Development of Polylactic Acid Films with Selenium Microparticles and Its Application for Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA and PLA/SeMPs Composite Membranes
2.3. Morphology Studies
2.4. Fourier-Transform Infrared Experiment (FTIR)
2.5. Density Test
2.6. Mechanical Property Analysis
2.7. Swelling Capacity and Solubility of PLA/SeMP Composite Membranes
2.8. Permeability Experiments
2.9. Color Measurements
2.10. DPPH Free Radical Scavenging Activity
2.11. Light Transmittance
2.12. Seal strength Determination
2.13. Antimicrobial Properties
2.14. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Surface Characteristics of PLA/SeMPs Films
3.2. Infrared Spectra
3.3. Density Tests
3.4. Tensile Properties
3.5. Swelling Capacity and Solubility
3.6. Water Vapor Permeability
3.7. Color Measurements
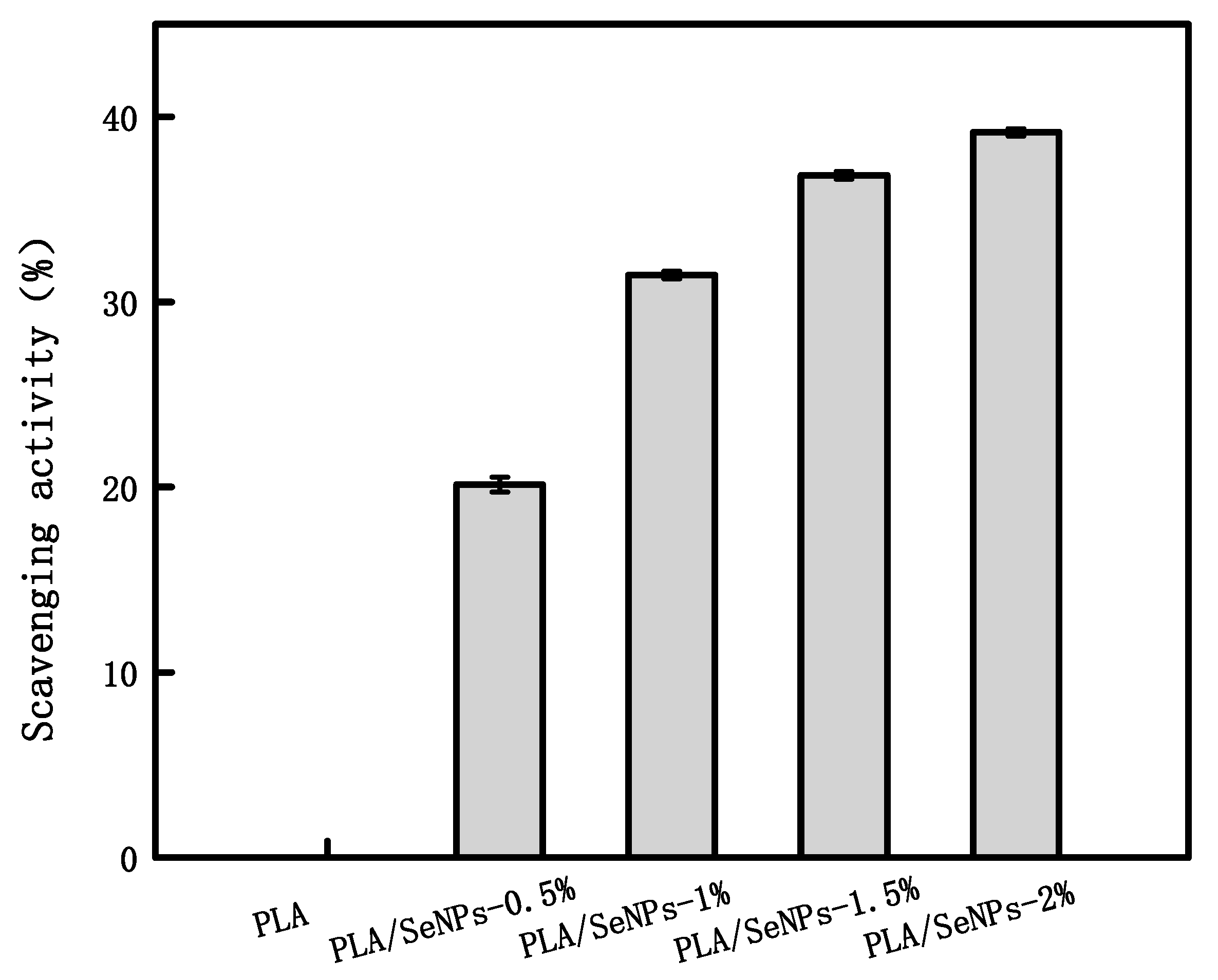
3.8. DPPH Free Radical Scavenging Activity
3.9. Light Transmittance
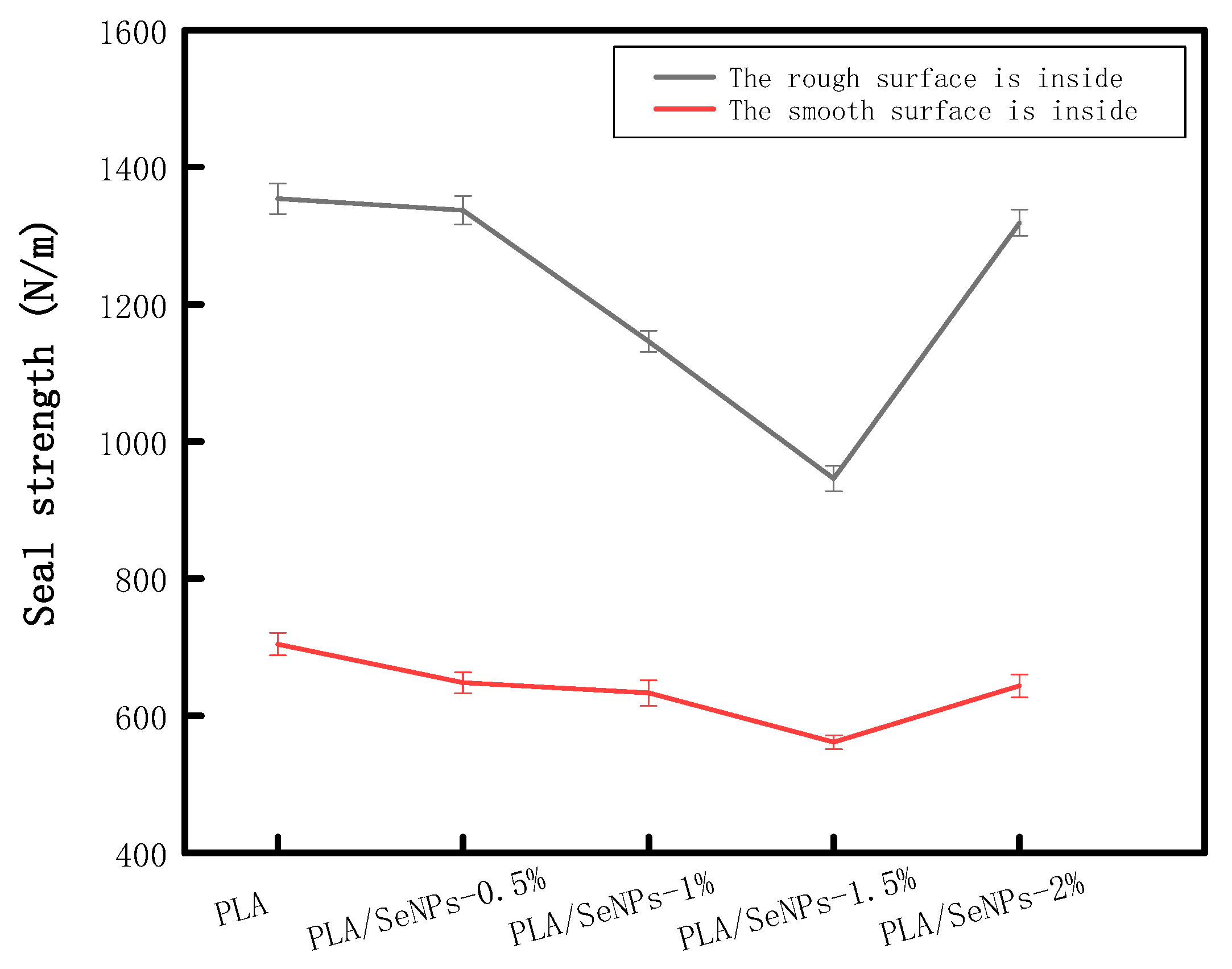
3.10. Seal Strength Determination
3.11. Antimicrobial Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Yusoff, R.B.; Takagi, H.; Nakagaito, A.N. Tensile and flexural properties of polylactic acid-based hybrid green composites reinforced by kenaf, bamboo and coir fibers. Ind. Crops Prod. 2016, 94, 562–573. [Google Scholar] [CrossRef]
- Norma, M.; Thanh, P.; Maria-Beatrice, C.; Patrizia, C.; Andrea, L. Poly(lactic acid) (PLA) based tear resistant and biodegradable flexible films by blown film extrusion. Materials 2018, 11, 148–162. [Google Scholar] [CrossRef]
- Agarwal, M.; Koelling, K.W.; Chalmers, J.J. Characterization of the degradation of polylactic acid polymer in a solid substrate environment. Biotechnol. Prog. 1998, 14, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Eslami, H.; Kamal, M.R. Elongational rheology of biodegradable poly(lactic acid)/poly[(butylene succinate)-co-adipate] binary blends and poly(lactic acid)/poly[(butylene succinate)-co-adipate]/clay ternary nanocomposites (pages 2290–2306). J. Appl. Polym. Sci. 2014, 127, 2290–2306. [Google Scholar] [CrossRef]
- Tawakkal, I.S.M.A.; Cran, M.J.; Miltz, J.; Bigger, S.W. A review of poly(lactic acid)-based materials for antimicrobial packaging. J. Food Sci. 2014, 79, R1477–R1490. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Tang, C.H.; Yin, S.W.; Yang, X.Q. Development and characterization of novel antimicrobial bilayer films based on polylactic acid (PLA)/pickering emulsions. Carbohydr. Polym. 2018, 181, 727–735. [Google Scholar] [CrossRef]
- Gordobil, O.; Delucis, R.; Egüés, I.; Labidia, J. Kraft lignin as filler in PLA to improve ductility and thermal properties. Ind. Crops Prod. 2015, 72, 46–53. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Therias, S.; Larché, J.-F.; Bussière, P.-O.; Gardette, J.L.; Dubois, P. Photochemical behavior of polylactide/ZnO nanocomposite films. Biomacromolecules 2012, 13, 3283–3291. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Kenny, M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef]
- Luo, Z.; Qin, Y.; Ye, Q. Effect of nano-TiO2-ldpe packaging on microbiological and physicochemical quality of pacific white shrimp during chilled storage. Int. J. Food Sci. Technol. 2015, 50, 1567–1573. [Google Scholar] [CrossRef]
- Silveira, A.C.; Moreira, G.C.; Artés, F.; Aguayo, E. Vanillin and cinnamic acid in aqueous solutions or in active modified packaging preserve the quality of fresh-cut cantaloupe melon. Sci. Hortic. 2015, 192, 271–278. [Google Scholar] [CrossRef]
- Athanasouliaa, I.G.; Mikropouloua, M.; Karapati, S.; Tarantili, P.; Trapalis, C. Study of thermomechanical and antibacterial properties of TiO2/Poly(lactic acid) nanocomposites. Mater. Today Proceed. 2018, 5, 27553–27562. [Google Scholar] [CrossRef]
- Shebi, A.; Lisa, S. Evaluation of biocompatibility and bactericidal activity of hierarchically porous PLA-TiO2 nanocomposite films fabricated by breath-figure method. Mater. Chem. Phys. 2019, 230, 308–318. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/Silver-NP/Vitamin E bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanopart. Res. 2014, 16, 2643. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Yunus, W.M.Z.W.; Ibrahim, N.A.; Rahman, R.A.; Jokar, M.; Darroudi, M. Silver/poly (lactic acid) nanocomposites: Preparation, characterization, and antibacterial activity. Int. J. Nanomed. 2010, 5, 573–579. [Google Scholar] [CrossRef]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Vallini, G. Biogenic selenium nanoparticles: Characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, S.; Mashreghi, M. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J. Trace Elem. Med. Biol. 2017, 39, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.A.; O’Brien-Simpson, N.; Reynolds, E.C.; Pantarat, N.; Biswas, D.P.; O’Connor, A.J. Low cytotoxic trace element selenium nanoparticles and their differential antimicrobial properties against S. aureus and E. coli. Nanotechnology 2015, 27, 045101. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Wang, Q.; Khachatryan, E.; Mimun, L.C.; Mendoza-Cruz, R.; Larese-Casanova, P.; Nash, K.L. Inhibition of E. coli and S. aureus with selenium nanoparticles synthesized by pulsed laser ablation in deionized water. Int. J. Nanomed. 2016, 11, 3731–3736. [Google Scholar] [CrossRef]
- Redman, C.; Scott, J.A.; Baines, A.T.; Basye, J.L.; Clark, L.C.; Calleye, C.; Nelson, M.A. Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998, 125, 103–110. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2013, 6, 25–54. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Muthukumaran, A. Comparative analysis of cardiovascular effects of selenium nanoparticles and sodium selenite in zebrafish embryos. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1–7. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Muthukumaran, A. A novel one-pot green synthesis of selenium nanoparticles and evaluation of its toxicity in zebrafish embryos. Artif. Cells Nanomed. Biotechnol. 2014, 44, 1–7. [Google Scholar] [CrossRef]
- Weekley, C.M.; Harris, H.H. Which form is that? the importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8910. [Google Scholar] [CrossRef]
- Huawei, Z.; Jay, C.; Gerald, C. Selenium in bone health: Roles in antioxidant protection and cell proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Egrise, D.; Neve, J.; Pasteels, J.L.; Schoutens, A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Miner. Res. 2001, 16, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.J.; Gregoire, B.R.; Zeng, H. Selenium deficiency decreases antioxidative capacity and is detrimental to bone microarchitecture in mice. J. Nutr. 2012, 142, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Mandrioli, J.; Borella, P.; Michalke, B.; Tsatsakis, A.; Finkelstein, Y. Selenium neurotoxicity in humans: Bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014, 230, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Crane, S. Selenium toxicity from a misformulated dietary supplement, adverse health effects, and the temporal response in the nail biologic monitor. Nutrients 2013, 5, 1024–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Yu, H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological effects of a nano red elemental selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Stevanović, M.; Filipović, N.; Djurdjević, J.; Lukić, M.; Milenković, M.; Boccaccini, A. 45S5Bioglass®-based scaffolds coated with selenium nanoparticles or with poly (lactide-co-glycolide)/selenium particles: Processing, evaluation and antibacterial activity. Colloids Surf. B Biointerfaces 2015, 132, 208–215. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication and testing of PVA/Chitosan bilayer films for strawberry packaging. Coatings 2017, 7, 109. [Google Scholar] [CrossRef]
- Gontard, N.; Duchez, C.; CUQ, J.L.; Guilbert, S. Edible composite films of wheat gluten and lipids: water vapour permeability and other physical properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Use of the electrohydrodynamic process to develop active/bioactive bilayer films for food packaging applications. Food Hydrocoll. 2016, 55, 11–18. [Google Scholar] [CrossRef]
- Luchese, C.L.; Uranga, J.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Valorisation of blueberry waste and use of compression to manufacture sustainable starch films with enhanced properties. Int. J. Biol. Macromol. 2018, 115, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P.J. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- Yu, Z.; Alsammarraie, F.K.; Nayigiziki, F.X.; Wang, W.; Vardhanabhuti, B.; Mustapha, A.; Lin, M. Effect and mechanism of cellulose nanofibrils on the active functions of biopolymer-based nanocomposite films. Food Res. Int. 2017, 99, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Mattoso, L.H. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Ren, J.; Chang, M.; He, B.; Zhang, C. Effects of nano-ZnO and nano-SiO2 particles on properties of PVA/xylan composite films. Int. J. Biol. Macromol. 2019, 132, 978–986. [Google Scholar] [CrossRef]
- Filipović, N.; Veselinović, L.; Ražić, S.; Jeremić, S.; Filipič, M.; Žegura, B.; Stevanović, M. Poly (ε-caprolactone) microspheres for prolonged release of selenium nanoparticles. Mater. Sci. Eng. C 2019, 96, 776–789. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljević, V.; Adam, V. Development and characterisation of furcellaran-gelatin films containing SeNPs and AgNPs that have antimicrobial activity. Food Hydrocoll. 2018, 83, 9–16. [Google Scholar] [CrossRef]
- Ahmed, J.; Arfat, Y.A.; Castro-Aguirre, E.; Auras, R. Mechanical, structural and thermal properties of Ag–Cu and ZnO reinforced polylactide nanocomposite films. Int. J. Biol. Macromol. 2016, 86, 885–892. [Google Scholar] [CrossRef]
- Li, S.C.; Li, Y.N. Mechanical and antibacterial properties of modified nano-ZnO/high-density polyethylene composite films with a low doped content of nano-ZnO. J. Appl. Polym. Sci. 2010, 116, 2965–2969. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chem. 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA films from environment friendly additives. Compos. Part B Eng. 2016, 102, 94–99. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Lan, W.; Qin, W.; Liu, Y. Preparation and properties of polylactic acid-tea polyphenol-chitosan composite membranes. Int. J. Biol. Macromol. 2018, 117, 632–639. [Google Scholar] [CrossRef]
- Hauri, J.F.; Niece, B.K. Leaching of silver from silver-impregnated food storage containers. J. Chem. Educ. 2011, 88, 1407–1409. [Google Scholar] [CrossRef]
- Xia, S.; Liu, X.; Wang, J.; Kan, Z.; Chen, H.; Fu, W.; Li, Z. Role of poly (ethylene glycol) grafted silica nanoparticle shape in toughened PLA-matrix nanocomposites. Compos. Part B Eng. 2019, 168, 398–405. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication of polylactic acid/carbon nanotubes/chitosan composite fibers by electrospinning for strawberry preservation. Int. J. Biol. Macromol. 2019, 121, 1329–1336. [Google Scholar] [CrossRef]
- Yoshida, C.M.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Tapia, M.S.; Pérez, E.; Famá, L. Structural and mechanical properties of edible films made from native and modified cush-cush yam and cassava starch. Food Hydrocoll. 2015, 45, 211–217. [Google Scholar] [CrossRef]
- Shankar, S.; Wang, L.F.; Rhim, J.W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
- Fan, C.; Chi, H.; Zhang, C.; Cui, R.; Lu, W.; Yuan, M.; Qin, Y. Effect of multiscale structure on the gas barrier properties of poly (lactic acid)/Ag nanocomposite films. Polym. Adv. Technol. 2019, 30, 1709–1715. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W.; Won, K. Preparation of poly (lactide)/lignin/silver nanoparticles composite films with UV light barrier and antibacterial properties. Int. J. Biol. Macromol. 2018, 107, 1724–1731. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Baek, J.B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Peñalba, M.; De La Caba, K.; Guerrero, P. Preparation and characterization of soy protein thin films: Processing–properties correlation. Mater. Lett. 2013, 105, 110–112. [Google Scholar] [CrossRef]
- Ramos, Ó.L.; Reinas, I.; Silva, S.I.; Fernandes, J.C.; Cerqueira, M.A.; Pereira, R.N.; Malcata, F.X. Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocoll. 2013, 30, 110–122. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Prodpran, T.; Benjakul, S.; Phatcharat, S. Effect of phenolic compounds on protein cross-linking and properties of film from fish myofibrillar protein. Int. J. Biol. Macromol. 2012, 51, 774–782. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Pisuchpen, S.; Osako, K. Mechanical, thermal and heat sealing properties of fish skin gelatin film containing palm oil and basil essential oil with different surfactants. Food Hydrocoll. 2016, 56, 93–107. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Hajji, S.; Salem, R.B.S.B.; Hamdi, M.; Jellouli, K.; Ayadi, W.; Nasri, M.; Boufi, S. Nanocomposite films based on chitosan–poly (vinyl alcohol) and silver nanoparticles with high antibacterial and antioxidant activities. Process Saf. Environ. Prot. 2017, 111, 112–121. [Google Scholar] [CrossRef]
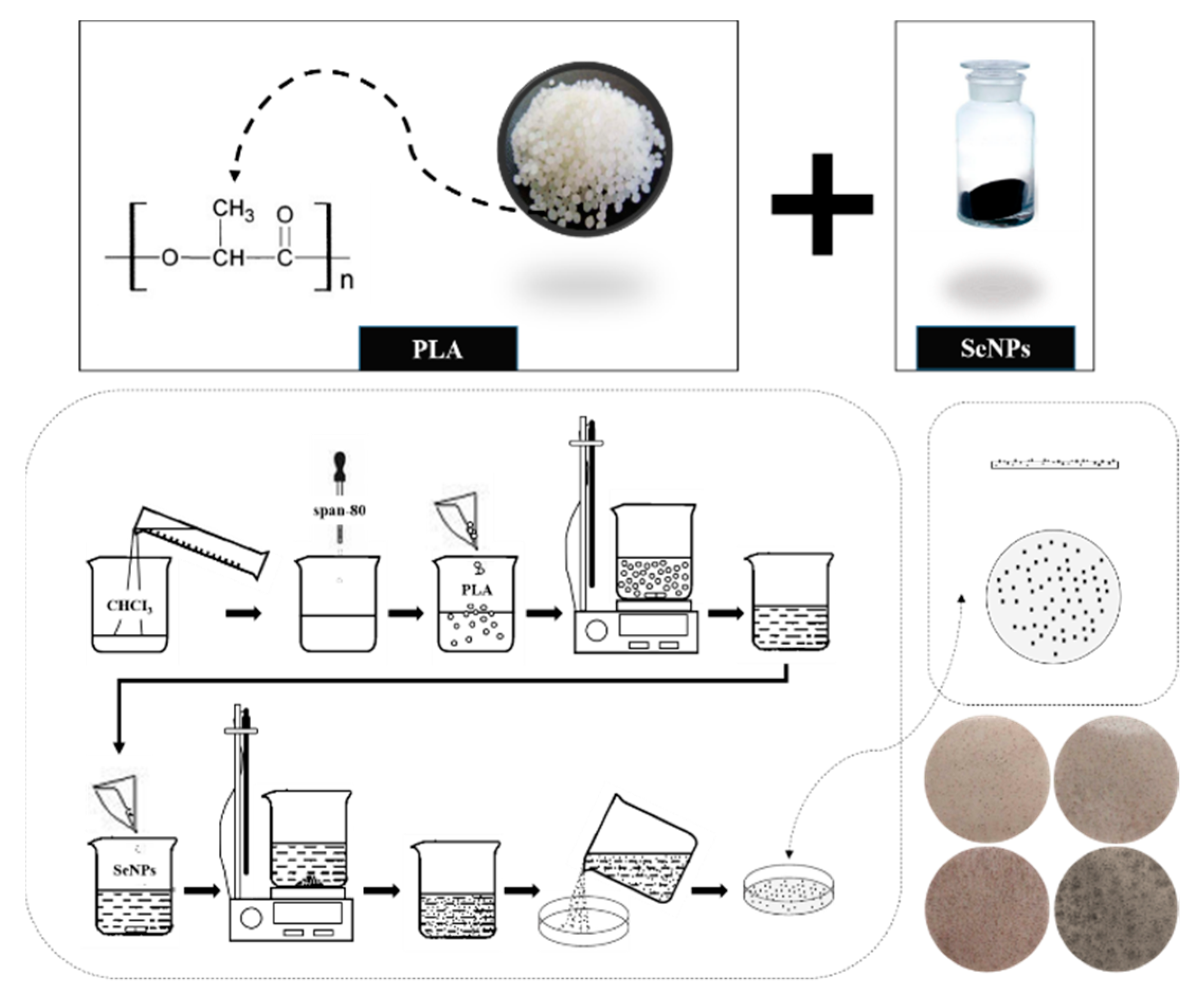
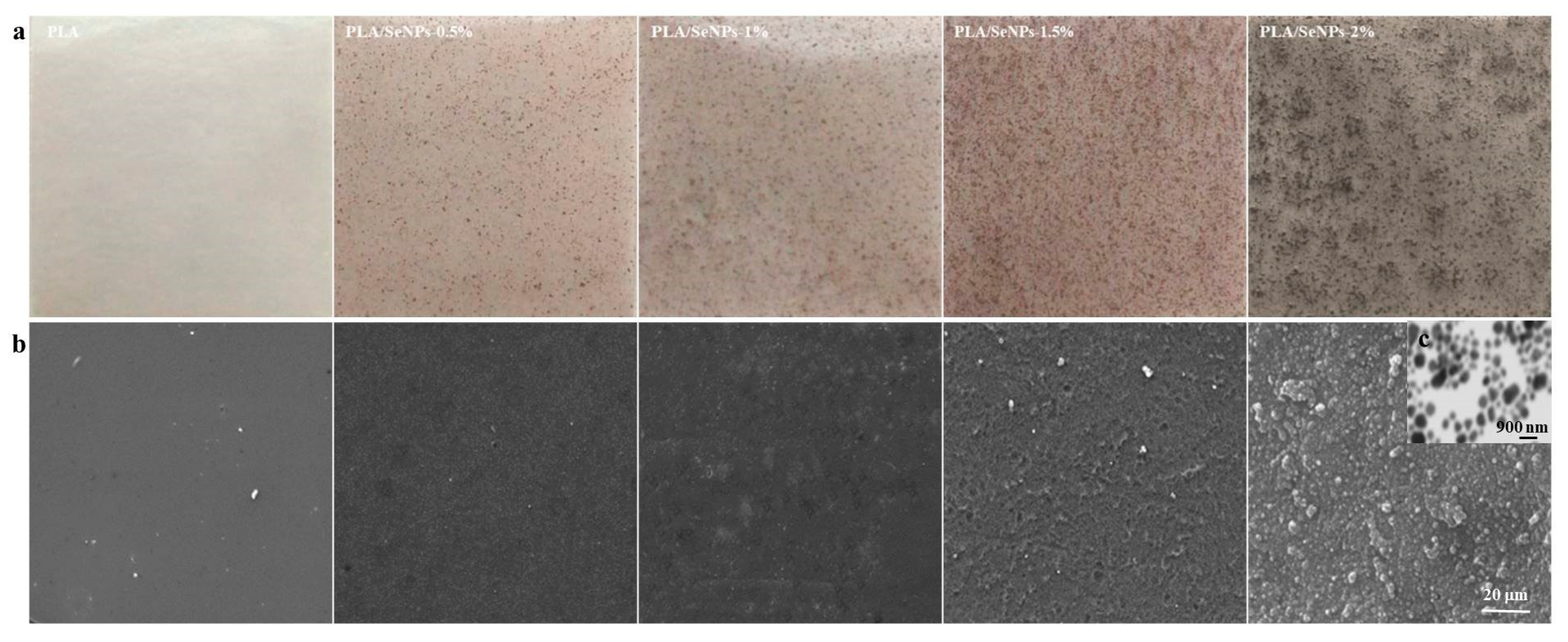
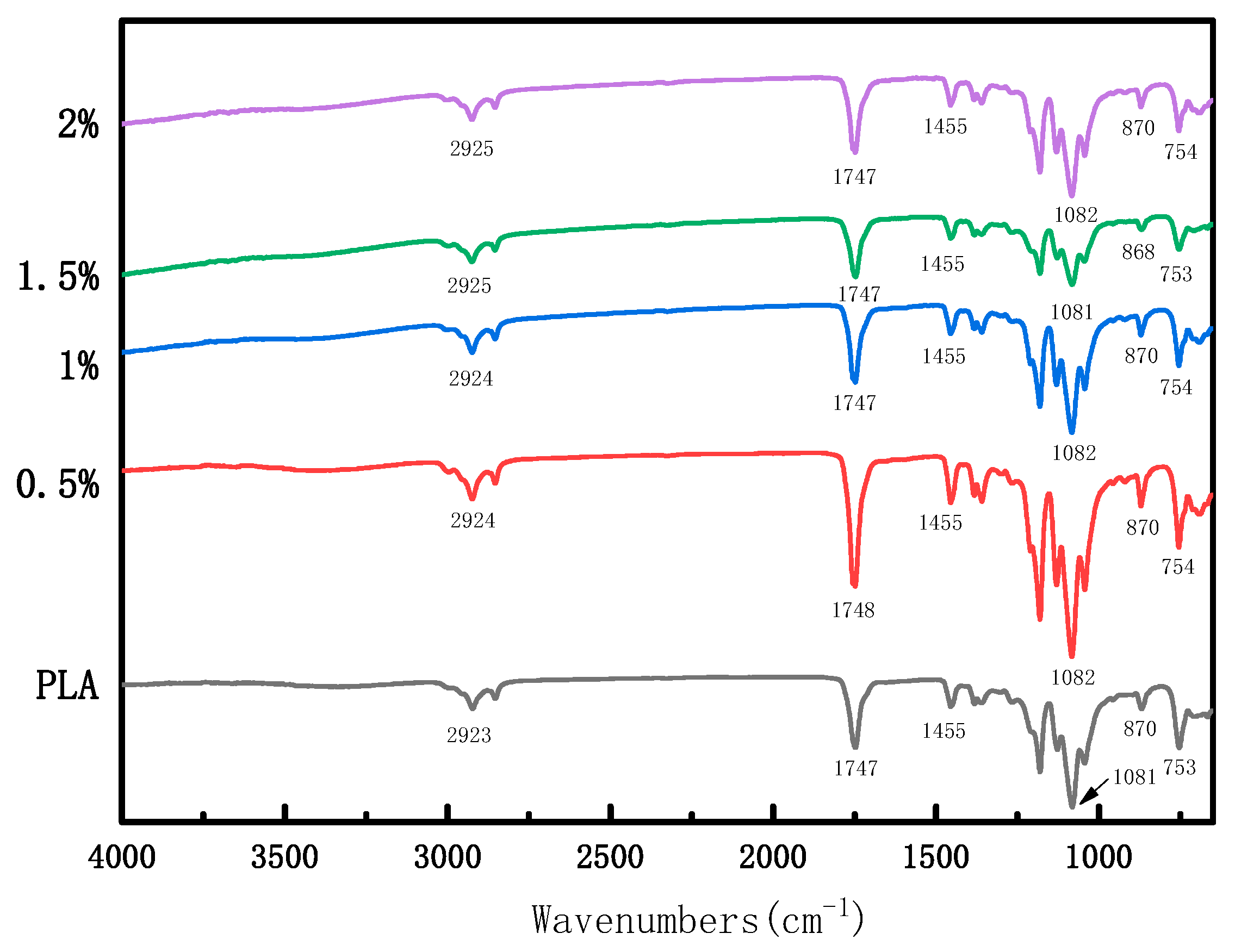

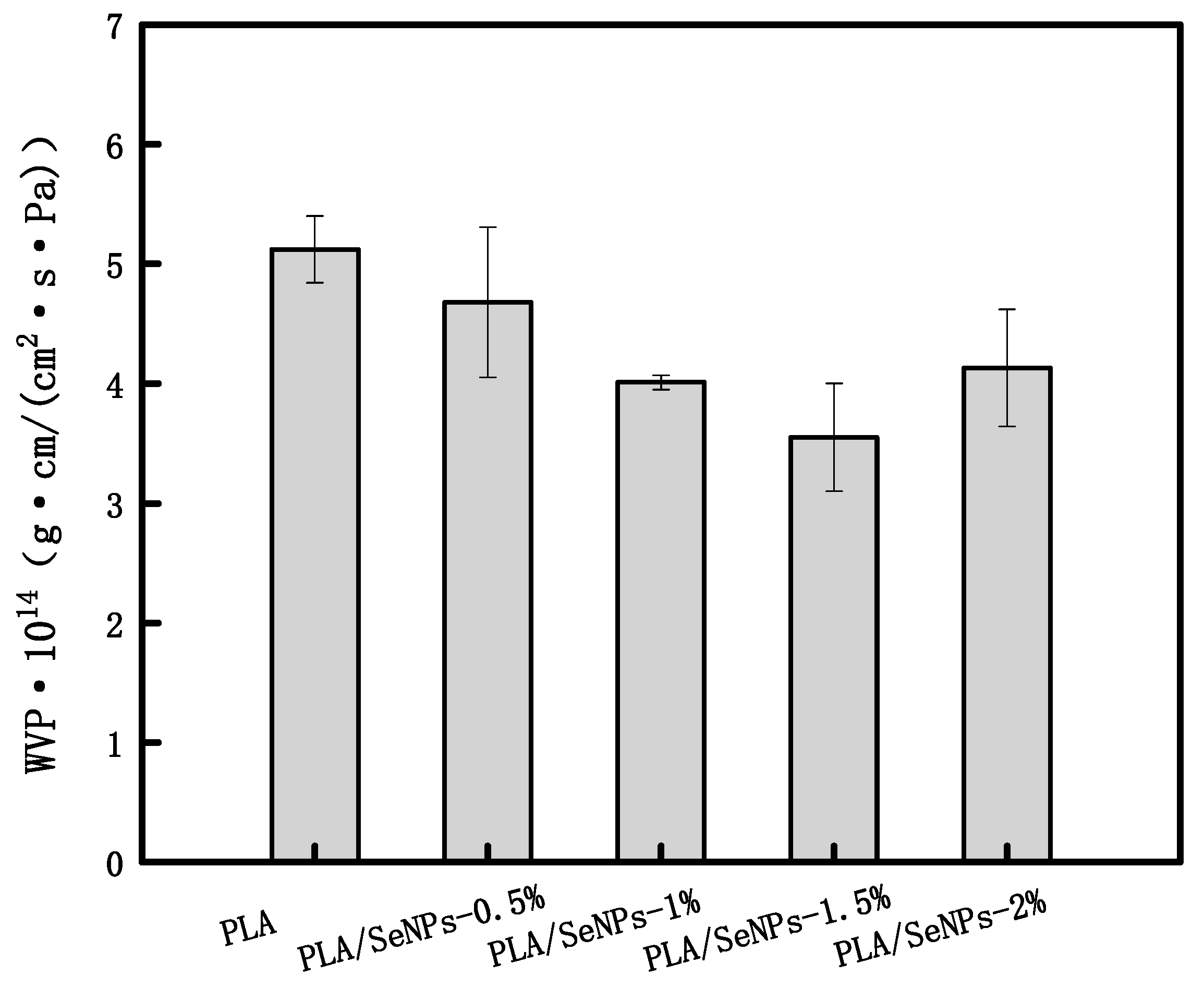
| Sample | SeMPs (g) | PLA (g) | Span-80 (g) |
|---|---|---|---|
| PLA | 0 | 20 | 4 |
| PLA/SeMPs-0.5% | 1 | 20 | 4 |
| PLA/SeMPs-1% | 2 | 20 | 4 |
| PLA/SeMPs-1.5% | 3 | 20 | 4 |
| PLA/SeMPs-2% | 4 | 20 | 4 |
| Sample | Thickness (mm) | Density (g/cm3) | TS (MPa) | %E (%) |
|---|---|---|---|---|
| PLA | 0.08 ± 0.01a | 1.14 ± 0.09a | 23.34 ± 1.81a | 272.16 ± 33.61a |
| PLA/SeMPs-0.5% | 0.10 ± 0.01ab | 0.99 ± 0.08ab | 13.47 ± 1.13b | 145.43 ± 13.54b |
| PLA/SeMPs-1% | 0.11 ± 0.01bc | 1.05 ± 0.03b | 11.45 ± 0.84c | 181.70 ± 20.51c |
| PLA/SeMPs-1.5% | 0.13 ± 0.02c | 1.10 ± 0.04c | 9.28 ± 0.52d | 111.64 ± 18.11cd |
| PLA/SeMPs-2% | 0.17 ± 0.03d | 0.91 ± 0.02c | 6.89 ± 0.39e | 96.70 ± 15.64e |
| Sample | L* | a* | b* | ΔE* |
|---|---|---|---|---|
| PLA | 85.91 ± 0.21a | −0.33 ± 0.01a | 0.40 ± 0.08a | 2.41 ± 0.10a |
| PLA/SeMPs-0.5% | 82.84 ± 0.23b | −0.22± 0.08b | −0.70 ± 0.09b | 6.12 ± 0.23b |
| PLA/SeMPs-1% | 77.96 ± 0.31c | −0.13 ± 0.03c | −0.91 ± 0.03c | 16.52 ± 0.35b |
| PLA/SeMPs-1.5% | 71.81 ± 0.54d | −0.03 ± 0.01d | −1.24 ± 0.10d | 16.78 ± 0.81c |
| PLA/SeMPs-2% | 58.72 ± 0.26e | 0.71 ± 0.08d | −2.67 ± 0.05e | 29.71 ± 0.81d |
| Sample | T660 (%) | T280 (%) |
|---|---|---|
| PLA | 62.51± 2.58a | 19.77 ± 0.86a |
| PLA/SeMPs-0.5% | 57.18 ± 1.38b | 17.67 ± 0.92b |
| PLA/SeMPs-1% | 43.68 ± 1.32c | 7.39 ± 0.34c |
| PLA/SeMPs-1.5% | 40.30 ± 0.93d | 6.03 ± 0.21d |
| PLA/SeMPs-2% | 34.97 ± 0.91e | 5.51 ± 0.19d |
| Sample | Inhibition Zone Diameter (mm) | |
|---|---|---|
| E. coli (−) | S. aureus (+) | |
| PLA | 0 | 0 |
| PLA/SeMPs-0.5% | 12.54 ± 0.03a | 12.30 ± 0.08a |
| PLA/SeMPs-1% | 12.92 ± 0.05b | 12.68 ± 0.05b |
| PLA/SeMPs-1.5% | 13.48 ± 0.11c | 13.17 ± 0.07c |
| PLA/SeMPs-2% | 13.65 ± 0.06d | 13.53 ± 0.03d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, R.; Sameen, D.E.; Qin, W.; Wu, D.; Dai, J.; Li, S.; Liu, Y. Development of Polylactic Acid Films with Selenium Microparticles and Its Application for Food Packaging. Coatings 2020, 10, 280. https://doi.org/10.3390/coatings10030280
Lu R, Sameen DE, Qin W, Wu D, Dai J, Li S, Liu Y. Development of Polylactic Acid Films with Selenium Microparticles and Its Application for Food Packaging. Coatings. 2020; 10(3):280. https://doi.org/10.3390/coatings10030280
Chicago/Turabian StyleLu, Rui, Dur E. Sameen, Wen Qin, Dingtao Wu, Jianwu Dai, Suqing Li, and Yaowen Liu. 2020. "Development of Polylactic Acid Films with Selenium Microparticles and Its Application for Food Packaging" Coatings 10, no. 3: 280. https://doi.org/10.3390/coatings10030280
APA StyleLu, R., Sameen, D. E., Qin, W., Wu, D., Dai, J., Li, S., & Liu, Y. (2020). Development of Polylactic Acid Films with Selenium Microparticles and Its Application for Food Packaging. Coatings, 10(3), 280. https://doi.org/10.3390/coatings10030280





