Abstract
In this paper, we report on a modified arc process to synthetize graphene, copper and zinc oxide graphene hybrids. The anode was made of pure graphite or graphite mixed with metals or metal oxides. After applying a controlled direct current, plasma is created in the interelectrode region and the anode is consumed by eroding. Continuous and abundant flux of small carbon, zinc or copper species, issued from the anode at a relatively high temperature, flows through the plasma and condenses in the vicinity of a water-cooled cathode leading to few-layered graphene sheets and highly ordered carbon structures. When the graphite rod is filled with copper or zinc oxide nanoparticles, few layers of curved graphene films were anchored with spherical Cu and ZnO nanoparticles leading to a one-step process synthesis of graphene hybrids, which combine the synergetic properties of graphene along with nanostructured metals or semiconducting materials. The as-prepared samples were characterized by Raman spectroscopy, X-ray diffraction (XRD), spatially resolved electron energy loss spectroscopy (EELS), energy filtered elemental mapping and transmission electron microscopy (TEM). In addition to the experimental study, numerical simulations were performed to determine the velocity, temperature and chemical species distributions in the arc plasma under specific graphene synthesis conditions, thereby providing valuable insight into growth mechanisms.
1. Introduction
Transformation of two-dimensional one-atom-thick sheet of graphene (Gr) into three-dimensional architectures extends the outstanding properties of graphene [1] to novel graphene hybrid (GrH) materials such as graphene-semiconducting (GrSC) or graphene metals (GrM). Indeed, graphene exhibits impressive conjunction of mechanical, thermal, photoelectric and electronic properties such as excellent thermal conductivity (as high as 5000 W m−1 K−1) [2], high specific surface area (as high as 3000 m2 g−1) [3], high chemical stability and high carrier mobility (as high as 350,000 cm2 V−1·s−1) [4]. Due to its unique band structure with a band-tuning ability, these properties are modular and can be combined endlessly. However, graphene properties are effective in the planar direction, limiting the graphene scope and applications. To extend the field of graphene applications, new hybrids are proposed by combining graphene’s large surface area with carbon nanotubes, nanometric metals or semiconducting metal oxides enhancing electron transfer kinetics within new 3D conducting networks. Synergy between axial properties of nanotubes [5], quantum confined metallic (M) or semiconducting (SC) nanoparticles [6] and graphene is hence the key issue in determining the performance of these hybrid nanostructures with great promise for applications. Hybrid materials can be categorized as structurally hybridized materials (composites) or functionally hybridized materials. In the composites, the matrix is composed of a metal as copper [7,8] and the graphene with low concentration from 0.5 wt % to 5 wt % is used as a filler to provide a higher degree of reinforcement of mechanical or thermal properties. Exceptional mechanical properties of graphene together with its large surface area, good hydrophobicity and enhanced wettability for organic reactants allows for several industrial applications. Hierarchical graphene, graphene oxide or reduced graphene oxide mixed with other compounds such as polymers [9,10,11,12], oxides [13] or urea [14] were proposed for the fabrication of new nanocomposites. This includes, but is not limited to, anion exchange membranes [9], nanocomposites membranes for CO2 separation [10], membranes for nanofiltration [11], electromagnetic interference shielding [12], batteries [13] and catalysts for biodiesel production by transesterification [14]. In functionally hybridized materials, the matrix is mainly composed of graphene, and the metal or semiconductor is used in smaller quantities but imperatively in nanometric sizes to improve specific functional properties. Such functionally hybrids are envisaged as an electrode in electronics [15], energy storage [16], sensing [17,18], photonics and optoelectronics [19,20,21,22,23,24,25,26,27] or as microwave absorbing materials [28]. The interaction characteristics through Gr-M or Gr-SC could dramatically induce a matter interaction with the incident electromagnetic radiation, in light harvesting, electric, dielectric or magnetic contexts. More specifically, metallic copper and semiconducting zinc oxide have great interest because they are inexpensive and their properties can be controlled during the process synthesis. Copper nanoparticles have relatively high thermal conductivity (400 W m−1 K−1) and high electrical conductivity. Due to the localized surface plasmon resonances, copper is a good alternative for noble metals such as silver or gold. Copper nanoparticles were hence proposed as plasmonic nanoparticles or as selective catalysts. However, poor chemical stability allows copper nanoparticles to be easily oxidized to form copper oxide and to agglomerate when attracting forces outweigh the repulsive one. Then intrinsic properties of copper nanoparticles are hindered, since the surfaces of the agglomerates become the reference for the transfer of properties, even if the powder crystallite sizes are in the nanometer range. To avoid these pitfalls, copper nanoparticles could be coated with graphene who acts as passivation layer to protect the underlying metal surface from oxidation. In addition, GrCu hybrids allow a strong localization of the field at the Gr–Cu interface. As a consequence, an enhanced intensity of the localized surface plasmon resonances supported by the hybrid nanostructures induces a much more enhanced fluorescent intensity from the dye coated hybrid nanostructure [18]. In the other hand, semiconducting zinc oxide nanomaterials has a wide bandgap of 3.37 eV, relatively high electron mobility (200 cm2 V−1 s−1 for ZnO as compared to 30 cm2 V−1 s−1 for TiO2), high exciton binding energy (60 meV), strong room-temperature luminescence, high transparency as well as UV filter, antifungal and antimicrobial activities. These distinctive physicochemical properties have the potential to allow the development of new systems, structures and devices to transform energy into optical or electrical signals for sensing. For example, zinc oxide based dye sensitized solar cells presents potential advantages including a fast charge transport with an electron mobility and conductivity of several orders of magnitude higher compared to conventional anatase titanium oxide [29]. For photochemical/optical sensors, the association of ZnO with graphene allows a better electric response, enhances adsorption of molecules and increases the signal-to-noise ratio [6]. GrZnO hybrids have been already proposed to sense molecular hydrogen [30], ethanol vapors [31] and sulfur hydrogen [32]. For microwave absorption, GrZnO hybrids interact with microwaves of specific frequencies providing responses to gigahertz-range electromagnetic radiation. This interaction is attributed to the functional capacity of the metal center to which the oxygen anion is bonded to [28]. Carbon-based nanocomposites formed between graphene and nanomaterials with a large number of free electrons such as n-type semiconductors tin oxide (SnO2), or tungsten oxide (WO3) was studied in [33,34]. The presence of graphene increases photoluminescence intensity of the semiconductors by creating an additional pathway for electron transfer from the conduction band of the excited semiconductors to graphene sheets. This improves the photocatalytic degradation of an organic pollutant using Gr-SC nanostructures under visible light irradiation. In order to expand the scope of these new graphene hybrids and to take advantage of these new abilities, synthesis methods require development and improvement. In this direction, synthesis strategies proposed for graphene hybrids (GrH) could be classified in two fundamentally different approaches [6]. The in situ method, in which metals M or semiconductors SC grow directly on the graphene Gr structures or vice versa. The assembly methods, in which Gr (with less than 10 layers) M and SC are synthesized separately and then assembled spontaneously via physical dispersion in solutions or by electrostatic or covalent assembly and subsequent solution processing. In these methods, the source of graphene is either graphene oxide (GO) produced by the Hummer’s method [35], reduced graphene oxide (rGO), CVD or plasma enhanced PECVD graphene. For some applications, defects can be intentionally induced in graphene by an electron beam irradiation in order to improve hydrophilic and photoelectrochemical graphene performances [36]. An alternative method to Hummer’s [35] and CVD [37,38,39,40,41,42,43] or PECVD [44,45] methods is the electric arc discharge in which carbon is evaporated by implementing direct current DC arc voltage across two graphite electrodes in an inert gas atmosphere. When the electrodes are brought together, discharge occurs by consumption of the anode and plasma formation. Arc discharge played an important role in the genesis and discovery of several carbon materials including carbon fibers and scroll-like whiskers [46,47,48], fullerenes [49], carbon nanotubes [50,51,52,53,54] and graphene [55,56,57,58,59,60,61,62]. In 2009, Subrahmanyam et al. [55] have reported for the first time the synthesis of graphene flakes through the arc-discharge method. They used a graphite anode rod with different H2-He mixtures to produce graphene flakes with 2–4 layers in the inner wall region of the arc chamber. As reported for multiwalled nanotube synthesis, reactive gases as H2 or CO2 could react with carbon radicals at the growing edge of nanotubes thus preventing their ends from enclosing [84,85,86]. Karmakar et al. [56] demonstrates that graphene parameters can be controlled by an external steady non-uniform magnetic field. They succeeded in bulk synthesis of few-layer graphene by sublimating the graphite anode in an argon atmosphere. The magnetic field, enhance stacking of carbon precursors preferably along the surface of the cathode, assisting in the formation of graphene-sheet-like structures. As discussed by Moravsky et al. [52], externally applied magnetic field can deflect the flow of positively charged particles to enhance arc discharge process. In graphene context, the uncompensated positive charges that contribute to the cathode deposit could be limited to improve the graphene yield. Wu et al. [57] synthesized large-scaled few-layered graphene using carbon dioxide (CO2) and helium (He) mixture as buffer gas, and direct currents of 100–200 A. The in-plane crystallite size obtained from Raman spectra was 6 nm. Wang et al. [58] synthesized graphene and carbon nanohorns in the atmosphere of air. The yield of graphene was found to be dependent on the air pressure, which was varied between 400 and 1000 torr. [59] Keidar et al. [59] discussed several approaches to improve the controllability of the arc discharge process for graphene and carbon nanotubes synthesis. This includes experimental improvements of the arc by applying external magnetic field as well as by proposing deterministic approaches to estimate from multispecies simulations electron density and temperature distribution. Single or few-layer graphene flakes with sizes in the order of 0.1–1 µm was obtained [59]. Shen et al. [60] studied the effect of several buffer gases at different pressures on the yield of few-layered graphene by arc. The graphene sheets produced in H2–He buffer gases exhibit the best crystallinity and the highest specific surface area.
In 2013, Huang et al. [61] reported a gram-scale and high-quality graphene sheets synthesis by an arc-discharge method using graphite as the carbon source and ZnO or ZnS as catalysts. Since zinc oxide (ZnO) has a similar hexagonal crystal structure as graphene, they supposed that carbon atoms prefer to graphitize on the surface of ZnO to form graphene sheets. Karmakar et al. [62] obtained few-layer graphene in an external magnetic field modulated DC carbon arc in different non-reactive buffer gases N2, Ar, He and their mixtures. The crystallite size of the as-synthesized sheets was estimated to be 136 nm. Cotul et al. [63] obtained high purity graphene nanoflakes by the electric arc discharge technique and helium, nitrogen and their mixtures were used as the reactor atmosphere. The degree of purity of the synthesized samples was increased by increasing both the current and the electrode diameter. Since the first arc discharge experiment of Krätschmer et al. in 1990, and in spite of three decades of intensive research, carbon nanomaterial growth by arc discharge has been hampered by the lack of quantitative understanding of the process [59]. The major inconvenience of this process remains the difficult in situ control of the final material purity and physical properties. Indeed, the non-equilibrium reacting gas flow leads to the emergence of interdependent fluxes and gradients over a wide range of time and space scales, making arc discharge modeling very complex [53].
In this paper, a modified arc process is proposed for one step synthesis of graphene and copper (GrCu) and zinc oxide (GrZnO) graphene hybrids. The issued materials have been investigated using high resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD) and Raman spectroscopy. Since all the parameters of the arc process are interdependent, an attempt to adjust only one of them will lead to inevitable variation of the others. Therefore we performed simulations of the arc conditions using the commercial computational fluid dynamics (CFD) code ANSYS Fluent in order to calculate the gas convection and temperature profiles under specific graphene conditions. When copper or zinc oxide is added to the anode material, plasma composition was estimated from chemical equilibrium calculations.
2. Materials and Methods
2.1. Materials
Graphene films were grown in the arc-discharge reactor. The cathode is made in pure graphite with external diameter of 27 mm and length of 20 mm. The movable anode is a graphite rod with external diameter of 6.15 mm and length of 76 mm (Alfa Aesar, with a spectral purity of 99.99 weight %) with a hole of 3 mm in diameter and 30 mm of length. For buffer gas we used helium from Air Liquide with a purity of 99.95%. For the graphite filler we used synthetic graphite powder with a size of 1–2 µm purchased from Sigma-Aldrich. For copper filler we used commercial powder (Merck powder, max. particle size 50 microns and purity of 99%). For zinc oxide, we used homemade nanorods with an average diameter of 35 nm and a length of 110 nm indexed (NR35) and synthesized by the polyol process. For their elaboration we used acetate dihydrate (Zn(OAc)2· 2H2O) with a purity of 99%, purchased from Sigma-Aldrich, sodium hydroxide NaOH pellets with 98% purity from Acros Organic, diethylene glycol DEG (O(CH2CH2OH)2) with a purity of 99.5% from Panreac. Solvents acetone and 96 vol. % ethanol are from VWR Chemical.
2.2. Characterization Techniques
To identify crystalline quality and graphene thickness, we used Raman spectroscopy (HR800, HORIBA Jobin-Yvon) working in a confocal mode in air and with the back-scattering configuration, excitation wavelength of 632.8 nm. To further confirm the graphene phase and morphology, X-ray diffraction was taken in the 2θ angle range from 20 to 120°. The XRD analysis was performed using 2INELTM diffractometers with Co-Kα radiation. Different locations were sampled in order to reliably describe each sample. Morphology and structure analysis of the samples was carried out on transmission electron microscope (JEOL 2011) (JEOL (Europe) SAS, Croissy-Sur-Seine, France) with an accelerating voltage of 200 kV and high-resolution transmission electron microscope HRTEM (JEM-ARM200F) (JEOL (Europe) SAS, Croissy-Sur-Seine, France). In addition, spatially resolved electron energy loss spectroscopy (EELS) (JEOL (Europe) SAS, Croissy-Sur-Seine, France) and energy filtered elemental mapping was used.
2.3. Methods
Experiments were designed and conducted to synthesize graphene (Gr) and graphene hybrids (GrH) using the same laboratory scale arc discharge reactor developed earlier for nanotube synthesis [53,54]. Obtaining high yields of graphene requires careful control of experimental conditions. The reactor chamber consists of a water-cooled stainless steel with two water-cooled electrodes as shown in Figure 1a. Both electrodes were in graphite fixed on the water-cooled copper holder. Due to its high thermal capacity and conductivity, water is suitable for the absorbing part of the 6 kW input power, to prevent damage that might otherwise occur when the plasma is in contact either with the cathode or the anode holder [52]. Anode was filled with graphite, copper or zinc oxide powders in different proportions as schematically shown in Figure 1c. The direct current (DC) arc operates in a 3 mm gap between the electrodes that are horizontally installed in a water-cooled chamber filled with helium gas at sub-atmospheric pressure. The pressure was kept at 530 mbar, and direct current of 120 A and 150 A was employed for the purpose of comparison.
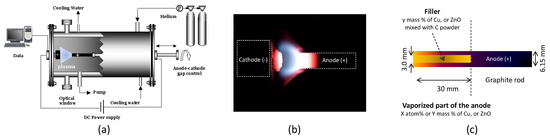
Figure 1.
Arc discharge set-up: (a) schematic; (b) photography of the luminous plasma zone created between the anode and the cathode and (c) anode composition.
The filled part of the anode is consumed in the arc, whereas a cylindrically shaped cathode deposit is grown on the cathode as shown in Figure 1b. Efficient operation is assumed to exist when the discharge is stable and the anode erosion rate is constant. This can be achieved by maintaining a constant voltage between the electrodes, which is closely related to the stabilization of the electrode spacing. Under these conditions the arc gap and the voltage drop across the gap remain constant, ensuring constant evaporation and condensation. The plasma is first ignited by contact between the cathode and the anode, which elevates the temperature of the contact point until evaporation of the anode material. Then the anode was manually moved toward the cathode by adjusting the desired distance between the electrodes. An active plasma zone is created and could be observed through a quartz window in the reaction chamber. A magnified image of the luminescent plasma is projected from this window on a screen and allowed to continuously adjust the distance between the anode and the cathode. This hot plasma zone produces carbon and metal or metal oxides vapors, which then diffuse to the cooled reactor regions rich in helium. The high temperature near the anode and the high energy density in the plasma ensure vaporization of most of the anode material. The water-cooled cathode and reactor walls lead to a high quench rates and high levels of super cooled or supersaturated mixed carbon and metal vapor with soot propagating away from the gap that deposit on the cold rector surfaces. The efficiency of graphene synthesis is determined primarily by the choice of the buffer gas and the intrinsic arc parameters. Since, the key aspects defining graphene properties are the atomically organized crystallite size and the grain boundaries structure, which could be obtained through TEM, Raman and XRD analysis of the collected samples.
For all experiments, the arc was ignited by contacting the anode and cathode. The contact between the electrodes elevates the temperature “instantaneously” to the sublimation temperature of carbon of 4098 K [64] and carbon flux starts to evaporate from the anode. Then the anode was moved back toward the cathode and maintained at a distance anode–cathode DAC of 3 mm where sufficiently high carbon vapor pressure is maintained. The internal part of the drilled hole in the graphite anode was filled by 200 ± 1 mg of material composed by pure graphite powder for Gr synthesis or by graphite mixed with y mass % of copper or zinc oxide for (GrH) synthesis. By considering the outer graphite shell, we calculated the total mass %Y and total atom %X of the filler Cu or ZnO in the vaporized anode part. For graphene and graphene hybrid, we conducted 6 experiments with experimental conditions reported in Table 1.

Table 1.
Experimental conditions for graphene and graphene hybrids synthesis.
3. Results
After arc discharge processing, the reactor was cooled down to the ambient temperature and soot formed on the reactor walls was collected and sieved by a 60 micron stainless steel sieve in order to exclude any heavy particles as graphite or metal ejected from the anode by spallation in which fragments of graphite called “spalls” are expelled from the body of the anode and survive to the plasma [52]. We performed structural and molecular analysis by Raman spectroscopy complemented by detailed crystallographic study via X-ray diffraction (XRD). TEM information is also proposed for morphological and elemental mapping studies. These measurements are complemented by XRD to provide better insight of structural properties at the macroscopic scale of the sample. Accordingly, the combined use of these techniques allows the identification of our compounds in both molecular and macroscopic scales. In particular, the Rietveld’s method is a very fine technique for analyzing powder diffraction patterns. It consists of completely describing the diagram including the background noise, the shape, the position and the intensities of the diffraction lines observed for the different phases using structural models. We proceeded by progressive refinement of the parameters of the models by least squares technique until obtaining an agreement as good as possible between the diagrams observed and calculated with the models. It requires knowledge of the phases present and their structure including the structural type and atomic positions. Figure 2 shows a typical room temperature Raman of Gr and GrH products prepared under conditions of Table 1. The main features in the graphene Raman spectra were peaks D generally observed at 1333 cm−1, G 1580 cm−1 and 2D 2660 cm−1, in correspondence with Raman shifts. The D’ peak (1620 cm−1) originated from the intra-valley one-phonon and was caused by a disordered structure of graphene. Hence both D and D’ peaks require a defect for their activation. Graphene edges also constitute a kind of defect due to the broken in translational symmetry. Besides the peaks D, D’, G and 2D, other weak Raman modes could also be observed including D’+TA and D’+LA in the range of 1700–1750 cm−1. They originate from phonon dispersion of in-plane longitudinal acoustic (LA) and transverse acoustic (TA) branches [65,66].
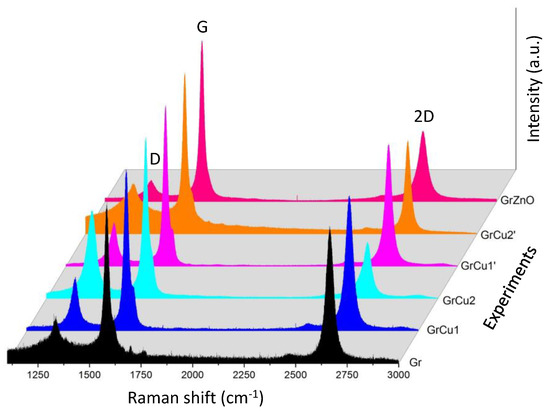
Figure 2.
Raman spectra of the synthesized graphene and graphene hybrids in the helium arc discharge method in the conditions of Table 1.
For graphene/copper samples, since metals contain only a single atom, they only have acoustic phonons and do not have first-order Raman bands. This is not the case if the primitive unit cell contains at least two atoms, as in copper oxides, for which optical phonons will also exist. For CuO there are three Raman first-order modes observed experimentally at 296–300 cm−1 (Ag) and 346–350 cm−1 (Bg) with 638 cm−1 (Bg). For Cu2O there are several Raman modes observed experimentally at 146–153 cm−1 (T1u), at 350 cm−1 (A2u), at 515 cm−1 (T2g) and at 638–665 cm−1 (T1u) [67].
In Figure 2, we could distinguish typical graphene bands G and 2D located at 1583 cm−1 and 2667 cm−1 respectively and a weak D peak located at 1337 cm−1. The D band, which is known as the disorder band or defect band, is generally very weak in graphite but becomes Raman active through a disorder-induced double resonance Raman process, which causes in-plane breathing vibrations of the aromatic ring structures (A1g symmetry). The intensity of the D-band is hence directly proportional to the level of defects in the sample [68,69]. The G band is assigned to the in-plane stretching vibration of sp2 carbon (E2g symmetry) related to phonon vibrations in sp2 carbon domains and is related to the tangential elongation modes in graphene [70,71]. It is almost identical regardless of the number of sheets. Finally, the 2D band, which is the second order of the D band, does not need to be activated by the proximity of a defect. As a result, it is always a strong band in graphene, and it is used to determine the number of layers of the graphene. The 2D band is caused by the second-order zone boundary phonons and it is very sensitive to the number of layers. The G-band has been used as a characteristic band for ordered graphite carbon sheets [71]. The intensity ratio of the D band to the G band ID/IG is used to characterize the structural quality of graphitic materials [60].
As reported in Table 2, Raman spectra give an ID/IG ratio of 0.13–0.55. Compared to the Raman spectra in reduced graphene oxide, arc discharge graphene exhibited the lower D band and sharper 2D band, which demonstrated the higher quality. Defects generated in graphene can be either zero-dimensional (0D), such as vacancies or dopants or one-dimensional (1D) defects, such as dislocations or crystallite borders and wrinkles [60,72]. In addition, structural amorphization induced by fast cooling rates in the arc could lead to horn-shaped sheath aggregate of graphene down to more complex structures.

Table 2.
Raman intensity ratios and in plane crystallite size.
At low copper contents in the vaporized anode, when the current was increased from 120 A (GrCu1) to 150 A (GrCu1’), the ID/IG ratio decreased by a factor of 20% from 0.34 to 0.27. The same conclusion could be drawn for higher copper contents, when the current was increased from 120 A (GrCu2) to 150 A (GrCu2’), the ID/IG ratio decreased by a factor of 45% from 0.55 to 0.3. This is probably due to faster graphene growth rates at higher current preventing defect formation. By almost doubling the amount of copper in vaporized anode, we increased the ID/IG ratio by a factor of 62% at 120 A. This may result from copper clusters/atoms attached to the graphene sheet during the growth inducing additional defects. Indeed, copper’s first ionization potential (7.72 eV) was low as compared to carbon (11.26 eV) and helium (24.58 eV). Increasing copper ions might radically change space charge, potential and electric field in the interelectrode space. At 150 A, the extent of the defects brought by an increase in the quantity of copper in the anode was reduced since the ID/IG ratio increased by only a factor of 11% when doubling the quantity of copper in the anode. Unlike copper, the zinc oxide filler did not seem to induce additional defects. On the contrary, the ID/IG ratio was improved for GrZnO as compared to the Gr experiment at the same current of 120 A. Probably since zinc’s low first ionization potential (9.39 eV) was higher than for copper, the discharge was hence less affected by ZnO addition. In Table 2, the I2D/IG ratio obtained from the Raman spectra provides information on the number of layers in the graphene sheets [73]. The ratio I2D/IG < 1 for multilayer graphene and I2D/IG > 1 for monolayer graphene [41]. By combining data from Table 1 and Table 2 for I = 120 A, we can clearly see that increasing atom% of Cu or ZnO decreased I2D/IG hence changing graphene flakes from few-layer to multilayer. One of the reasons could be the quantity of added material, even a few atom%, represents up to 20 wt %. This inevitably affects the energy and mass balance in the arc, thereby changing species and temperature distribution and graphene growth mechanism as well. In our samples, 0.36 < I2D/IG < 0.88 thus corresponding graphene had between 4 and 5 layers as discussed by Wu et al. [57]. According to the literature, the intensity ratio of the 2D peak to G peak, I2D/IG, decreases with the layer number in graphene [73]. In the case of I2D/IG less than one, the graphene is multilayer [74]. The calculated full width at half maximum (FWHM) of peak 2D in our samples was between 30.3 and 53.0 cm−1. Combining these features with the high-intensity peak G, the relatively weak peak D and the sharp and near-symmetric peak 2D, we could conclude that a reasonable quality of few-layer graphene was achieved. In addition, the ID/IG ratio obtained from Raman spectra could be used to estimate crystallite sizes. Indeed, since most of the potential applications of graphene are dependent on a large area sample production, a theoretical model supported by experimental results was proposed to correlate the IG/ID ratio between the C–C stretching (G band) and the defect-induced (D band) modes with the crystallite sizes only for samples with sizes larger than the phonon coherence length, which is found equal to 32 nm [75]. Hence, Raman spectroscopy could be proposed as a quick technique to measure the in plane crystallite size (La) of nanostructured graphitic samples using Equation (1) where λ is the laser excitation in (nm) [76].
La(nm)= (2.4 × 10−10) λ4 (ID/IG) −1,
By applying Equation (1) to the Gr sample, we estimated the in plane crystallite size (La) of nanostructured graphitic at 124 nm, which is confirmed by the size of graphene sheet in HRTEM image of Figure 3. It should be noted that this comparison only gives access to an order of magnitude of the sizes of the crystallites. Exact validation of crystallite sizes La obtained from Raman at the micron scale would require further study at HRTEM to determine the average size of non-defective domains. Adding copper to the graphite at 120 A, reduced crystallite sizes by a factor of 9% (from 124 to 113 nm at low copper charge) and by a factor of 43% (from 124 to 70 nm at higher copper charge). On the other hand, the presence of ZnO as filler seemed to improve the size of graphene crystallites by a factor of 138% (from 124 to 296 nm). As reported by Huang et al. [61] catalytic role of ZnO to promote graphene’s growth is not excluded.
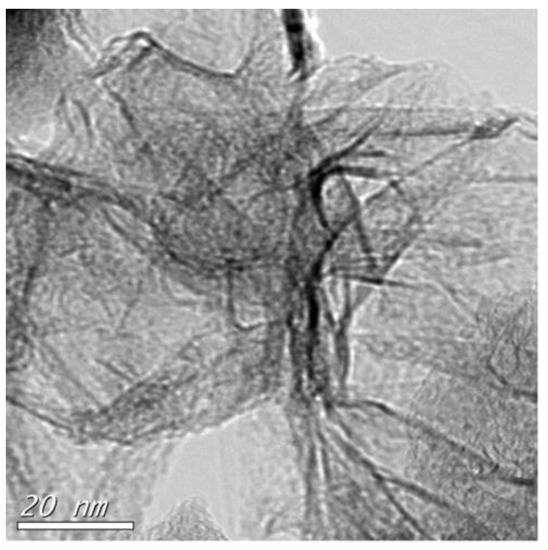
Figure 3.
TEM image of multilayered graphene sheets in (Gr) sample. Produced by DC arc-discharge (scale bar 20 nm).
When copper is added as a filler, spherical copper nanoparticles with a predominant diameter of 20–60 nm and mostly encapsulated in carbon graphitic shells were present in the product.
Figure 4a shows typical dark-field HRTEM image characterization of the Cu nanoparticles in (GrCu2) sample with an average diameter of 53 nm. The low carbon solubility of copper of 0.008 wt % at 1084 °C [77] leads to a carbon shells capping the copper nanoparticle core. From the high-magnification view of Figure 4b, the number of layers was estimated to be four layers.
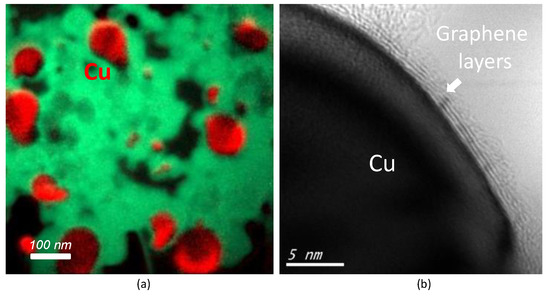
Figure 4.
HRTEM characterization of the Cu nanoparticles in the GrCu2 sample: (a) elemental mapping of copper (red)-scale bar 100 nm and (b) HRTEM image of Cu nanoparticles mostly encapsulated in carbon graphitic shells—scale bar 5 nm.
To verify that no oxide phases were formed, Raman spectra were recorded for the GrCu1 sample exposed in ambient air at room temperature for several months. From Figure 5 we can notice the absence of characteristic Cu2O and CuO Raman peaks in the range 150–650 cm−1, attesting of long time and efficient graphene protection of copper nanoparticles against oxidation.
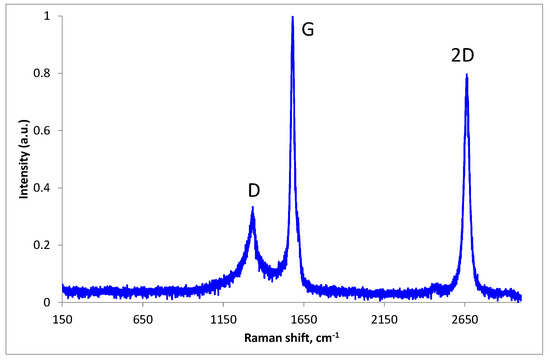
Figure 5.
Raman spectra of the GrCu1 sample exposed in ambient air at room temperature for several months.
For zinc oxide preparation, zinc acetate dihydrate (Zn(OAc)2·2H2O), sodium hydroxide and diethylene glycol DEG (O(CH2CH2OH)2) was mixed with distilled water in appropriate concentrations. The mixture was then heated under reflux at a specified temperature for 1 h. After hydrolysis, a white precipitate, identified as zinc oxide nanorods by X-ray diffraction, was centrifuged, washed several times with ethanol and acetone and dried at 60 °C. The size and morphology of the particles were controlled by adjusting the zinc concentration, the hydrolysis ratio and the basicity ratio in our pilot-scale reactor. As shown in the HRTEM of Figure 6a, these nanorods are of excellent structural crystallinity. This is better perceived from high-magnification view in Figure 6b where the growth of the individual single-crystalline Würtzite-type ZnO occurred in the [001] direction as confirmed by the selected-area electron diffraction selected-area electron diffraction (SAED) pattern (inset of Figure 6b). Detailed description of ZnO nanoparticles synthesis by polyol process could be found in our previous works [25].
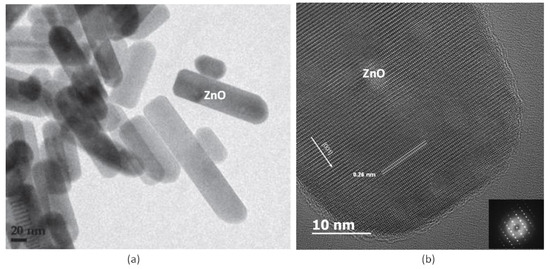
Figure 6.
High resolution TEM pictures showing the NR35 ZnO nanorods used as a filler in the anode for GrZnO synthesis: (a) low magnification—scale bar 20 nm and (b) high magnification—scale bar 10 nm. The lattice spacing of 0.26 nm corresponds to the ZnO (0001) plane. Inset of Figure 6b is the corresponding selected-area electron diffraction (SAED) pattern.
When ZnO is added as a filler in the anode, a bright-field TEM image of Figure 7a shows the presence of shaped nanoparticles. Performing spatially resolved electron energy loss spectroscopy (EELS) and energy filtered elemental mapping on the same area revealed the presence of oxygen (red part of the mapping of Figure 7b). The oxygen K-edge is represented in Figure 7c and corresponded to oxygen around 540 eV. Since no oxygen was added in the discharge, the scattering at the vicinity of the oxygen atoms was attributed to ZnO clusters. From TEM images of Figure 6 and Figure 7a we can notice that the nanorod shape of the ZnO used as a filler was not conserved and their average size was reduced to 40 nm. Their smaller size and strong and short-range dipole–dipole interaction led inevitably to agglomeration.
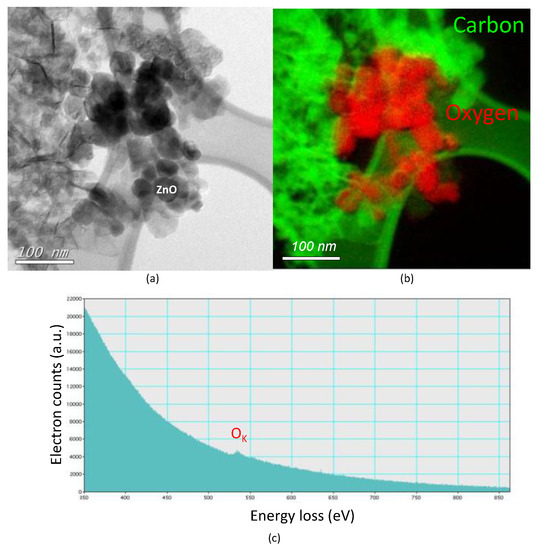
Figure 7.
Characterization of the GrZnO sample: (a) bright-field TEM image—scale bar 100 nm; (b) dark-field TEM image for the same area as in (a) with electron energy loss spectroscopy (EELS) spectral imaging showing the presence of carbon (green) and oxygen (red) and (c) representative EELS profile.
To further confirm the crystallographic structure, X-ray diffraction was used. The representative XRD patterns of Gr, GrCu and GrZnO samples are shown in Figure 8 and Figure 9 where most peaks were identified indicating a good degree of purity that verify the crystallinity of our products.
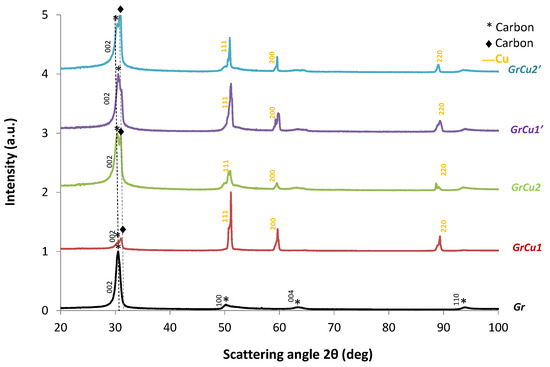
Figure 8.
X-ray diffraction patterns of Gr and GrCu samples synthesized following the experimental conditions of Table 1, taken with Cobalt-Kα radiation (λ = 1.7889 Å).
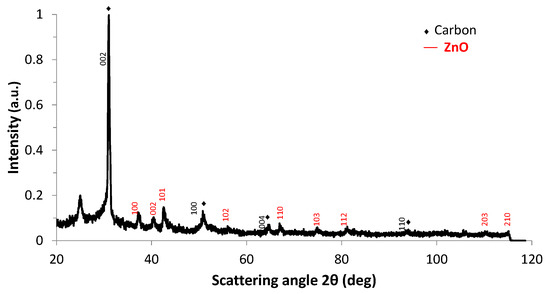
Figure 9.
X-ray diffraction pattern of GrZnO sample obtained using Cobalt-Kα radiation (λ = 1.7889 Å).
As shown in Figure 8, XRD peaks of the Gr sample were centered at 2θ of 30.5° corresponding to the (002) crystal graphite plane with interlayer spacing of 0.34 nm. Secondary reflections from (Gr) sample, corresponded to 2θ of 50.3°, 63.4° and 93.8° that could be indexed to (100), (004) and (110) crystal planes of graphite respectively as reported in the Joint Committee on Powder Diffraction Standards database (JCPDS-01-075-1621 labeled by the star symbol * in Figure 8). When copper was added in the anode, a secondary peak centered at 2θ of 31.0° corresponding to (002) crystal graphite plane appeared in GrCu samples (JCPDS-04-016-6288 labeled by the diamond symbol ♦ in Figure 8). The relative intensities of these peaks varied with the copper content in the anode as well as with the applied current. This could be attributed to graphitic distorted microstructures induced by the presence of copper, including disordered stacking of graphene layers, increased interlayer spacing at the edges of flakes, curved graphene layers on the copper nanoparticles and other structural defects [78]. Similar microstructural variation on the X-ray diffraction intensities was discussed by Li et al. [7], during ball milling of hexagonal graphite (h-graphite) and formation of turbostratic carbon (t-carbon). In Figure 8, peaks observed for GrCu samples at 2θ values of 50.9°, 59.53° and 88.19° corresponded to (111), (200) and (220) planes of metallic Cu respectively. These peaks were consistent with those of the standard spectrum of the pure fcc (face centered cubic) of metallic copper (JCPDS-04-0009-2090). We can notice the absence of diffraction peaks from copper oxide as CuO or Cu2O attesting that these nanoparticles were oxygen free and perfectly protected against oxidation by the graphene layer covering them. Using the Rietveld refinement, we analyzed the structural evolution of samples with varying current and copper concentration in the anode. We refined the structure of compounds using the Maud program [79] and we found the unit cell parameters and crystallite size reported in Table 3. For all graphene copper hybrids obtained in GrCu samples, the lattice constant confirmed the cubic structure of copper characterized by a lattice parameter a = 3.6035 Å (JCPDS-04-0009-2090). At low current of 120 A, the crystallite size slightly decreased from 53.5 (GrCu1) to 48.9 nm (GrCu2) with the increase of atom % of Cu. Increasing the current to 150 A, decreased the crystallite size by a factor of 60% from 53.5 (GrCu1) to 21.6 nm (GrCu1’) for low atom % of Cu. This effect was less spectacular at higher atom % of Cu in the anode since the crystallite size decreased only by a factor of 12% from 48.9 (GrCu2) to 42.9 nm (GrCu2’). For enhanced functionality of copper hybrids, it is possible to tune nanoparticle size by adjusting the appropriate arc parameters. Smaller nanoparticles were obtained at higher current and lower atom % of Cu. Additional experiments within Taguchi factorial designs are needed for signal-to-noise ratio estimation in the measurements.

Table 3.
Copper and zinc oxide peak positions, lattice parameters and crystallite sizes obtained from X-ray diffraction patterns of graphene hybrids GrCu and GrZnO.
In Figure 9, XRD peaks of (GrZnO) sample were centered at 2θ of 30.5° corresponding to the (002) crystal graphite plane with interlayer spacing of 0.34 nm (JCPDS-01-075-1621 labeled ♦ in Figure 9). The peaks observed at 2θ values of 37.2°, 40.4° and 42.6° corresponded to (100), (002) and (101) planes of ZnO respectively. These peaks matched well with the Würtzite hexagonal zinc oxide structure characterized by lattice parameters a = 3.25 Å and c = 5.2 Å (JCPDS-04-013-6608). The data of Table 3 were consistent with the literature reviewed data [80,81]. Using the Rietveld refinement, the crystallite size of ZnO in the hybrid was 42.3 nm confirming HRTEM observations. In Figure 9, the peak at 2θ~25.2° was observed in fullerene-reduced graphene oxides hybrids C60/rGO [A13] and in C9O2, (JCPDS-01-075-1621). This peak could eventually be attributed to graphene oxides structures formed when ZnO is added.
Extraordinary structures and properties of graphene with remarkable chemical and physical properties allow a broad range of promising applications and the arc-discharge remains the easiest and cheapest technique to obtain significant quantities of graphene and graphene hybrids. We can also extend the field of arc-discharge synthesis to boron and/or nitrogen doped graphene by changing either the gaseous atmosphere or the composition of the anode. Additionally, the potential for manufacturing hybrid materials is, largely under-exploited. Elements, either metallic (Cu, Ag and Au) or semiconductors (ZnO, TiO2, WO3 and SnO2) could be associated with graphene and fabricated by arc discharge. Coinage metals as copper, silver and gold with their d-orbital almost saturated leads to a low solubility of the carbon and hence are of particular interest to grow graphene. A reproduction of the Hummer method could also be envisaged for mass production of graphene oxide by using for example KMnO4 as a filler in the anode. Regarding the other 2D graphene analogues, in a band gap scale between 0 (Graphene) and 6 eV (h-BN), we found among others, transition metal dichalcogenides (TMDC) such as WS2, MoS2, WSe2 and MoSe2 as well as black phosphorus. The perspective to use the arc process to produce such materials was already mentioned [82]. The synthesis of these materials by arc discharge however raises a certain number of scientific and technical difficulties, as their weak electrical conductivity limiting material erosion from the anode and plasma creation. However, they can be mixed with graphite to obtain hybrid structures composed of 2D materials associated with graphene.
Nevertheless, even if the products of the arc are of excellent crystallinity, the selectivity is reputed to be poor. This is mainly due to the complex chemical mixture of species and spatiotemporal variations of process parameters that can change from one experiment to the other. Temperature in the arc-discharge reactors varied from 10,000 K down to few hundreds K in a millisecond, leading to extremely high quench rates and high levels of super cooled and supersaturated vapors. Experiences provided reproducible results thus demonstrating the method’s reproducibility within no more than 10% standard deviation. Extreme conditions with three dimensional transient flows and steep spatial gradients of temperature and concentrations, makes it difficult to selectively control the number of layers. Selective syntheses of a monolayer graphene are still also a real challenge. Overlapping and aggregation, together with other carbonaceous byproducts brings additional difficulties. There is a real need to develop models for quantitative understanding of thermochemical processes under specific graphene synthesis conditions.
4. Modeling
The arc vaporizes graphite anode containing copper or zinc oxide in a background gas of helium. This vapor flowing from a sufficiently narrow arc gap can be idealized as a turbulent jet subjected to chemical reactions as well as heat and mass transfer in the reactor chamber [52]. These transfer phenomena control the dynamics of carbon vapor mixing with helium gas and the resulting cooling. In order to estimate the temperature profiles and species distribution in the plasma arc reactor under our typical conditions of graphene synthesis, a two-dimensional CFD modeling was performed by the commercial software ANSYS Fluent (version 15.0) [83]. Thermochemical and transport properties of the gas species as a function of temperature have been taken from Chemkin thermodynamic database [84,85,86] and NASA Reference publication [87]. The model uses the finite volume method to solve the governing equations, i.e., conservation of total mass, momentum and energy, and the individual species conservation equations. The 2-D axisymmetric computational domain, presented in Figure 10 was restricted to a limited part of the reactor including the plasma zone. The chosen dimensions referred to the experimental setup. The distance between the anode and the cathode was fixed at 3 mm. The geometry was created using ANSYS Design Modeler and the mesh was generated using ANSYS Meshing application. The grid was composed of an unstructured quadrilateral mesh. The total number of cells was 8166 leading to a final grid with 7975 nodes, an average grid skewness of 0.2 and an orthogonal quality of 0.998. The very fine grid of the plasma zone was chosen to compute correctly the gradients of all transport variables between the two electrodes.
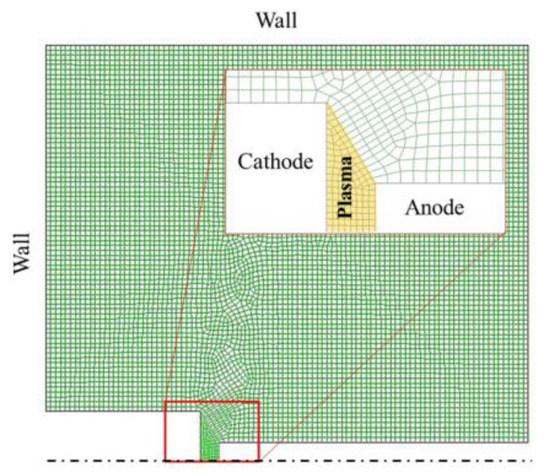
Figure 10.
Computational domain and grid with a zoom of the gap region.
The model was developed based on the following assumptions:
(i) The plasma was modeled using a steady state formulation. This assumption is justified by the continuous adjustment of the interelectrode gap, leading to a constant anode erosion rate.
(ii) The plasma is assumed in local thermodynamic equilibrium (LTE). This hypothesis was based on the model of Bilodeau et al. [88] for fullerene synthesis by arc discharge in the same range of pressure as the graphene synthesis. Indeed, the arc plasma deviates from carbon LTE, but the abundance of carbon species in the arc region increases the collision frequency of carbons. This increases the electrical conductivity of the gas and reduces deviations from LTE.
There are hundreds of species possible, ranging from atomic carbon to large clusters of carbonaceous soot Cn. In the case of a circular graphene sheet of 124 nm diameter as found in our experiments, their surface area is 12,000 nm2. Considering the C–C bond length of d = 1.421 Å, the area of an individual hexagon of the honeycomb is 0.369 nm2. Since each hexagon in the lattice contains 2 full atoms (6 atoms with a third of each inside the hexagon), the monolayer graphene sheets will have a stoichiometry C16,400. Due to the significant computer time required for models containing large numbers of species used in computational fluid dynamic (CFD) simulations, large models are impractical for simulating. Hence, for the simulation of the graphene condition, eleven neutral carbon species (from atomic carbon C to the cluster C11) were involved in the gas-phase chemistry. The ions and electrons were not considered. This assumption affected the accuracy of the calculations. However, an effort was made here to only estimate the plasma temperature and major gas species concentrations. The considered gas-phase reversible reactions between the carbon species and rate coefficients are given in Table 4 from Krestinin et al. [89].

Table 4.
Gas phase chemistry used for graphene simulation.
Helium (He) was used as inert gas and we assumed a dilution factor of τ = 20. This factor accounts for the mixing of carbon coming from the anode erosion with the inert atmosphere. It is defined by the ratio:
For initial and boundary conditions, the inlet was specified as a uniform inflow with axial velocity estimated on the basis of the measured anode erosion rate. The inlet temperature at the anode was fixed at 4000 K—the temperature of vaporization of the graphite. The water-cooled reactor walls, the anode and the cathode were modeled as wall boundary conditions at a constant temperature of 600 K for the reactor walls and 1000 K for the electrodes. The plasma heat was generated by constant volumetric source dependent on the input power and the plasma volume. The power density was homogeneously distributed inside the active plasma zone showed in Figure 11. The radiative losses were neglected. The total pressure was fixed at 530 mbar. Turbulence was modeled using the k-ε turbulence approach. The simple method for pressure–velocity coupling was selected. Simulations were carried out for two different current intensities, 120 A and 150 A. The 2-D calculated temperature contours of arc discharge plasma and 1-D temperature profiles along the centerline of plasma in the interelectrode gap are shown in Figure 11.
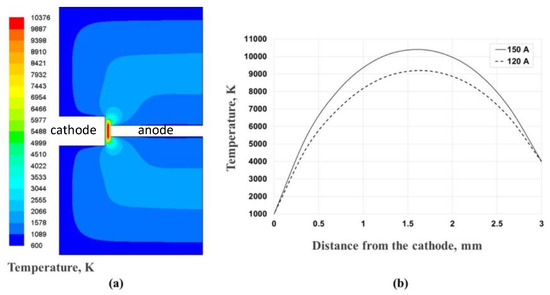
Figure 11.
(a) Simulated temperature distribution inside the reactor at 150 A and (b) one dimensional (1D) temperature profiles along the centerline between the anode and the cathode at 120 A (dashed line) and 150 A (continuous line).
It can be seen that the maximal temperature in the center of the plasma reaches 9200 K at 120 A and 10,400 K at 150 A. These values are in the same order of magnitude of measurements of the optical emission spectra obtained in our setup for various combinations of gap width, position in the gap, radius, arc current and gas pressure. The thermal balance of plasma heating and cathode cooling determines the cathode temperature. Hence, there is a steep temperature gradient between the cathode and the region where the temperature is the highest. The flow and trajectories of gas species are visualized on Figure 12 by using the velocity path lines. The maximum velocity is 4 m/s, which is sufficient to ensure a fully turbulent jet in the narrow arc gap. The presence of vortices around the electrodes is supposed to control the dynamics of carbon vapor mixing with helium gas and the resulting cooling thereby increasing the mass flux gas species from the plasma zone to the cold cathode region.
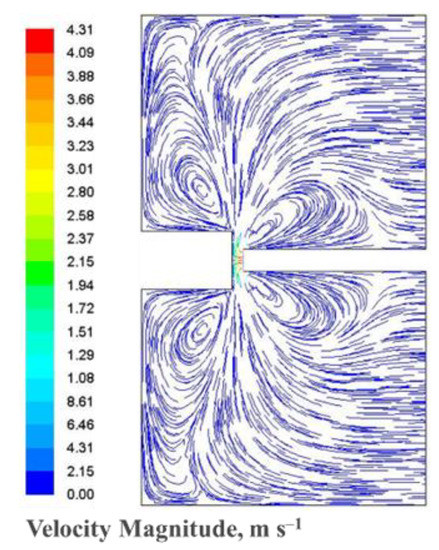
Figure 12.
Calculated velocity path lines in the reactor at 150 A.
The simulated molar fraction profiles of small carbon clusters C–C3 and higher mass carbon clusters C9–C11 at 150 A are presented in Figure 13. This figure shows that atomic C was the major carbon specie in the plasma. Other species such as C2 and C3 had also a rather important contribution to the graphene synthesis. They were formed on the front face of the cathode as well as in the region close to the plasma. Higher mass carbon clusters such as C9, C10 and C11 were relegated to the cold walls of the reactor (see Figure 13) where graphene was expected to form. Similar profiles were also found for a current of 120 A.
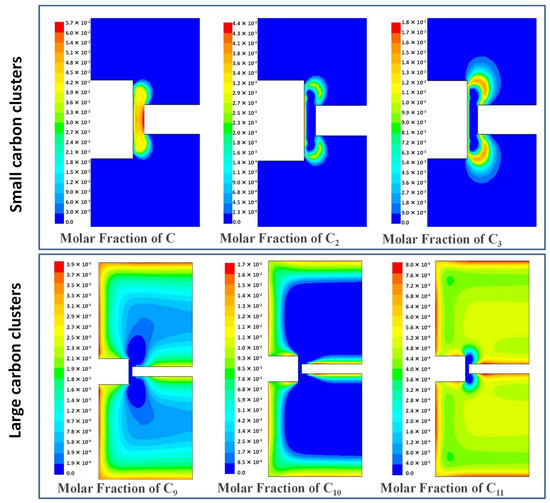
Figure 13.
Simulated mole fractions of small carbon clusters C–C3 and higher mass carbon cluster C9–C11 at 150 A.
Finally, the plasma composition during the growth of graphene and graphene hybrids was predicted assuming chemical equilibrium calculations. Computation of equilibrium compositions was determined by minimizing the Gibbs free energy of each chemical system using the Ivtanthermo computer code [90]. These calculations constitutes a first approximation since they did not take into account neither homogeneous chemistry nor transport of the species. In our case, the plasma was modeled by a mixture of graphite and Cu or ZnO evaporated from the anode and mixed with He present in the buffer gas. The neutral and ions species considered for each system are listed in Table 5 and the chemical composition in the plasma was estimated under the assumption of local thermal equilibrium as discussed above.

Table 5.
Neutral and ion species considered in equilibrium calculations.
The calculated mole fractions of neutral and ions corresponding to graphene (Gr) condition are plotted in Figure 14. Due to the high temperature that exists throughout most of the gap, the discharge was dominated by helium and C atoms with the major ion C+ balanced by e−. The lower temperature (close to the cathode) enhanced the carbon species recombination until up to C5.
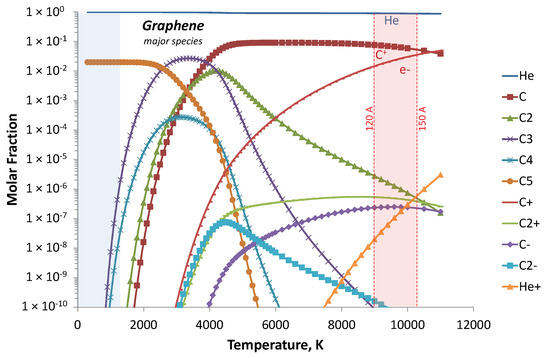
Figure 14.
Calculated equilibrium species in the interelectrode gap (red zone) and cold cathode (blue zone) at a total pressure of 530 mbar.
Adding copper slightly modified species distribution with the appearance of copper atoms in the hot zone as shown in Figure 15a. The condensation of these vapors in cold regions led to C5 and Cu2 clusters that could serve as precursors for the growth of graphene hybrid (GrCu). We can notice from Figure 15b the apparition of Cu+ ion together with C+, which was the major ion in the gap.
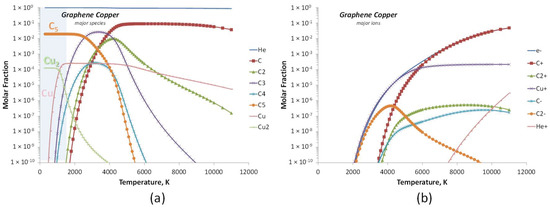
Figure 15.
Calculated equilibrium species for GrCu conditions at a total pressure of 530 mbar: (a) major species with the cold cathode region schematized in the blue area and (b) major ions.
When zinc oxide is added, the hot zone of the discharge became dominated by He, C and O atoms as shown in Figure 16a. The condensation of these vapors in cold regions led to C5 and Zn clusters together with carbon monoxide CO and no more O atoms. We could finally notice from Figure 16b the apparition of Zn+ ion together with C+, which was the major ion in the gap. Based on our equilibrium calculations, and since no zinc phases were obtained in XRD patterns, the transformation of atomic zinc into ZnO could not be explained by classical thermodynamics.
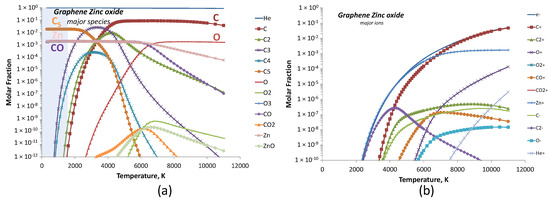
Figure 16.
Calculated equilibrium species for GrZnO conditions at a total pressure of 530 mbar: (a) major species with the cold cathode region schematized in blue area and (b) major ions.
Nevertheless, the presence of zinc oxide in the soot and the changes in their size and shape as observed in HRTEM could be explained by the spallation of the original nanorods into smaller fragments that were expelled from the body of the anode and survived to the plasma. Since the anode surface was subjected to a high temperature gradient that could induce high pressures with a tensile stress wave exceeding the local tensile strength of the anode material.
5. Conclusions
In conclusion, a one-step arc-discharge process was proposed to synthesize graphene, copper and zinc oxide graphene hybrids. The relatively weak Raman peak D and the sharp and near-symmetric peak 2D attested that a reasonable quality of few-layer graphene was achieved. The temperature in the gap region was estimated from our numerical simulations to 10,000 K. This high temperature ensures vaporization of the anode and graphene, where 124 nm crystallite sizes are thought to precipitate from carbon vapor during fast cooling. Adding copper to the graphite composing the anode material reduced the graphene crystallite size down to 70 nm and induced defects. High resolution, spatially resolved EELS and energy filtered elemental mapping together with XRD confirmed that GrCu hybrids were composed of spherical copper nanoparticles, free of any copper oxide. Copper nanoparticles with a predominant diameter of 53 nm and mostly encapsulated in carbon graphitic shells were stable even after their exposition in ambient air at room temperature for several months. Unlike copper, zinc oxide nanorods used as a filler did not seem to induce additional defects and acted as a catalyst to promote the graphene crystallite size up to 296 nm as compared to the Gr experiment. Nevertheless, their small size and strong and short-range dipole–dipole interaction led inevitably to agglomeration.
Due to the difficulties associated with in situ measurements during the arc discharge operation, modeling remains a key element in the effort to improve product quality. Our modeling gave a better understanding of the behavior of the species distribution. It is especially important to understand how the species behaves upon vaporization from the anode down to the cool regions of the reactor. We limited the carbon atoms in the model to C11, whereas, this is much fewer than the actual graphene sheet stoichiometry estimated at C16,400. The calculated flow and trajectories attested a fully turbulent jet in the narrow arc gap. The presence of vortices around the electrodes controlled the dynamics of carbon vapor mixing with helium gas thereby increasing the mass flux gas species from the plasma zone to the cold cathode region. Small carbon clusters, e.g., C2 are envisioned to form higher carbonaceous species that would tend to grow fragments of graphene. Since complete fluid dynamics in three dimensions plus large models presents a formidable computational task, it may be possible to improve the actual chemical modeling by lumping many clusters into a representative clusters Cn and consider their interaction with small carbon fragments Cm (m = 1,2,..,) via reactions Cn+Cm→Gr.
Author Contributions
Conceptualization, S.F. and I.H.; software, I.H.; investigation, A.K.; data curation, A.K.; writing—original draft preparation, A.K.; writing—review and editing, All authors; visualization, O.B.; M.H. and A.K.; supervision, S.F.; S.M.C. and A.H.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANR (Agence Nationale de la Recherche) grant numbers ANR 11 IDEX 05 02, and ANR-14CE08-0018.
Acknowledgments
ANR (Agence Nationale de la Recherche), and CGI (Commissariat à l’Investissement d’Avenir) are gratefully acknowledged for their financial support of this work through Labex SEAM (Science and Engineering for Advanced Materials and devices) ANR 11 LABX 086, ANR 11 IDEX 05 02, and ANR-14CE08-0018. Embassy of France in Nouakchott, Mauritania and University of Nouakchott AL-Assriya are gratefully acknowledged for funding through international mobility fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Iakunkov, A.; Skrypnychuk, V.; Nordenström, A.; Shilayev, E.A.; Korobov, M.; Prodana, M.; Enachescu, M.; Larsson, S.H.; Talyzin, A.V. Activated graphene as a material for supercapacitor electrodes: Effects of surface area, pore size distribution and hydrophilicity. Phys. Chem. Chem. Phys. 2019, 21, 17901–17912. [Google Scholar] [CrossRef] [PubMed]
- Banszerus, L.; Sohier, T.; Epping, A.; Winkler, F.; Libisch, F.; Haupt, F.; Watanabe, K.; Taniguchi, T.; Müller-Caspary, K.; Marzari, N.; et al. Extraordinary high room-temperature carrier mobility in graphene-WSe2 heterostructures. arXiv 2019, arXiv:1909.09523. [Google Scholar]
- Kim, S.H.; Song, W.; Jung, M.W.; Kang, M.-A.; Kim, K.; Chang, S.-J.; Lee, S.S.; Lim, J.; Hwang, J.; Myung, S.; et al. Carbon Nanotube and graphene hybrid thin film for transparent electrodes and field effect transistors. Adv. Mater. 2014, 26, 4247–4252. [Google Scholar] [CrossRef]
- Badhulika, S.; Thakoor, T.T.; Villarreal, C.; Mulchandani, A. Graphene hybrids: Synthesis strategies and applications in sensors and sensitized solar cells. Front. Chem. 2015, 3, 38. [Google Scholar] [CrossRef]
- Hidalgo-Manrique, P.; Lei, X.; Xu, R.; Zhou, M.; Kinloch, I.A.; Young, R.J. Copper/graphene composites: A review. J. Mater. Sci. 2019, 54, 12236–12289. [Google Scholar] [CrossRef]
- Xiao, Q.; Yi, X.; Jiang, B.; Qin, Z.; Hu, J.; Jiang, Y.; Liu, H.; Wang, B.; Yi, D. In-situ synthesis of graphene on surface of copper powder by rotary CVD and its application in fabrication of reinforced Cu-matrix composites. Adv. Mater. Sci. 2017, 2, 5–6. [Google Scholar] [CrossRef]
- Cseri, L.; Baugh, J.; Alabi, A.; AlHajaj, A.; Zou, L.; Dryfe, R.A.W.; Budd, P.M.; Szekely, G. Graphene oxide-polybenzimidazolium nanocomposite anion exchange membranes for electrodialysis. J. Mater. Chem. A 2018, 6, 24728. [Google Scholar] [CrossRef]
- Kweon, H.; Lin, C.W.; Hasan, M.M.F.; Kaner, R.; Sant, G.N. Highly permeable polyaniline-graphene oxide nanocomposite membranes for CO2 separations. ACS Appl. Polym. Mater. 2019, 1, 12. [Google Scholar] [CrossRef]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust covalently crosslinked polybenzimidazole/graphene oxide membranes for high-flux organic solvent nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Vallés, C.; Zhang, X.; Cao, J.; Lin, F.; Young, R.J.; Lombardo, A.; Ferrari, A.C.; Burk, L.; Rolf Mülhaupt, R.; Kinloch, I.A. Graphene/polyelectrolyte layer-by-layer coatings for electromagnetic interference shielding. ACS Appl. Nano Mater. 2019, 2, 5272–5281. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, J.; Yun, X.; Zhou, W.; Xi, L.; Li, N.; Hu, Z. Hydrothermal synthesis of nanoflakes-assembled (Ni0.5Co0.5)0.85Se microspheres as cathode and reduced graphene oxide/porous Fe2O3 nanospheres composite as anode for novel alkaline aqueous batteries. ACS Sustain. Chem. Eng. 2020, 8, 561–572. [Google Scholar] [CrossRef]
- Kong, W.; Liu, F.; Liu, Y. Design of nitrogen-doped graphitized 2D hierarchical porous carbons as efficient solid base catalysts for transesterification to biodiesel. Green Chem. 2020, 22, 903–912. [Google Scholar] [CrossRef]
- Prasad, K.P.; Chen, Y.; Chen, P. Three-dimensional graphene-carbon nanotube hybrid for high-performance enzymatic biofuel cells. ACS Appl. Mater. Interfaces 2014, 6, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, X.; Wang, L.; Song, H.; Zhang, H.; Huang, W.; Chen, P. 3D Graphene foam as a monolithic and macroporous carbon electrode for electrochemical sensing. ACS Appl. Mater. Interfaces 2012, 4, 3129–3133. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Subramani, B. Synthesis of zinc oxide nanoparticles on graphene-carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef]
- Li, Y.F.; Dong, F.X.; Chen, Y.; Zhang, X.L.; Wang, L.; Bi, Y.G.; Tian, Z.N.; Liu, Y.F.; Feng, J.; Sun, H.B. As-grown graphene/copper nanoparticles hybrid nanostructures for enhanced intensity and stability of surface plasmon resonance. Sci. Rep. 2016, 6, 37190. [Google Scholar] [CrossRef]
- Cho, B.; Yoonb, J.; Hahm, M.G.; Kim, D.H.; Kim, A.R.; Kahng, Y.H.; Park, S.W.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; et al. Graphene-based gas sensor: Metal decoration effect and application to a flexible device. J. Mater. Chem. C2 2014, 2, 5280–5285. [Google Scholar] [CrossRef]
- Echtermeyer, T.J.; Britnell, L.; Jasnos, P.K.; Lombardo, A.; Gorbachev, R.V.; Grigorenko, A.N.; Geim, A.K.; Ferrari, A.C.; Novoselov, K.S. Strong plasmonic enhancement of photovoltage in graphene. Nat. Commun. 2011, 2, 458. [Google Scholar] [CrossRef]
- Emani, N.K.; Chung, T.F.; Ni, X.; Kildishev, A.V.; Chen, Y.P.; Boltasseva, A. Electrically tunable damping of plasmonic resonances with graphene. Nano Lett. 2012, 12, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.; Steiner, M.; Lombardo, A.; Ferrari, A.C.; Löhneysen, H.V.; Avouris, P.; Krupke, R. Light-matter interaction in a microcavity-controlled graphene transistor. Nat. Commun. 2012, 3, 906. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Choy, W.C.H.; Ren, X.G.; Zhang, D.; Lu, H.F. Highly intensity surface enhanced raman scattering by usinig monolayer graphene as the nanospacer of metal film-metal nanoparticle coupling system. Adv. Funct. Mater. 2014, 24, 3114–3122. [Google Scholar] [CrossRef]
- Reckinger, N.; Vlad, A.; Melinte, S.; Colomer, J.F.; Sarrazin, M. Graphene-coated holey metal films: Tunable molecular sensing by surface plasmon resonance. Appl. Phys. Lett. 2013, 102, 211108. [Google Scholar] [CrossRef]
- Xu, M.; Feng, J.; Liu, Y.S.; Jin, Y.; Wang, H.Y.; Sun, H.B. Effective and tunable light trapping in bulk heterojunction organic solar cells by employing Au-Ag alloy nanoparticles. Appl. Phys. Lett. 2014, 105, 153303. [Google Scholar] [CrossRef]
- Leem, J.; Wang, M.C.; Kang, P.; Nam, S. Mechanically self-assembled, three-dimensional graphene-gold hybrid nanostructures for advanced nanoplasmonic sensors. Nano Lett. 2015, 15, 7684–7690. [Google Scholar] [CrossRef]
- Xie, H.; Lee, H.Y.; Youn, W.; Choi, M. Nanofluids containing multiwalled carbon nanotubes and their enhanced thermal conductivities. J. Appl. Phys. 2003, 94, 4967–4971. [Google Scholar] [CrossRef]
- Green, M.; Chen, X. Recent progress of nanomaterials for microwave absorption. JMAT 2019, 5, 503–541. [Google Scholar] [CrossRef]
- Hosni, M.; Kusumawati, Y.; Farhat, S.; Jouini, N.; Pauporté, T. Effects of oxide nanoparticle size and shape on electronic structure, charge transport, and recombination in dye-sensitized solar cell photoelectrodes. J. Phys. Chem. C 2013, 118, 16791–16798. [Google Scholar] [CrossRef]
- Liu, J.W.; Wu, J.; Ahmad, M.Z.; Wlodarski, W. Hybrid aligned zinc oxide nanowires array on CVD graphene for hydrogen sensing. In Proceedings of the 2013 Transducers & Eurosensors XXVII: 17th International Conference on Solid State Sensors, Actuators and Microsystems (Transducers & Eurosensors XXVII), Barcelona, Spain, 16–20 June 2013; pp. 194–197. [Google Scholar]
- Yi, J.; Lee, J.M.; Park, W. Vertically aligned ZnO nanorods and graphene hybrid architectures for high-sensitive flexible gas sensors. Sens. Actuators B 2011, 155, 264–269. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.H.; Chung, J.S.; Shin, E.W.; Yoo, D.H.; Hahn, S.H.; Huh, J.S.; Rue, G.H.; Kim, E.J.; Hur, S.H.; et al. Solution-processed ZnO-chemically converted graphene gas sensor. Mater. Lett. 2010, 64, 2479–2482. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Fabrication of WO3 nanorods on graphene nanosheets for improved visible light-induced photocapacitive and photocatalytic performance. RSC Adv. 2016, 6, 20824–20833. [Google Scholar] [CrossRef]
- Mohammada, A.; Khan, M.E.; Karima, M.R.; Choa, M.H. Synergistically effective and highly visible light responsive SnO2-g-C3N4 nanostructures for improved photocatalytic and photoelectrochemical performance. Appl. Surf. Sci. 2019, 495, 143432. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, J.R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Defected graphene nano-platelets for enhanced hydrophilic nature and visible light-induced photoelectrochemical performances. J. Phys. Chem. Solids 2017, 104, 233–242. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Kim, E.; An, H.; Jang, H.; Cho, W.J.; Lee, N.; Lee, W.G.; Jung, J. Growth of few-layer graphene on a thin cobalt film on a Si/SiO2 substrate. Chem. Vap. Depos. 2011, 17, 9–14. [Google Scholar] [CrossRef]
- Coraux, J.; N’Diaye, T.; Engler, M.; Busse, C.; Wall, D.; Buckanie, N.; Heringdorf, F.J.M.Z.; Gaste, R.V.; Poelsema, B.; Michely, T. Growth of graphene on Ir(111). New J. Phys. 2009, 11, 023006. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Won, S.Y.; Ciobanu, C.V.; Petrova, V.; Shenoy, V.B.; Bareno, J.; Gambin, V.; Petroy, I.; Kodambaka, S. Growth of semiconducting graphene on palladium. Nano Lett. 2009, 9, 3985–3990. [Google Scholar] [CrossRef]
- Fujita, T.; Kobayashi, W.; Oshima, C. Novel structures of carbon layers on a Pt(111) surface. Surf. Interface Anal. 2005, 37, 120–123. [Google Scholar] [CrossRef]
- Mehedi, H.A.; Baudrillart, B.; Alloyeau, D.; Mouhoub, O.; Ricolleau, C.; Pham, V.D.; Chacon, C.; Gicquel, A.; Lagoute, J.; Farhat, S. Synthesis of graphene by cobalt-catalyzed decomposition of methane in plasma-enhanced CVD: Optimization of experimental parameters with Taguchi method. J. Appl. Phys. 2016, 120, 065304. [Google Scholar] [CrossRef]
- Pashova, K.; Hinkov, I.; Aubert, X.; Prasanna, S.; Bénédic, F.; Farhat, S. Graphene synthesis by microwave plasma chemical vapor deposition: Analysis of the emission spectra and modeling. Plasma Sources Sci. Technol. 2019, 28, 045001. [Google Scholar] [CrossRef]
- Bacon, R. Growth, structure, and properties of graphite whiskers. J. Appl. Phys. 1960, 31, 283. [Google Scholar] [CrossRef]
- Wiles, P.G.; Abrahamson, J. Carbon fibre layers on arc electrodes—I: Their properties and cool-down behaviour. Carbon 1978, 16, 341. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Carbon Nanotubes, Preparation and Properties; Ebbesen, T.W., Ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ebbsen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Moravsky, A.P.; Wexler, E.M.; Loutfy, R.O. Growth of carbon nanotubes by arc discharge and laser ablation. In Carbon Nanotubes Science and Applications; Meyyappan, M., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 65–97. [Google Scholar]
- Farhat, S.; Scott, C. Review of the arc process modeling for fullerene and nanotube production. J. Nanosci. Nanotechnol. 2006, 6, 1189–1210. [Google Scholar] [CrossRef]
- Farhat, S.; Lamy de la Chapelle, M.; Loiseau, A.; Scott, C.D.; Lefrant, S.; Journet, C.; Bernier, P. Diameter control of single-walled carbon nanotubes using argon-helium mixture gases. J. Chem. Phys. 2001, 15, 6752–6759. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple method of preparing graphene flakes by an arc-discharge method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Karmakar, S.; Kulkarni, N.V.; Nawale, A.B.; Lalla, N.P.; Mishra, R.; Sathe, V.G.; Bhoraskar, S.V.; Das, A.K. A novel approach towards selective bulk synthesis of few-layer graphenes in an electric arc. J. Phys. D Appl. Phys. 2009, 42, 115201. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Ma, Y.; Huang, Y.; Li, N.; Zhang, F.; Chen, Y. Efficient and large-scale synthesis of few-layered graphene using an arc-discharge method and conductivity studies of the resulting films. Nano Res. 2010, 3, 661–669. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Shi, Z.; Gu, Z. Low-cost and large-scale synthesis of graphene nanosheets by arc discharge in air. Nanotechnology 2010, 21, 175602. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Li, J.; Volotskova, O.; Kundrapu, M.; Zhuang, T.S. Arc plasma synthesis of carbon nanostructures: Where is the frontier? J. Phys. D Appl. Phys. 2011, 44, 174006. [Google Scholar] [CrossRef]
- Shen, B.; Ding, J.; Yan, X.; Feng, W.; Li, J.; Xue, Q. Influence of different buffer gases on synthesis of few-layered graphene by arc discharge method. Appl. Surf. Sci. 2012, 258, 4523–4531. [Google Scholar] [CrossRef]
- Huang, L.; Wu, B.; Chen, J.; Xue, Y.; Geng, D.; Guo, Y.; Yu, G.; Liu, Y. Gram-scale synthesis of graphene sheets by a catalytic arc-discharge method. Small 2013, 9, 1330–1335. [Google Scholar] [CrossRef]
- Karmakar, S.; Nawale, A.B.; Lalla, N.P.; Sathe, V.G.; Kolekar, S.K.; Mathe, V.L.; Das, A.K.; Bhoraskar, S.V. Gas phase condensation of few-layer graphene with rotational stacking faults in an electric-arc. Carbon 2013, 55, 209–220. [Google Scholar] [CrossRef]
- Cotula, U.; Parmaka, E.D.S.; Kaykilarlia, C.; Sarayb, O.; Colakc, O.; Uzunsoya, D. Development of high purity, few-layer graphene synthesis by electric arc discharge technique. Acta Phys. Pol. A 2018, 134, 289–291. [Google Scholar] [CrossRef]
- David, R.L. CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press Inc.: Boca Raton, FL, USA, 2009; p. 2804. [Google Scholar]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Hu, C.Y.; Dong, J.; Shen, W.C.; Zhang, B. Polarization properties, high-order Raman spectra, and frequency asymmetry between Stokes and anti-Stokes scattering of Raman modes in a graphite whisker. Phys. Rev. B 2001, 64, 214301. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Kruger, P. Vibrational properties of CuO and Cu4O3 from first-principles calculations, and Raman and infrared spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Kastner, J.; Pichler, T.; Kuzmany, H.; Curran, S.; Blau, W.; Weldon, D.N.; Delamesiere, M.; Draper, S.; Zandbergen, H. Resonance Raman and infrared spectroscopy of carbon nanotubes. Chem. Phys. Lett. 1994, 221, 53–58. [Google Scholar] [CrossRef]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K.S.; Basko, D.M.; Ferrari, A.C. Raman spectroscopy of graphene edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.S.; Vivekchand, S.R.C.; Govindaraj, A.; Rao, C.N.R. A study of graphenes prepared by different methods: Characterization, properties and solubilization. J. Mater. Chem. 2008, 18, 1517–1523. [Google Scholar] [CrossRef]
- Athanassiou, E.K.; Grass, R.N.; Stark, W.J. Large-scale production of carbon-coated copper nanoparticles for sensor applications. Nanotechnology 2006, 17, 1668–1673. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Speziali, N.L.; Jorio, A. Measuring the degree of stacking order in graphite by Raman spectroscopy. Carbon 2008, 46, 272–275. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Robertson, A.W.; Warner, J.H. Hexagonal single crystal domains of few-layer graphene on copper foils. Nano Lett. 2011, 11, 1182–1189. [Google Scholar] [CrossRef]
- Soares, J.R.; Olivero, M.E.; Garin, C.; David, M.V.; Martins, L.G.P.; Almeida, C.A.; Ferreira, E.H.M.; Takai, K.; Enoki, T.; Paniago, R.M.; et al. Structural analysis of polycrystalline graphene systems by Raman spectroscopy. Carbon 2015, 95, 646. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cancado, L.G.; Jorio, A.; Saitoe, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef] [PubMed]
- Oshima, C.; Nagashima, A. Ultra-thin epitaxial films of graphite and hexagonal boron nitride on solid surfaces. J. Phys. Condens. Matter 1997, 9, 1–20. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Lutterotti, L. Maud: A Rietveld analysis program designed for the internet and experiment integration. Acta Crystallogr. A 2000, 56, s54. [Google Scholar] [CrossRef]
- Sowa, H.; Ahsbahs, H. High-pressure X-ray investigation of zincite ZnO single crystals using diamond anvils with an improved shape. J. Appl. Crystallogr. 2006, 39, 169–175. [Google Scholar] [CrossRef]
- Desgreniers, S. High-density phases of ZnO: Structural and compressive parameters. Phys. Rev. B 1998, 58, 14102–14105. [Google Scholar] [CrossRef]
- Shashurin, A.; Keidar, M. Synthesis of 2D materials in arc plasmas. J. Phys. D Appl. Phys. 2015, 48, 31400. [Google Scholar] [CrossRef]
- ANSYS Fluent User’s Guide, Release 15.0; ANSYS, Inc.: Canonsburg, PA, USA, 2013.
- Kee, R.J.; Rupley, F.M.; Miller, J.A.; Coltrin, M.E.; Grcar, J.F.; Meeks, E.; Moffat, H.K.; Lutz, A.E.; Lewis, G.D.; Smooke, M.D.; et al. CHEMKIN Collection, Release 3.6; Reaction Design, Inc.: San Diego, CA, USA, 2001. [Google Scholar]
- JANAF. Report NSRDS-NBS: Dow Chemikal Company, Clearinghouse for Federal Scientific and Technical Information; PB168370; Springfield: Virginia, VA, USA, 1965.
- Kee, R.J.; Lewis, G.D.; Warnatz, J.; Miller, J.A. Technical Report SAND86-8426; Sandia National Laboratories: Albuquerque, NM, USA, 1986.
- Gupta, R.; Yos, J.; Thompson, R.; Lee, K. A Review of Reaction Rates and Thermodynamic and Transport Properties for 11-Species Air Model for Chemical and Thermal Non-Equilibrium Calculations to 30000 K; NASARP-1232; NASA Reference Publication: Hampton, VA, USA, 1990.
- Bilodeau, J.F.; Pousse, J.; Gleizes, A. A mathematical model of the carbon arc reactor for fullerene synthesis. Plasma Chem. Plasma Process. 1998, 18, 285–303. [Google Scholar] [CrossRef]
- Krestinin, A.V.; Moravskii, A.P.; Tesner, P.A. A kinetic modeling of formation of fullerenes C60 and C70 in condensation of carbon vapor. Chem. Phys. Rep. 1998, 17, 1687–1707. [Google Scholar]
- Gurvich, L.V.; Iorish, V.S. Ivtanthermo—A Thermodynamic Database and Software System for the Personal Computer. User’s Guide; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).