. Growth, structure, and properties of graphite whiskers. J. Appl. Phys. 1960, 31, 283. [Google Scholar] [CrossRef]
- Wiles, P.G.; Abrahamson, J. Carbon fibre layers on arc electrodes—I: Their properties and cool-down behaviour. Carbon 1978, 16, 341. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Carbon Nanotubes, Preparation and Properties; Ebbesen, T.W., Ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ebbsen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Moravsky, A.P.; Wexler, E.M.; Loutfy, R.O. Growth of carbon nanotubes by arc discharge and laser ablation. In Carbon Nanotubes Science and Applications; Meyyappan, M., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 65–97. [Google Scholar]
- Farhat, S.; Scott, C. Review of the arc process modeling for fullerene and nanotube production. J. Nanosci. Nanotechnol. 2006, 6, 1189–1210. [Google Scholar] [CrossRef]
- Farhat, S.; Lamy de la Chapelle, M.; Loiseau, A.; Scott, C.D.; Lefrant, S.; Journet, C.; Bernier, P. Diameter control of single-walled carbon nanotubes using argon-helium mixture gases. J. Chem. Phys. 2001, 15, 6752–6759. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple method of preparing graphene flakes by an arc-discharge method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Karmakar, S.; Kulkarni, N.V.; Nawale, A.B.; Lalla, N.P.; Mishra, R.; Sathe, V.G.; Bhoraskar, S.V.; Das, A.K. A novel approach towards selective bulk synthesis of few-layer graphenes in an electric arc. J. Phys. D Appl. Phys. 2009, 42, 115201. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Ma, Y.; Huang, Y.; Li, N.; Zhang, F.; Chen, Y. Efficient and large-scale synthesis of few-layered graphene using an arc-discharge method and conductivity studies of the resulting films. Nano Res. 2010, 3, 661–669. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Shi, Z.; Gu, Z. Low-cost and large-scale synthesis of graphene nanosheets by arc discharge in air. Nanotechnology 2010, 21, 175602. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Li, J.; Volotskova, O.; Kundrapu, M.; Zhuang, T.S. Arc plasma synthesis of carbon nanostructures: Where is the frontier? J. Phys. D Appl. Phys. 2011, 44, 174006. [Google Scholar] [CrossRef]
- Shen, B.; Ding, J.; Yan, X.; Feng, W.; Li, J.; Xue, Q. Influence of different buffer gases on synthesis of few-layered graphene by arc discharge method. Appl. Surf. Sci. 2012, 258, 4523–4531. [Google Scholar] [CrossRef]
- Huang, L.; Wu, B.; Chen, J.; Xue, Y.; Geng, D.; Guo, Y.; Yu, G.; Liu, Y. Gram-scale synthesis of graphene sheets by a catalytic arc-discharge method. Small 2013, 9, 1330–1335. [Google Scholar] [CrossRef]
- Karmakar, S.; Nawale, A.B.; Lalla, N.P.; Sathe, V.G.; Kolekar, S.K.; Mathe, V.L.; Das, A.K.; Bhoraskar, S.V. Gas phase condensation of few-layer graphene with rotational stacking faults in an electric-arc. Carbon 2013, 55, 209–220. [Google Scholar] [CrossRef]
- Cotula, U.; Parmaka, E.D.S.; Kaykilarlia, C.; Sarayb, O.; Colakc, O.; Uzunsoya, D. Development of high purity, few-layer graphene synthesis by electric arc discharge technique. Acta Phys. Pol. A 2018, 134, 289–291. [Google Scholar] [CrossRef]
- David, R.L. CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press Inc.: Boca Raton, FL, USA, 2009; p. 2804. [Google Scholar]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Hu, C.Y.; Dong, J.; Shen, W.C.; Zhang, B. Polarization properties, high-order Raman spectra, and frequency asymmetry between Stokes and anti-Stokes scattering of Raman modes in a graphite whisker. Phys. Rev. B 2001, 64, 214301. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Kruger, P. Vibrational properties of CuO and Cu4O3 from first-principles calculations, and Raman and infrared spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Kastner, J.; Pichler, T.; Kuzmany, H.; Curran, S.; Blau, W.; Weldon, D.N.; Delamesiere, M.; Draper, S.; Zandbergen, H. Resonance Raman and infrared spectroscopy of carbon nanotubes. Chem. Phys. Lett. 1994, 221, 53–58. [Google Scholar] [CrossRef]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K.S.; Basko, D.M.; Ferrari, A.C. Raman spectroscopy of graphene edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.S.; Vivekchand, S.R.C.; Govindaraj, A.; Rao, C.N.R. A study of graphenes prepared by different methods: Characterization, properties and solubilization. J. Mater. Chem. 2008, 18, 1517–1523. [Google Scholar] [CrossRef]
- Athanassiou, E.K.; Grass, R.N.; Stark, W.J. Large-scale production of carbon-coated copper nanoparticles for sensor applications. Nanotechnology 2006, 17, 1668–1673. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Speziali, N.L.; Jorio, A. Measuring the degree of stacking order in graphite by Raman spectroscopy. Carbon 2008, 46, 272–275. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Robertson, A.W.; Warner, J.H. Hexagonal single crystal domains of few-layer graphene on copper foils. Nano Lett. 2011, 11, 1182–1189. [Google Scholar] [CrossRef]
- Soares, J.R.; Olivero, M.E.; Garin, C.; David, M.V.; Martins, L.G.P.; Almeida, C.A.; Ferreira, E.H.M.; Takai, K.; Enoki, T.; Paniago, R.M.; et al. Structural analysis of polycrystalline graphene systems by Raman spectroscopy. Carbon 2015, 95, 646. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saitoe, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Oshima, C.; Nagashima, A. Ultra-thin epitaxial films of graphite and hexagonal boron nitride on solid surfaces. J. Phys. Condens. Matter 1997, 9, 1–20. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Lutterotti, L. Maud: A Rietveld analysis program designed for the internet and experiment integration. Acta Crystallogr. A 2000, 56, s54. [Google Scholar] [CrossRef]
- Sowa, H.; Ahsbahs, H. High-pressure X-ray investigation of zincite ZnO single crystals using diamond anvils with an improved shape. J. Appl. Crystallogr. 2006, 39, 169–175. [Google Scholar] [CrossRef]
- Desgreniers, S. High-density phases of ZnO: Structural and compressive parameters. Phys. Rev. B 1998, 58, 14102–14105. [Google Scholar] [CrossRef]
- Shashurin, A.; Keidar, M. Synthesis of 2D materials in arc plasmas. J. Phys. D Appl. Phys. 2015, 48, 31400. [Google Scholar] [CrossRef]
- ANSYS Fluent User’s Guide, Release 15.0; ANSYS, Inc.: Canonsburg, PA, USA, 2013.
- Kee, R.J.; Rupley, F.M.; Miller, J.A.; Coltrin, M.E.; Grcar, J.F.; Meeks, E.; Moffat, H.K.; Lutz, A.E.; Lewis, G.D.; Smooke, M.D.; et al. CHEMKIN Collection, Release 3.6; Reaction Design, Inc.: San Diego, CA, USA, 2001. [Google Scholar]
- JANAF. Report NSRDS-NBS: Dow Chemikal Company, Clearinghouse for Federal Scientific and Technical Information; PB168370; Springfield: Virginia, VA, USA, 1965.
- Kee, R.J.; Lewis, G.D.; Warnatz, J.; Miller, J.A. Technical Report SAND86-8426; Sandia National Laboratories: Albuquerque, NM, USA, 1986.
- Gupta, R.; Yos, J.; Thompson, R.; Lee, K. A Review of Reaction Rates and Thermodynamic and Transport Properties for 11-Species Air Model for Chemical and Thermal Non-Equilibrium Calculations to 30000 K; NASARP-1232; NASA Reference Publication: Hampton, VA, USA, 1990.
- Bilodeau, J.F.; Pousse, J.; Gleizes, A. A mathematical model of the carbon arc reactor for fullerene synthesis. Plasma Chem. Plasma Process. 1998, 18, 285–303. [Google Scholar] [CrossRef]
- Krestinin, A.V.; Moravskii, A.P.; Tesner, P.A. A kinetic modeling of formation of fullerenes C60 and C70 in condensation of carbon vapor. Chem. Phys. Rep. 1998, 17, 1687–1707. [Google Scholar]
- Gurvich, L.V.; Iorish, V.S. Ivtanthermo—A Thermodynamic Database and Software System for the Personal Computer. User’s Guide; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]

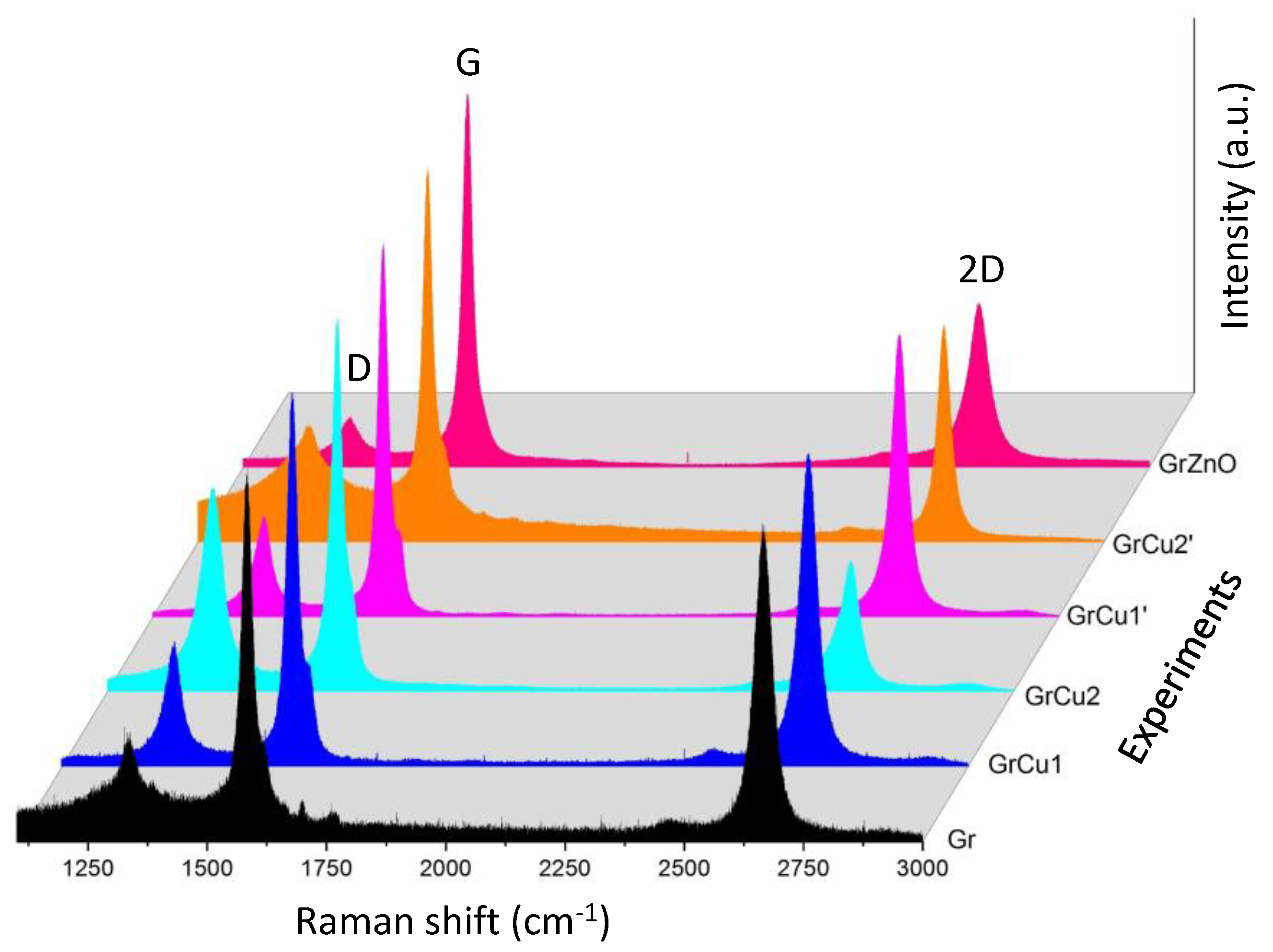



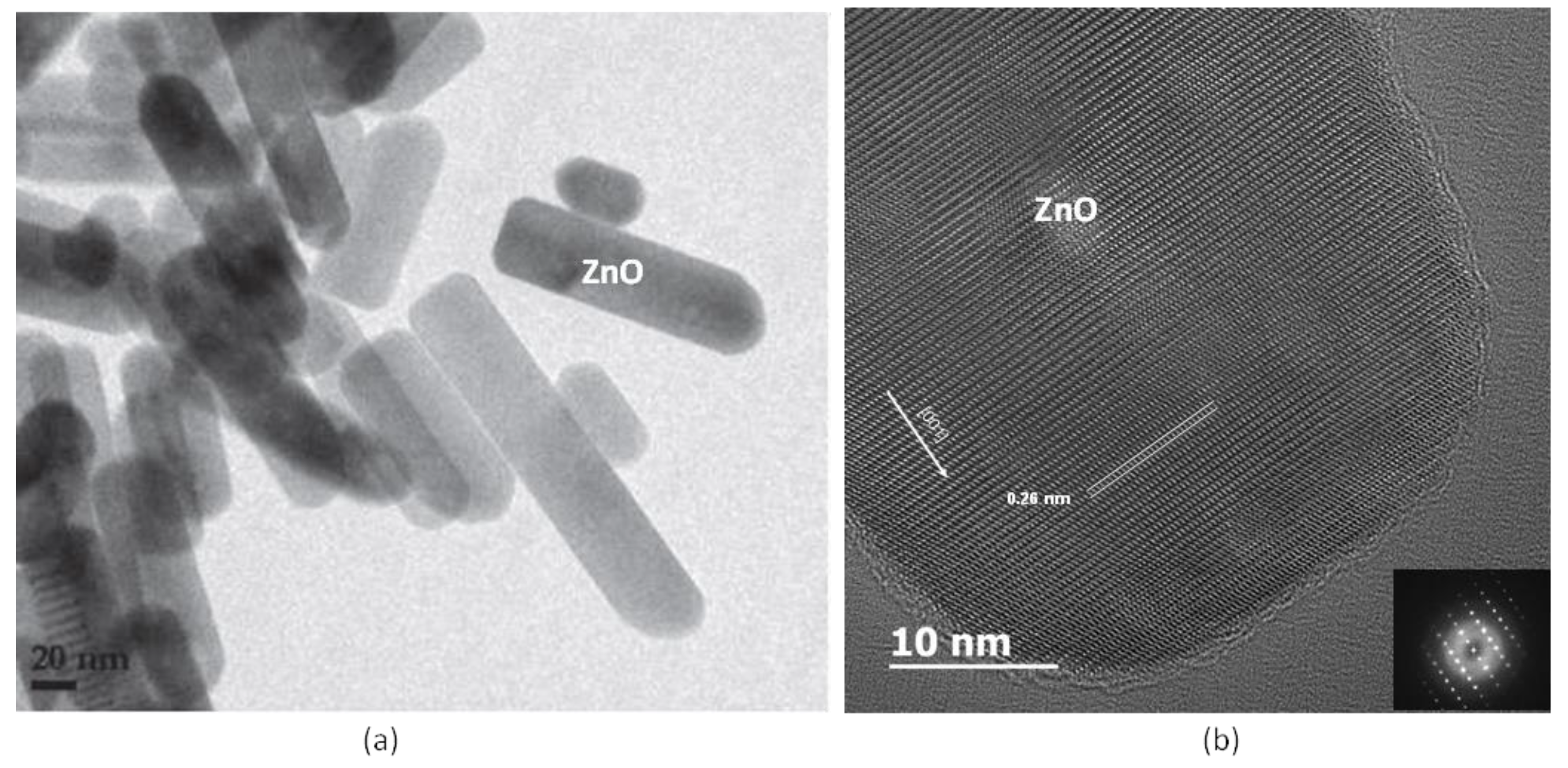


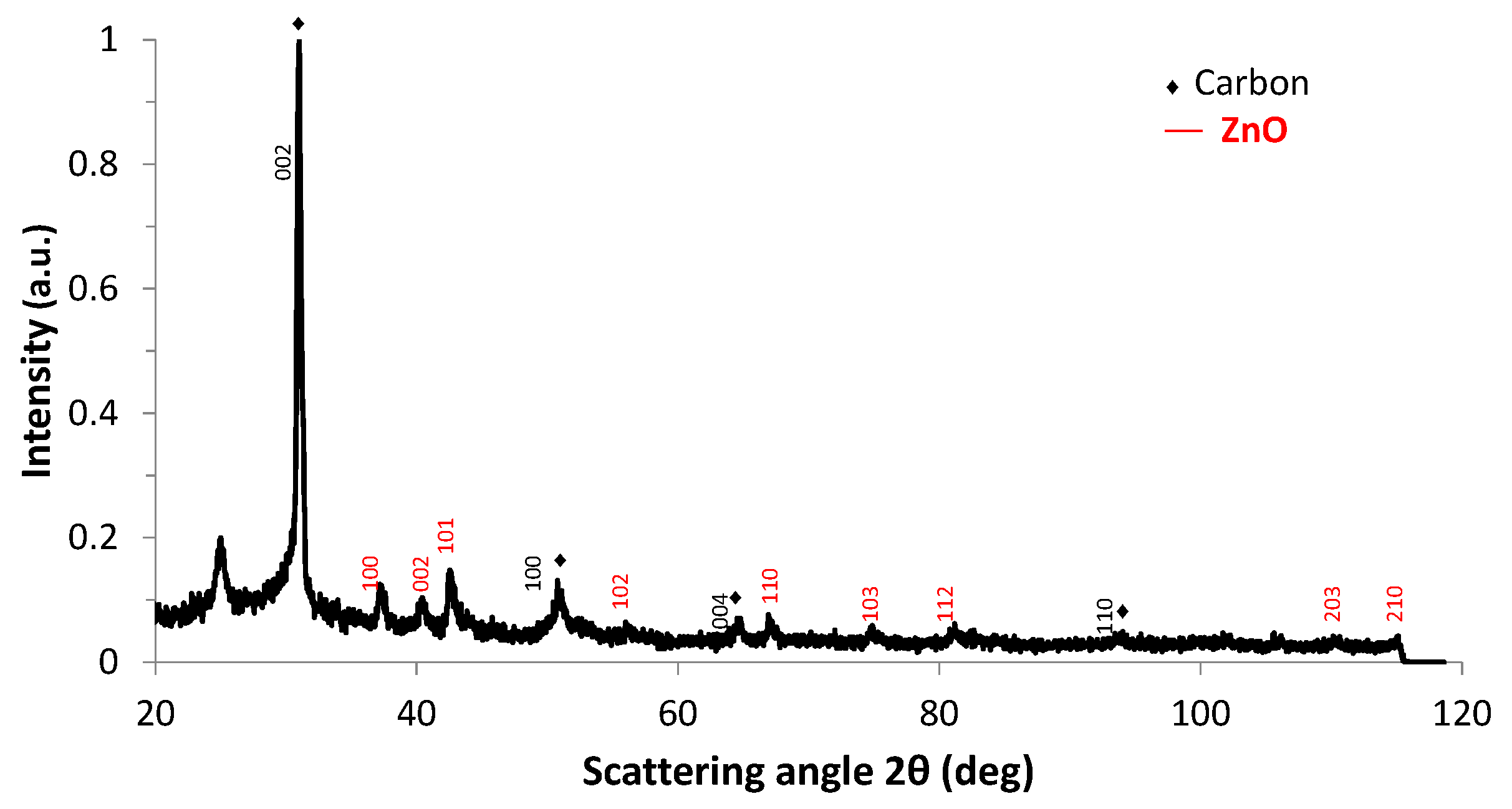

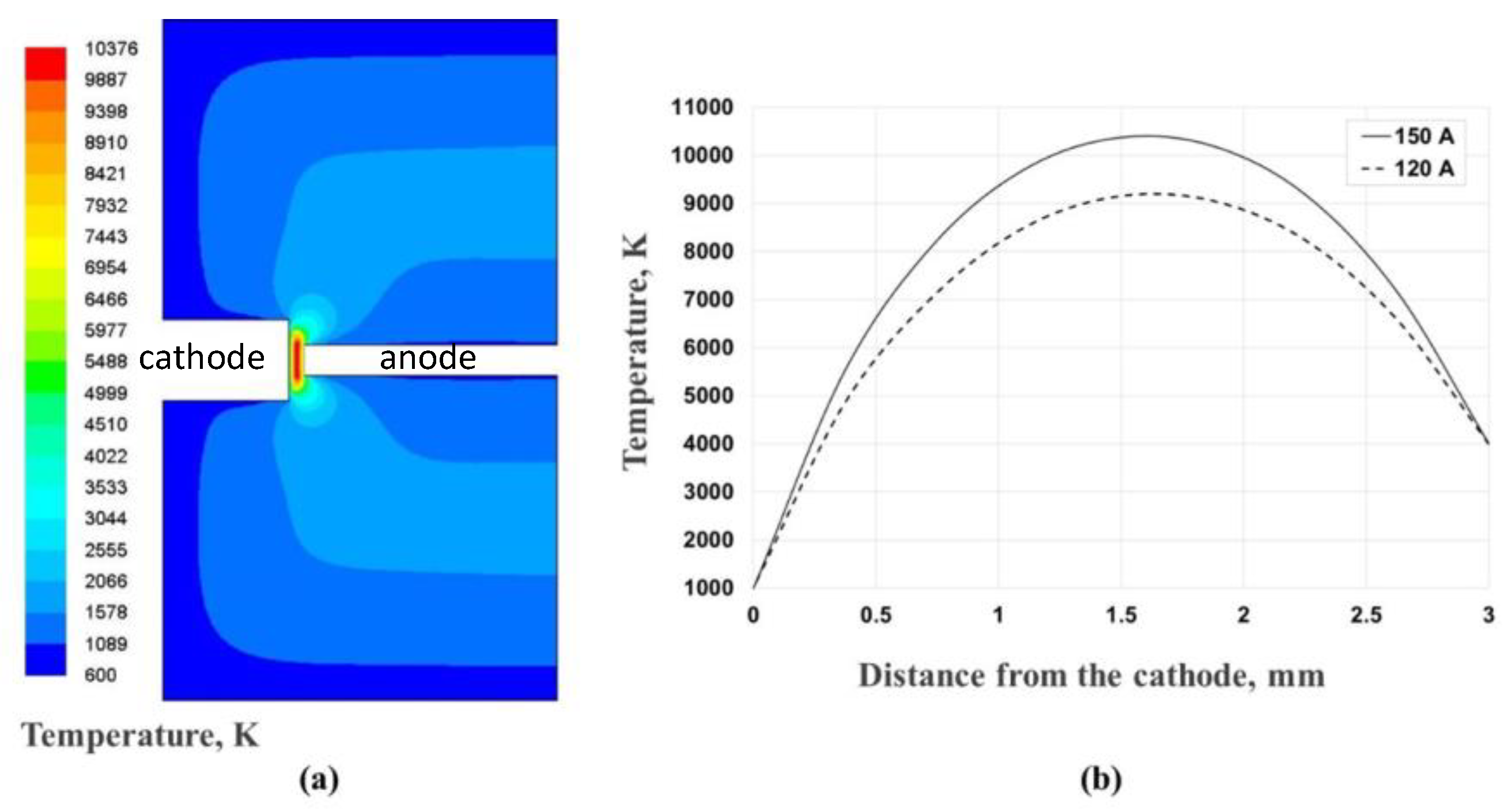
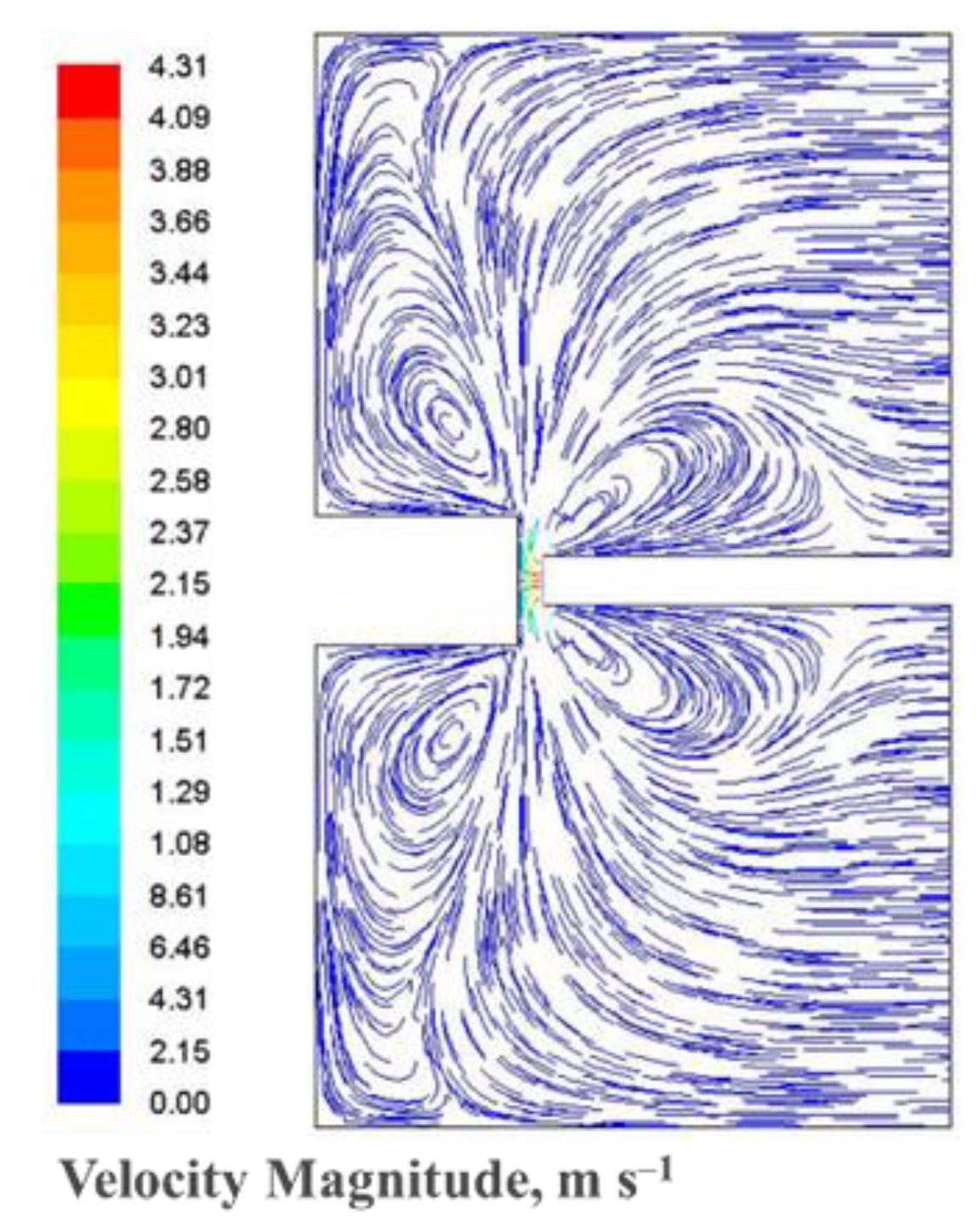
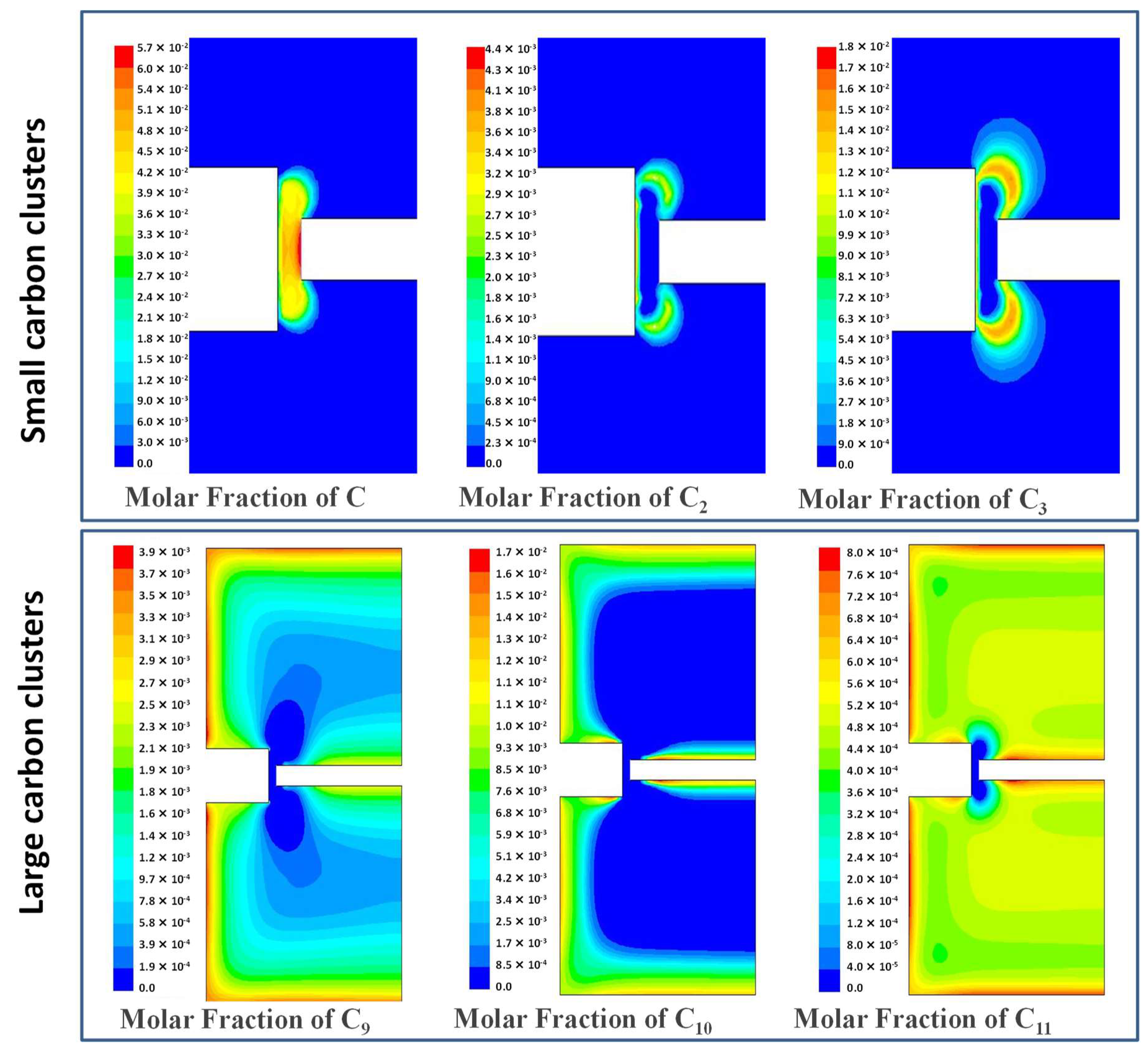

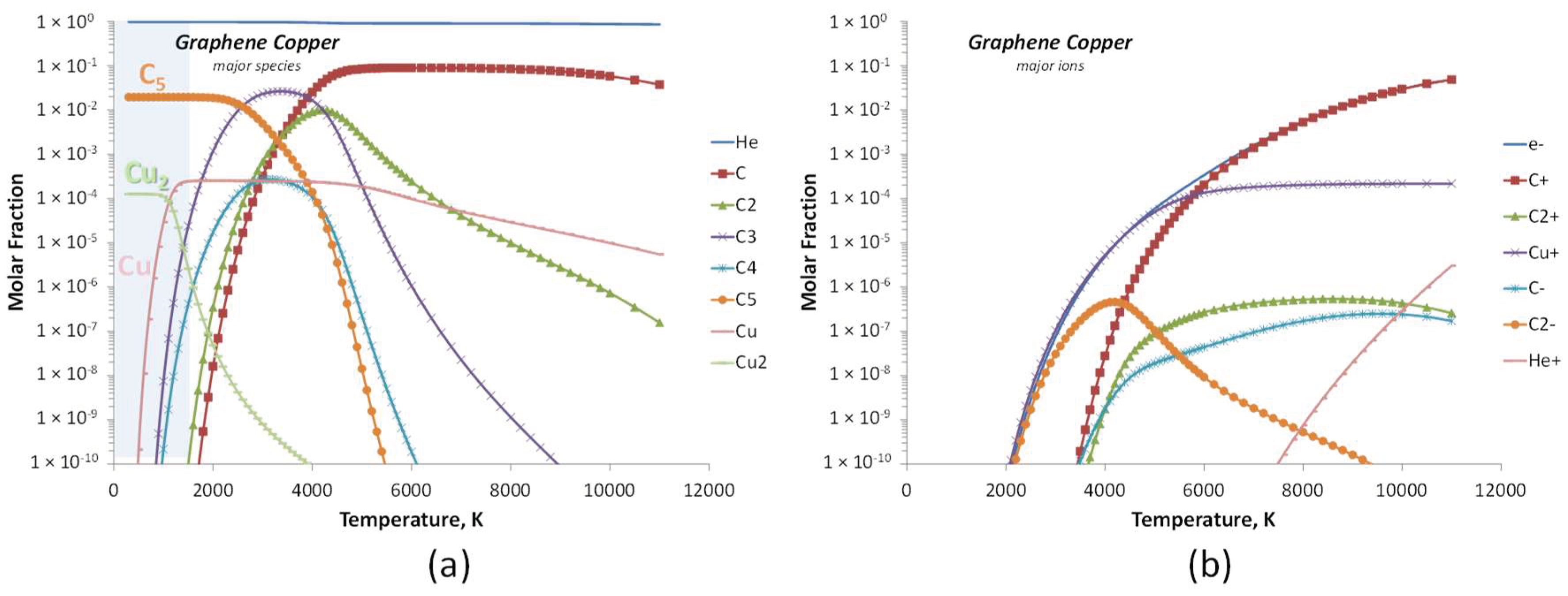
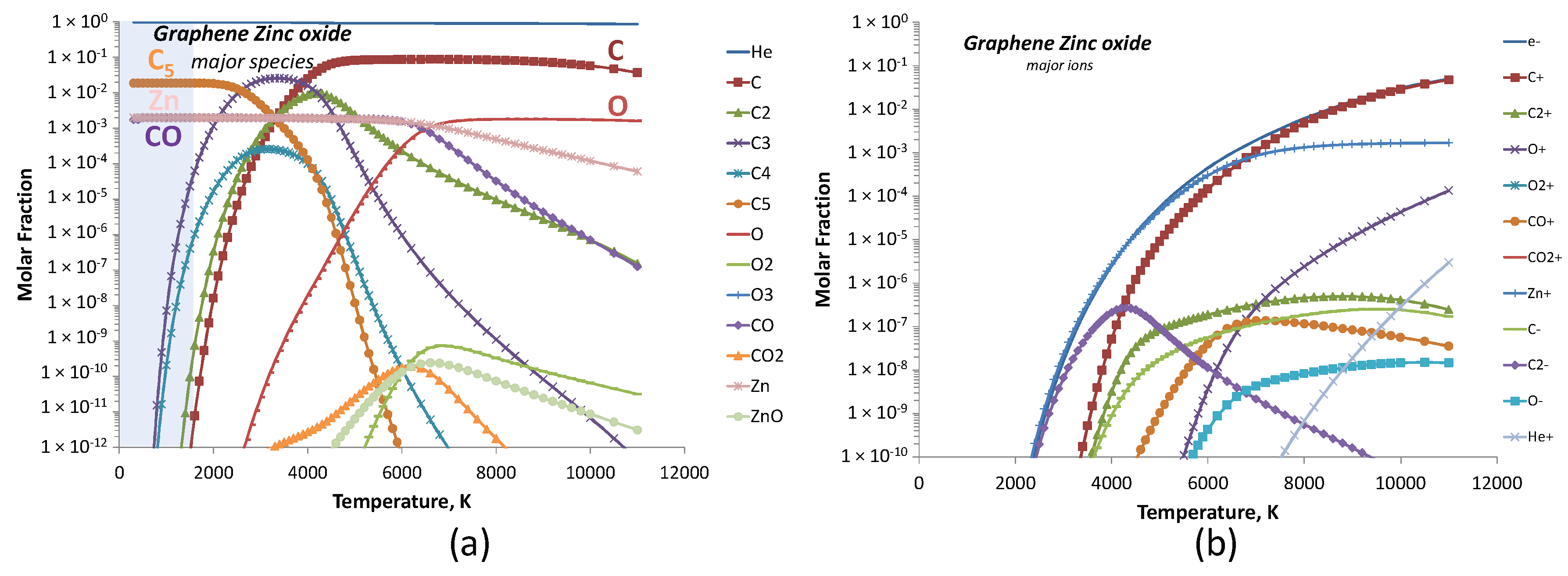
| Experiment 1 | Hybrid filler | Weight%, y | Weight%, Y | Atom%, X | Current (A) |
|---|---|---|---|---|---|
| Gr | - | - | - | - | 120 |
| GrCu1 | Cu | 75 | 11.65 | 2.43 | 120 |
| GrCu2 | Cu | 100 | 20.74 | 4.71 | 120 |
| GrCu1’ | Cu | 75 | 11.65 | 2.43 | 150 |
| GrCu2’ | Cu | 100 | 20.74 | 4.71 | 150 |
| GrZnO | ZnO | 100 | 12.26 | 2.02 | 120 |
| Sample | ID/IG | I2D/IG | La (nm) 1 | ΔLa (nm) | FWMH (cm−1) 2 |
|---|---|---|---|---|---|
| Gr | 0.31 | 0.88 | 124 | 3 | 39.8 |
| GrCu1 | 0.34 | 0.85 | 113 | 2 | 41.8 |
| GrCu2 | 0.55 | 0.36 | 70 | 1 | 53.1 |
| GrCu1’ | 0.27 | 0.76 | 142 | 1 | 38.6 |
| GrCu2’ | 0.3 | 0.58 | 128 | 3 | 30.3 |
| GrZnO | 0.13 | 0.44 | 296 | 13 | 53.4 |
| Sample | 2θ° | Crystal System | Lattice Parameters (Å) | Crystallite Size D (nm) 1 | ||
|---|---|---|---|---|---|---|
| Cu(111) | Cu(200) | Cu(220) | ||||
| GrCu1 | 51.13 | 59.67 | 88.87 | cubic | a = 3.6036 ± 1 × 10−4 | 53.5 ± 1.1 |
| GrCu2 | 50.98 | 59.53 | 88.61 | cubic | a = 3.6081 ± 2 × 10−4 | 48.9 ± 2.9 |
| GrCu1’ | 51.16 | 59.81 | 89.37 | cubic | a = 3.6005 ± 2 × 10−4 | 21.6 ± 0.3 |
| GrCu2’ | 50.92 | 59.58 | 89.04 | cubic | a = 3.6072 ± 2 × 10−4 | 42.9 ± 0.9 |
| ZnO(100) | ZnO(002) | ZnO(101) | ||||
| GrZnO | 37.18 | 40.43 | 42.53 | hexagonal | a = 3.2300 ± 9 × 10−4 c = 5.1725 ± 2.7 × 10−3 | 42.3 ± 1.3 |
| Gas Phase Reactions 1 | A (cm3/s/mol) | β (–) | E (K) |
|---|---|---|---|
| C + C = C2 | 2 × 1014 | 0 | 0 |
| C + C2 = C3 | 2 × 1014 | 0 | 0 |
| C2 + C2 = C3 + C | 2 × 1015 | 0 | 9040 |
| C2 + C2 = C4 | 2 × 1014 | 0 | 0 |
| C + C3 = C4 | 2 × 1014 | 0 | 0 |
| C + C4 = C5 | 2 × 1014 | 0 | 0 |
| C2 + C3 = C5 | 2 × 1014 | 0 | 0 |
| C5 + C = C6 | 2 × 1014 | 0 | 0 |
| C6 + C = C7 | 2 × 1014 | 0 | 0 |
| C7 + C = C8 | 2 × 1014 | 0 | 0 |
| C8 + C = C9 | 2 × 1014 | 0 | 0 |
| C9 + C = C10 | 2 × 1014 | 0 | 0 |
| C4 + C2 = C6 | 2 × 1014 | 0 | 0 |
| C5 + C2 = C7 | 2 × 1014 | 0 | 0 |
| C6 + C2 = C8 | 2 × 1014 | 0 | 0 |
| C7 + C2 = C9 | 2 × 1014 | 0 | 0 |
| C8 + C2 = C10 | 2 × 1014 | 0 | 0 |
| C9 + C2 = C11 | 2 × 1014 | 0 | 0 |
| C3 + C3 = C6 | 2 × 1014 | 0 | 0 |
| C4 + C3 = C7 | 2 × 1014 | 0 | 0 |
| C5 + C3 = C8 | 2 × 1014 | 0 | 0 |
| C6 + C3 = C9 | 2 × 1014 | 0 | 0 |
| C7 + C3 = C10 | 2 × 1014 | 0 | 0 |
| C4 + C4 = C8 | 2 × 1014 | 0 | 0 |
| C5 + C4 = C9 | 2 × 1014 | 0 | 0 |
| C6 + C4 = C10 | 2 × 1014 | 0 | 0 |
| C5 + C5 = C10 | 2 × 1014 | 0 | 0 |
| Chemical Systems and Species |
|---|
| Helium-Carbon (12 species) 6 neutral: He, C, C2, C3, C4, C5 6 ions: e−, C−, C2−, He+, C+, C2+ |
| Helium-Carbon-Copper (15 species) 8 neutral: He, C, C2, C3, C4, C5, Cu, Cu2 7 ions: e−, C+, C2+, Cu+, C−, C2−, He+ |
| Helium-Carbon-Zinc Oxide (28 species) 15 neutral: He, C, C2, C3, C4, C5, O, O2, O3, CO, C2O, CO2, C3O2, Zn, ZnO 13 ions: e−, C−, C2−, O−, O2−, C+, C2+, O+, O2+, CO+, CO2+, Zn+, He+ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kane, A.; Hinkov, I.; Brinza, O.; Hosni, M.; Barry, A.H.; Cherif, S.M.; Farhat, S. One-Step Synthesis of Graphene, Copper and Zinc Oxide Graphene Hybrids via Arc Discharge: Experiments and Modeling. Coatings 2020, 10, 308. https://doi.org/10.3390/coatings10040308
Kane A, Hinkov I, Brinza O, Hosni M, Barry AH, Cherif SM, Farhat S. One-Step Synthesis of Graphene, Copper and Zinc Oxide Graphene Hybrids via Arc Discharge: Experiments and Modeling. Coatings. 2020; 10(4):308. https://doi.org/10.3390/coatings10040308
Chicago/Turabian StyleKane, Aichata, Ivaylo Hinkov, Ovidiu Brinza, Mongia Hosni, Aliou Hamady Barry, Salim Mourad Cherif, and Samir Farhat. 2020. "One-Step Synthesis of Graphene, Copper and Zinc Oxide Graphene Hybrids via Arc Discharge: Experiments and Modeling" Coatings 10, no. 4: 308. https://doi.org/10.3390/coatings10040308
APA StyleKane, A., Hinkov, I., Brinza, O., Hosni, M., Barry, A. H., Cherif, S. M., & Farhat, S. (2020). One-Step Synthesis of Graphene, Copper and Zinc Oxide Graphene Hybrids via Arc Discharge: Experiments and Modeling. Coatings, 10(4), 308. https://doi.org/10.3390/coatings10040308





