Flame-Retardant Wood Composites Based on Immobilizing with Chitosan/Sodium Phytate/Nano-TiO2-ZnO Coatings via Layer-by-Layer Self-Assembly
Abstract
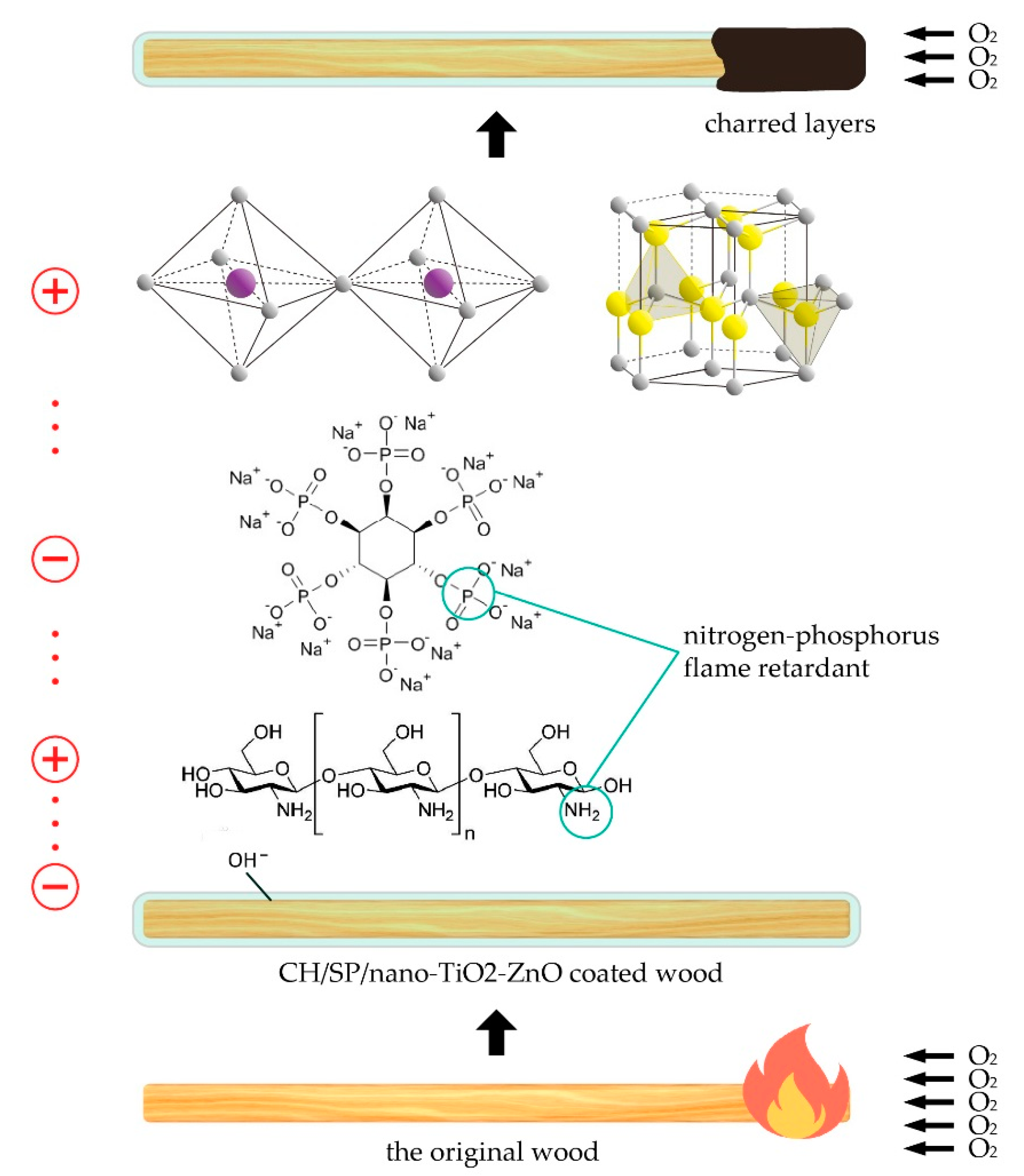
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wood Substrate and Solution Preparation
2.3. Formation of Flame Retardant Coatings on Wood Surfaces via Layer-by-Layer Self-Assembly
2.4. Characterization
2.5. Thermogravimetric Analysis
2.6. Limiting Oxygen Index Test
2.7. Combustion Test
3. Results and Discussion
3.1. Surface Morphologies Analysis
3.2. Thermal Stability and Fire Resistance Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Papadopoulos, A.N.; Taghiyari, H.R. Innovative wood surface treatments based on nanotechnology. Coatings 2019, 9, 866. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Bikiaris, A.N.; Mitropoulos, A.C.; Kyzas, G.Z. Nanomaterials and chemical modifications for enhanced key wood properties: A review. Nanomaterials 2019, 9, 607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, T.; Yan, H.; Peng, M.; Fang, Z. Modification of ramie fabric with a metal-ion-doped flame-retardant coating. J. Appl. Polym. Sci. 2013, 129, 2986–2997. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, Z.; Bai, L. Layer-by-layer assembly of polyelectrolyte and gold nanoparticle for highly reproducible and stable SERS substrate. Appl. Surf. Sci. 2016, 360, 437–441. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Ruch, D. Layer-by-layer deposition of a TiO2-filled intumescent coating and its effect on the flame retardancy of polyamide and polyester fabrics. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 1–10. [Google Scholar] [CrossRef]
- Srivastava, S.; Kotov, N.A. Layer-by-layer (LBL) assembly with semiconductor nanoparticles and nanowires. In Semiconductor Nanocrystal Quantum Dots; Springer: Vienna, Austria, 2008; pp. 197–216. [Google Scholar]
- Malucelli, G. Surface-engineered fire protective coatings for fabrics through sol-gel and layer-by-layer methods: An overview. Coatings 2016, 6, 33. [Google Scholar] [CrossRef]
- Renneckar, S.; Zhou, Y. Nanoscale coatings on wood: Polyelectrolyte adsorption and layer-by-layer assembled film formation. ACS Appl. Mater. Interfaces 2009, 1, 559–566. [Google Scholar] [CrossRef]
- Lozhechnikova, A.; Bellanger, H.; Michen, B.; Burgert, I.; Österberg, M. Surfactant-free carnauba wax dispersion and its use for layer-by-layer assembled protective surface coatings on wood. Appl. Surf. Sci. 2017, 396, 1273–1281. [Google Scholar] [CrossRef]
- Rao, X.; Liu, Y.; Fu, Y.; Liu, Y.; Yu, H. Formation and properties of polyelectrolytes/TiO2 composite coating on wood surfaces through layer-by-layer assembly method. Holzforschung 2016, 70, 361–367. [Google Scholar] [CrossRef]
- Lu, X.; Hu, Y. Layer-by-layer deposition of TiO2 nanoparticles in the wood surface and its superhydrophobic performance. BioResources 2016, 11, 4605–4620. [Google Scholar] [CrossRef]
- Bellanger, H.; Casdorff, K.; Muff, L.F.; Ammann, R.; Burgert, I.; Michen, B. Layer-by-layer deposition on a heterogeneous surface: Effect of sorption kinetics on the growth of polyelectrolyte multilayers. J. Colloid Interface Sci. 2017, 500, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rassam, G.; Abdi, Y.; Abdi, A. Deposition of TiO2 nano-particles on wood surfaces for UV and moisture protection. J. Exp. Nanosci. 2012, 7, 468–476. [Google Scholar] [CrossRef]
- Nine, M.J.; Tran, D.N.H.; Tung, T.T.; Kabiri, S.; Losic, D. Graphene-borate as an efficient fire retardant for cellulosic materials with multiple and synergetic modes of action. ACS Appl. Mater. Interfaces 2017, 9, 10160–10168. [Google Scholar] [CrossRef] [PubMed]
- Nine, M.J.; Tran, D.N.H.; ElMekawy, A.; Losic, D. Interlayer growth of borates for highly adhesive graphene coatings with enhanced abrasion resistance, fire-retardant and antibacterial ability. Carbon 2017, 117, 252–262. [Google Scholar] [CrossRef]
- Holder, K.M.; Huff, M.E.; Cosio, M.N.; Grunlan, J.C. Intumescing multilayer thin film deposited on clay-based nanobrick wall to produce self-extinguishing flame retardant polyurethane. J. Mater. Sci. 2014, 50, 2451–2458. [Google Scholar] [CrossRef]
- Köklükaya, O.; Carosio, F.; Grunlan, J.C.; Wågberg, L. Flame-retardant paper from wood fibers functionalized via layer-by-layer assembly. ACS Appl. Mater. Interfaces 2015, 7, 23750–23759. [Google Scholar] [CrossRef]
- Köklükaya, O.; Carosio, F.; Durán, V.L.; Wågberg, L. Layer-by-layer modified low density cellulose fiber networks: A sustainable and fireproof alternative to petroleum based foams. Carbohydr. Polym. 2020, 230, 115616. [Google Scholar] [CrossRef]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Huang, C.; Sun, S.; Zhang, M.; Umemura, K.; Yong, Q. Further exploration of sucrose–citric acid adhesive: Investigation of optimal hot-pressing conditions for plywood and curing behavior. Polymers 2020, 12, 216. [Google Scholar] [CrossRef]
- Sun, Q.; Yu, H.; Liu, Y.; Li, J.; Cui, Y.; Lu, Y. Prolonging the combustion duration of wood by TiO2 coating synthesized using cosolvent-controlled hydrothermal method. J. Mater. Sci. 2010, 45, 6661–6667. [Google Scholar] [CrossRef]
- Kong, L.; Tu, K.; Guan, H.; Wang, X. Growth of high-density ZnO nanorods on wood with enhanced photostability, flame retardancy and water repellency. Appl. Surf. Sci. 2017, 407, 479–484. [Google Scholar] [CrossRef]
- Carosio, F.; Laufer, G.; Alongi, J.; Camino, G.; Grunlan, J.C. Layer-by-layer assembly of silica-based flame retardant thin film on PET fabric. Polym. Degrad. Stab. 2011, 96, 745–750. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J. Buildup of ultrathin multilayer films by a self-assembly process, I. consecutive adsorption of anionic and cationic bipolar amphiphiles on charged surfaces. Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, J.; Yang, B.; Fu, F.; Tang, H.; Zhang, J.; Zhao, Y.; Zhang, Y.; Liu, L.; Zhu, Y.; et al. Self-assembly of chitosan and cellulose chains into a 3D porous polysaccharide alloy films: Co-dissolving, structure and biological properties. Appl. Surf. Sci. 2019, 493, 1032–1041. [Google Scholar] [CrossRef]
- Tian, M.; Hu, X.; Qu, L.; Du, M.; Zhu, S.; Sun, Y.; Han, G. Ultraviolet protection cotton fabric achieved via layer-by-layer self-assembly of graphene oxide and chitosan. Appl. Surf. Sci. 2016, 377, 141–148. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, C.J.; Cui, L.; Zhu, P.; Yan, C.; Liu, Y. Fire retardant and thermal degradation properties of cotton fabrics based on APTES and sodium phytate through layer-by-layer assembly. J. Anal. Appl. Pyrolysis 2017, 123, 216–223. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Layer by Layer ammonium polyphosphate-based coatings for flame retardancy of polyester—Cotton blends. Carbohydr. Polym. 2012, 88, 1460–1469. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, W.; Huang, H.; Chang, W.; Zhang, S.; Zhang, R.; Zhao, R.; Zhang, Y. Investigation of corrosion resistance and formation mechanism of calcium-containing coatings on AZ31B magnesium alloy. Appl. Surf. Sci. 2019, 487, 581–592. [Google Scholar] [CrossRef]
- Zhang, R.F.; Shi, H.W.; Liu, Z.L.; Zhang, S.F.; Zhang, Y.Q.; Guo, S.B. Property of anodic coatings obtained in an organic, environmental friendly electrolyte on aluminum alloy 2024-T3. Appl. Surf. Sci. 2014, 289, 326–331. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Wang, W.; Song, L.; Fan, W.; Gao, X.; Xiong, C. Synthesis and characterization of pH-responsive mesoporous chitosan microspheres loaded with sodium phytate for smart water-based coatings. Mater. Corros. 2018, 69, 736–748. [Google Scholar] [CrossRef]
- Li, J.; Ren, D.; Wu, Z.; Xu, J.; Bao, Y.; He, S.; Chen, Y. Flame retardant and visible light-activated Fe-doped TiO2 thin films anchored to wood surfaces for the photocatalytic degradation of gaseous formaldehyde. J. Colloid Interface Sci. 2018, 530, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Carosio, F.; Alongi, J.; Malucelli, G. Flammability and combustion properties of ammonium polyphosphate-/poly(acrylic acid)-based layer by layer architectures deposited on cotton, polyester and their blends. Polym. Degrad. Stab. 2013, 98, 1626–1637. [Google Scholar] [CrossRef]
- Carosio, F.; Ghanadpour, M.; Alongi, J.; Wagberg, L. Layer-by-layer-assembled chitosan/phosphorylated cellulose nanofibrils as a bio-based and flame protecting nano-exoskeleton on PU foams. Carbohydr. Polym. 2018, 9, 479–487. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Martinasso, G. Intumescent fire-retardant systems. Polymer 1989, 23, 359–376. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020. [Google Scholar] [CrossRef]
- GB2406.2-2009 Plastics-Determination of Burning Behaviour by Oxygen Index-Part 2: Ambient-Temperature Test; Standards Press of China: Beijing, China, 2009.
- Liu, Y.; Wang, Q.Q.; Jiang, Z.M.; Zhang, C.J.; Li, Z.F.; Chen, H.Q.; Zhu, P. Effect of chitosan on the fire retardancy and thermal degradation properties of coated cotton fabrics with sodium phytate and APTES by LBL assembly. J. Anal. Appl. Pyrolysis 2018, 135, 289–298. [Google Scholar] [CrossRef]
- Yuste, M.; Escobar-Galindo, R.; Benito, N.; Palacio, C.; Martínez, O.; Albella, J.M.; Sánchez, O. Effect of the incorporation of titanium on the optical properties of ZnO thin films: From doping to mixed oxide formation. Coatings 2019, 9, 180. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Effect of nanoparticles on the improvement in fire-resistant and anti-ageing properties of flame-retardant coating. Surf. Coat. Technol. 2006, 200, 5706–5716. [Google Scholar] [CrossRef]
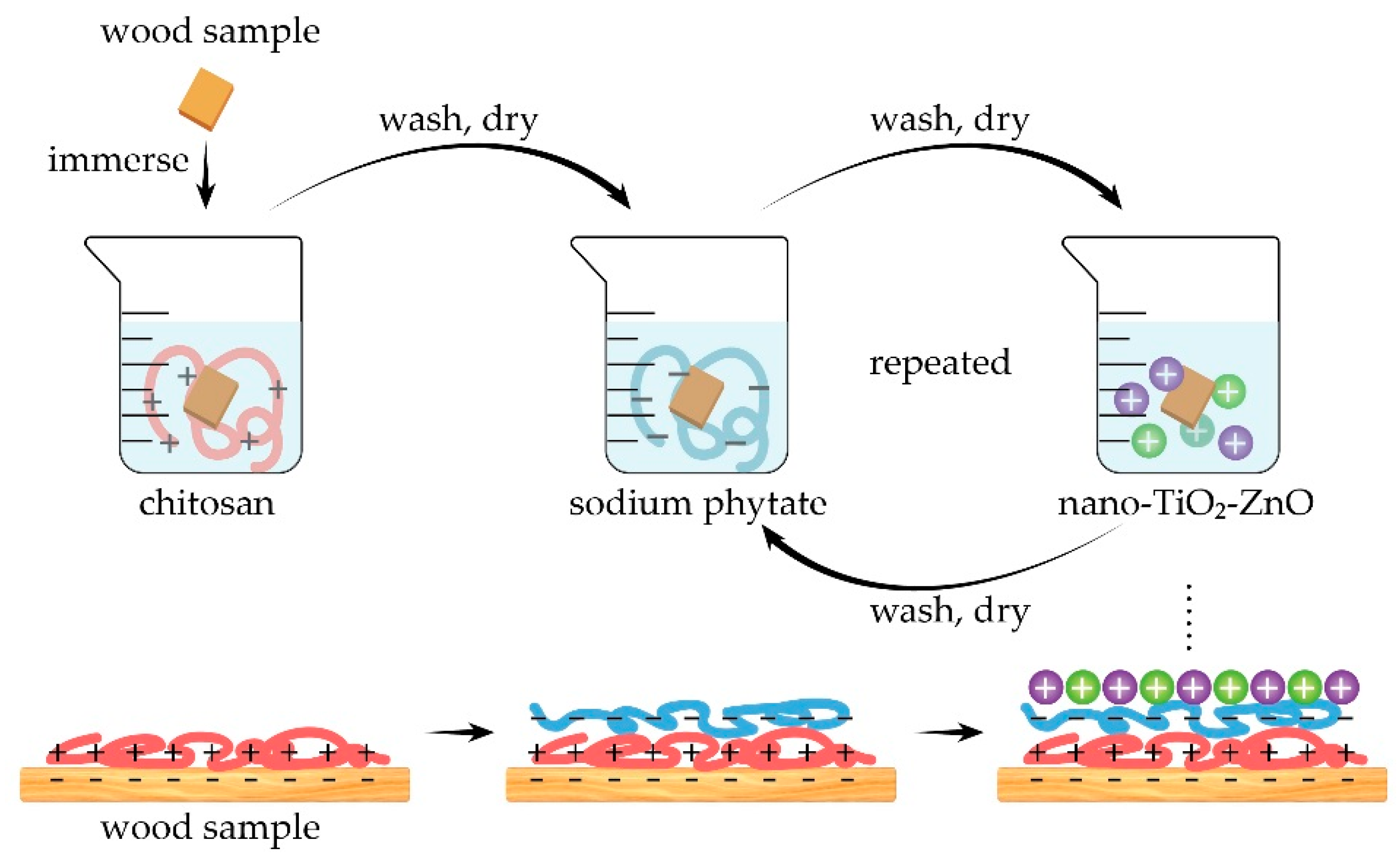
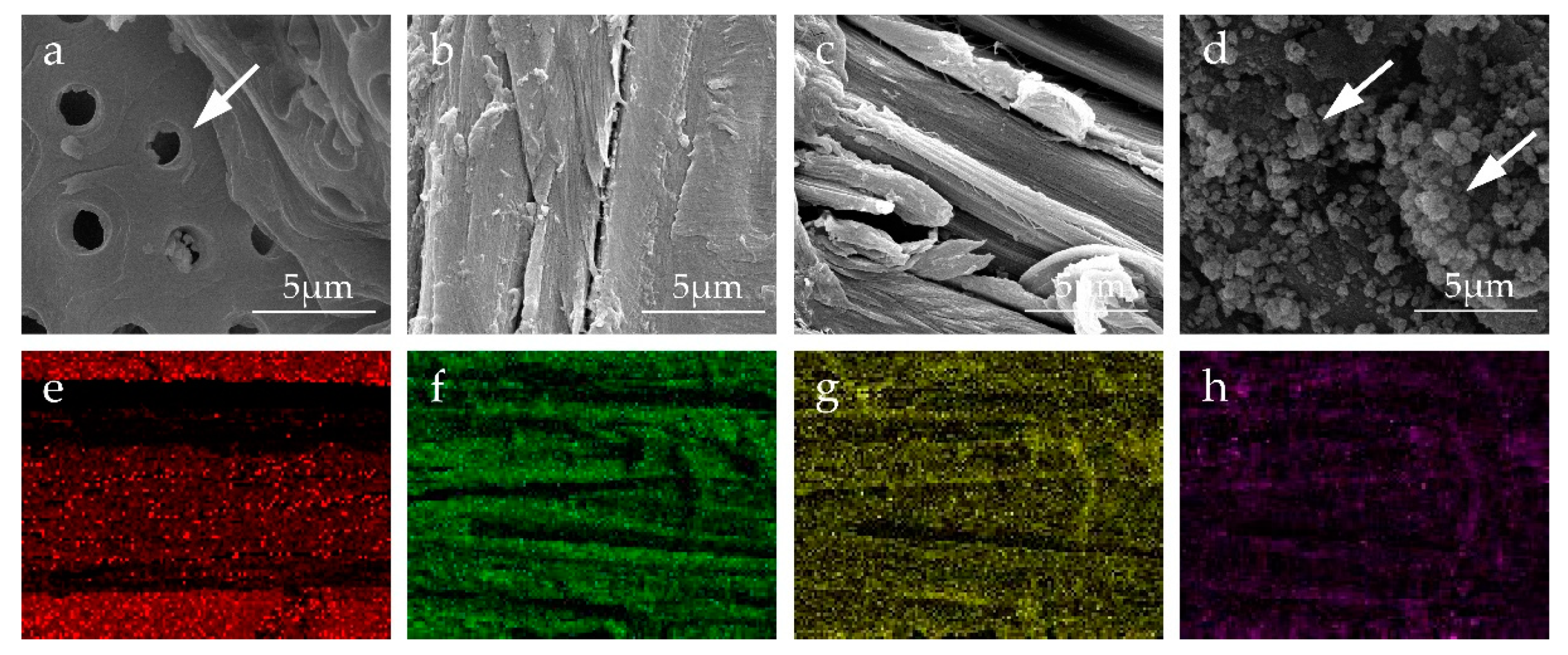
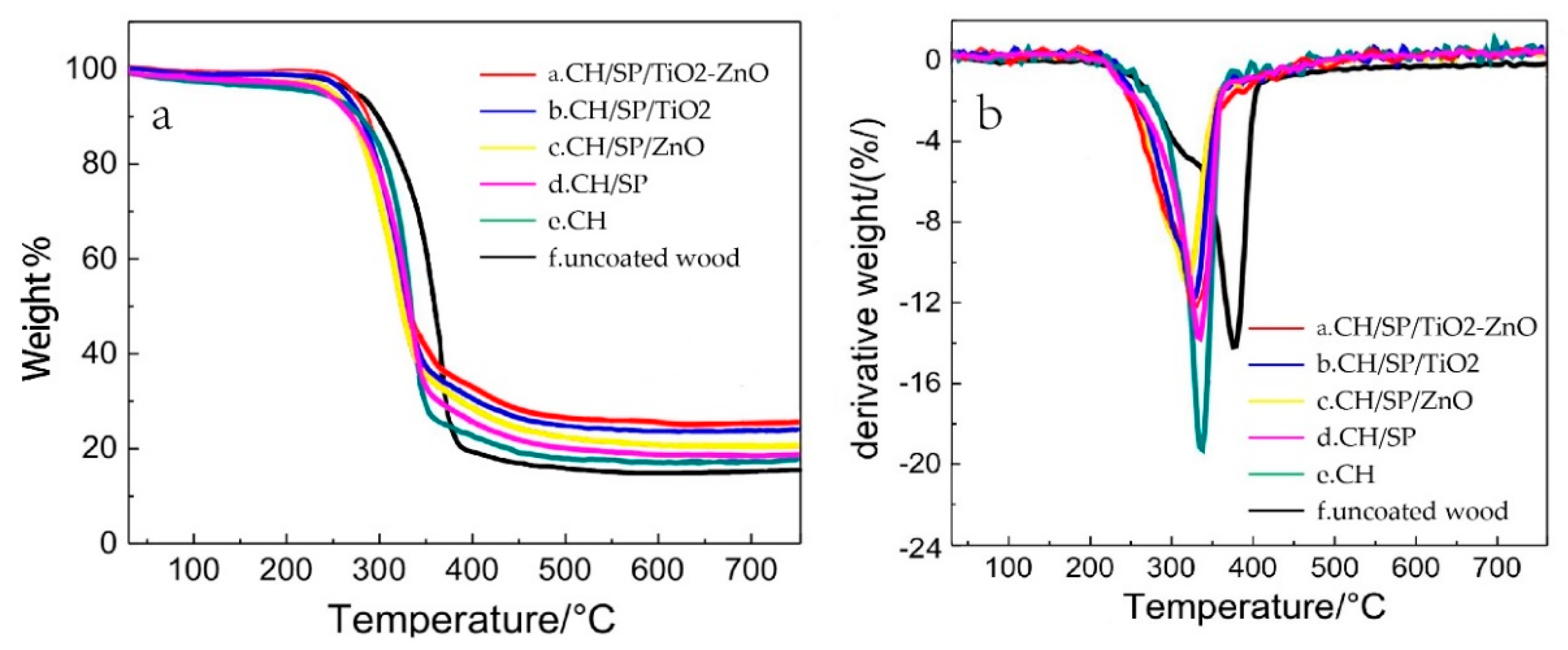

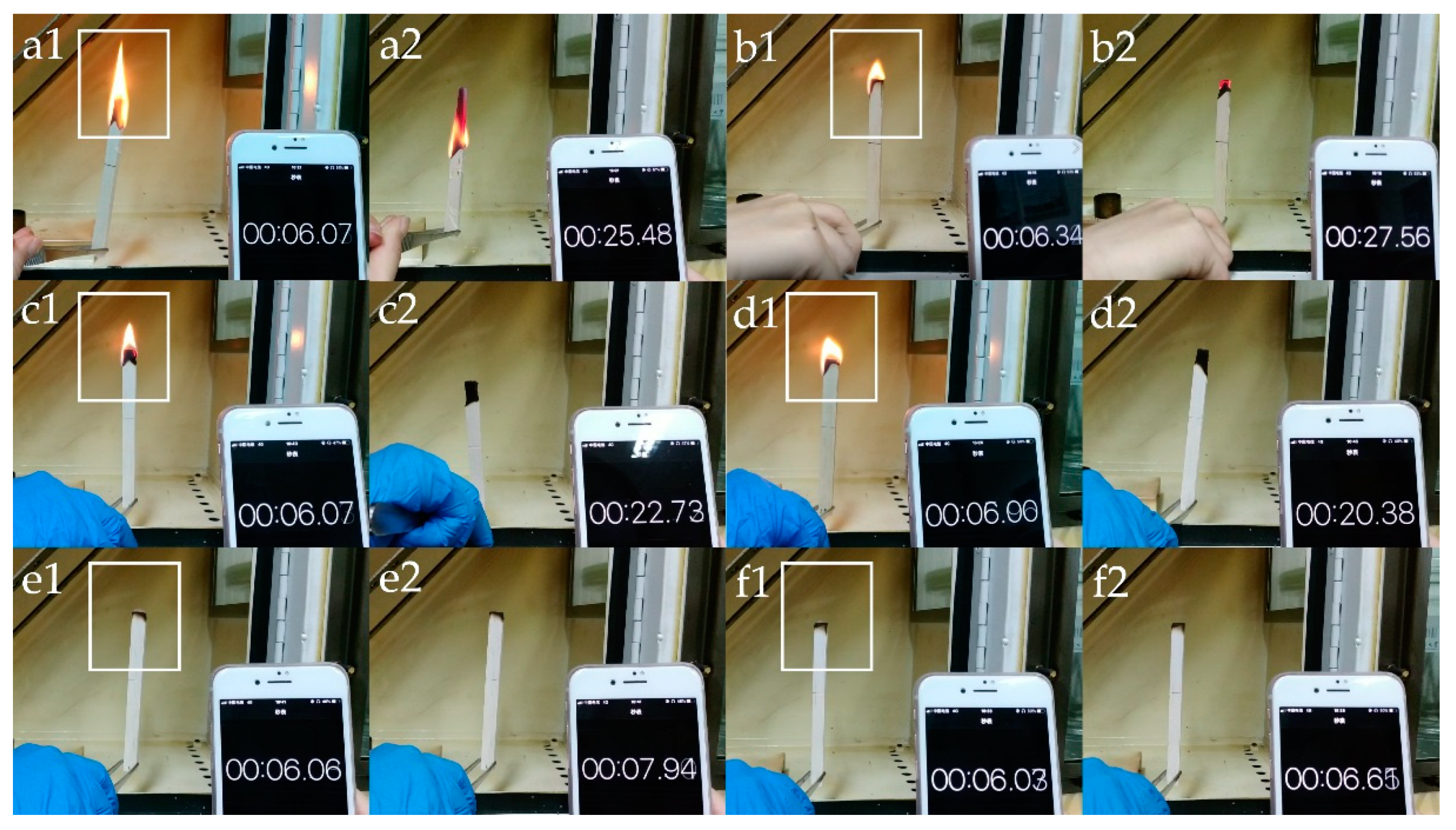
| Sample Name | Assembled Structure |
|---|---|
| CH | Wood/CH/CH/… |
| CH/SP | Wood/CH/SP/CH/SP/… |
| CH/SP/nano-TiO2 | Wood/CH/SP/TiO2/SP/TiO2/… |
| CH/SP/nano-ZnO | Wood/CH/SP/ZnO/SP/ZnO/… |
| CH/SP/nano-TiO2-ZnO | Wood/CH/SP/TiO2-ZnO/SP/TiO2-ZnO/… |
| Invariants | |
| Soaking time: 90 min; Deposition cycle: 10; Chitosan, sodium phytate, nano-TiO2, nano-ZnO, nano-TiO2-ZnO concentration: 1%. | |
| Sample Name | Initial Mass (g) | Mass Change (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| CH | 0.80 | 0.68 | 1.27 | 0.89 | 1.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| CH/SP | 0.27 | 1.17 | 0.88 | 0.91 | 0.85 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| CH/SP/nano-TiO2 | 0.46 | 0.49 | 1.07 | 0.93 | 0.74 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| CH/SP/nano-ZnO | 0.67 | 0.97 | 0.97 | 0.44 | 0.42 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 |
| CH/SP/nano-TiO2-ZnO | 0.90 | 1.34 | 0.97 | 0.67 | 1.13 | 0.03 | 0.04 | 0.03 | 0.02 | 0.03 |
| Sample Name | Quantity | Mean | Std. Deviation | Variance | ||
|---|---|---|---|---|---|---|
| CH | 5 | 28.08 | 1.89 | 3.587 | ||
| CH/SP | 5 | 22.96 | 1.64 | 2.683 | ||
| CH/SP/nano-TiO2 | 5 | 20.26 | 4.80 | 23.058 | ||
| CH/SP/nano-ZnO | 5 | 7.52 | 0.73 | 0.532 | ||
| CH/SP/nano-TiO2-ZnO | 5 | 6.58 | 1.07 | 1.137 | ||
| ANOVA | ||||||
| Sources | SS | df | MS | F | p-Value | F Crit |
| Between groups | 1836.652 | 4 | 459.163 | 74.06571604 | 1.06047E-11 | 2.886081 |
| Within groups | 123.988 | 20 | 6.1994 | – | – | – |
| Total | 1960.64 | 24 | – | – | – | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Fu, Y. Flame-Retardant Wood Composites Based on Immobilizing with Chitosan/Sodium Phytate/Nano-TiO2-ZnO Coatings via Layer-by-Layer Self-Assembly. Coatings 2020, 10, 296. https://doi.org/10.3390/coatings10030296
Zhou L, Fu Y. Flame-Retardant Wood Composites Based on Immobilizing with Chitosan/Sodium Phytate/Nano-TiO2-ZnO Coatings via Layer-by-Layer Self-Assembly. Coatings. 2020; 10(3):296. https://doi.org/10.3390/coatings10030296
Chicago/Turabian StyleZhou, Lin, and Yanchun Fu. 2020. "Flame-Retardant Wood Composites Based on Immobilizing with Chitosan/Sodium Phytate/Nano-TiO2-ZnO Coatings via Layer-by-Layer Self-Assembly" Coatings 10, no. 3: 296. https://doi.org/10.3390/coatings10030296
APA StyleZhou, L., & Fu, Y. (2020). Flame-Retardant Wood Composites Based on Immobilizing with Chitosan/Sodium Phytate/Nano-TiO2-ZnO Coatings via Layer-by-Layer Self-Assembly. Coatings, 10(3), 296. https://doi.org/10.3390/coatings10030296




