Biomimetic Sensitive Elements for 2,4,6-Trinitrotoluene Tested on Multi-Layered Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of Molecularly Imprinted Polymer (MIP) Films for TNT Recognition
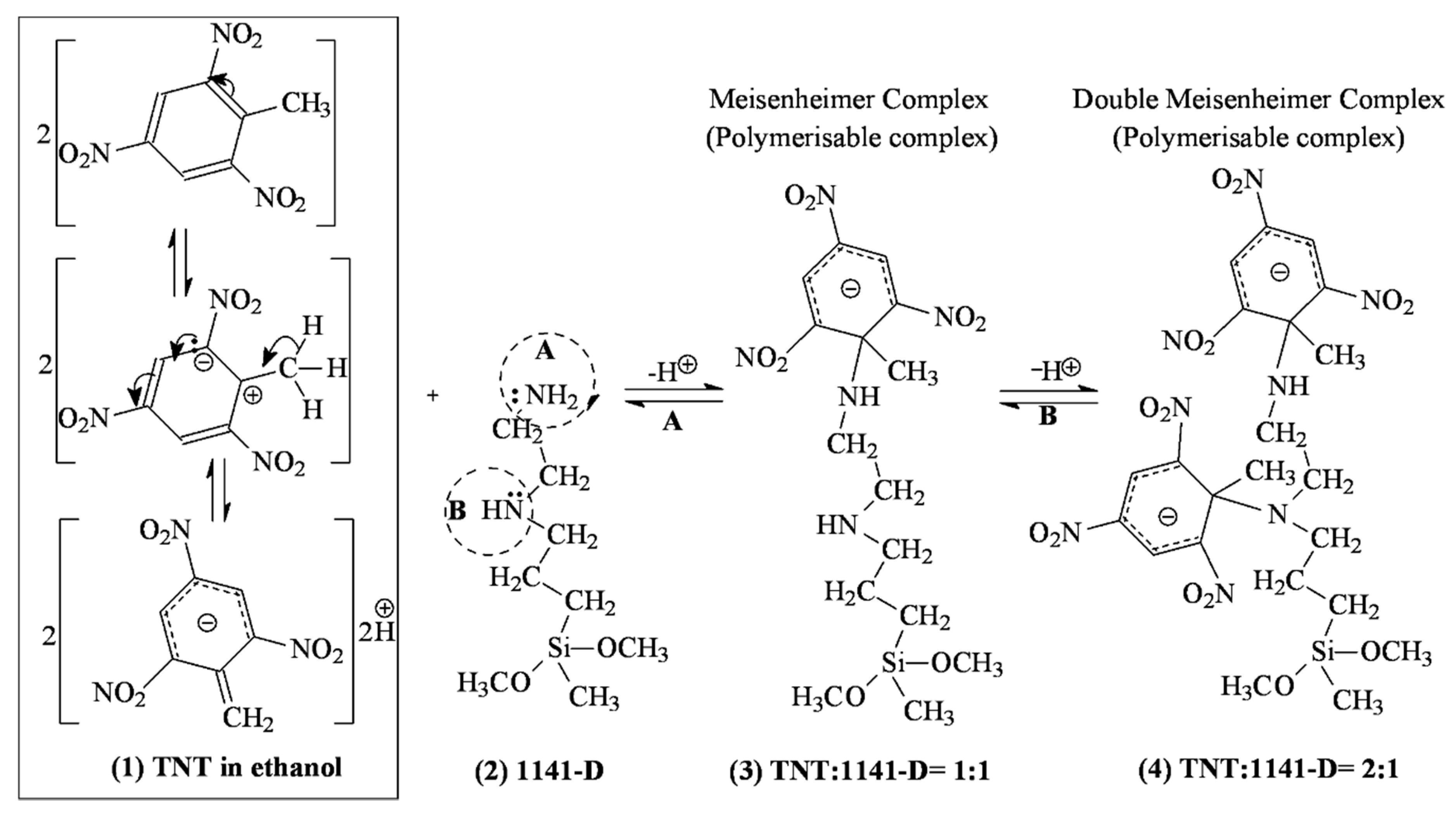
2.2.1. Study of Monomer-Template Auto-Assembly Via Double Meisenheimer Complexes
2.2.2. Films Preparation by Sol-Gel Derived Techniques
2.3. Preparation of Quartz-Cr-Au-TiO2-MIP Sensors
2.4. Employed Characterisation Methods
2.5. Protocols in Batch Rebinding Experiments of TNT
2.6. Testing Platform for EM-Quartz-Cr-Au-TiO2-MIP Sensors
3. Results and Discussion
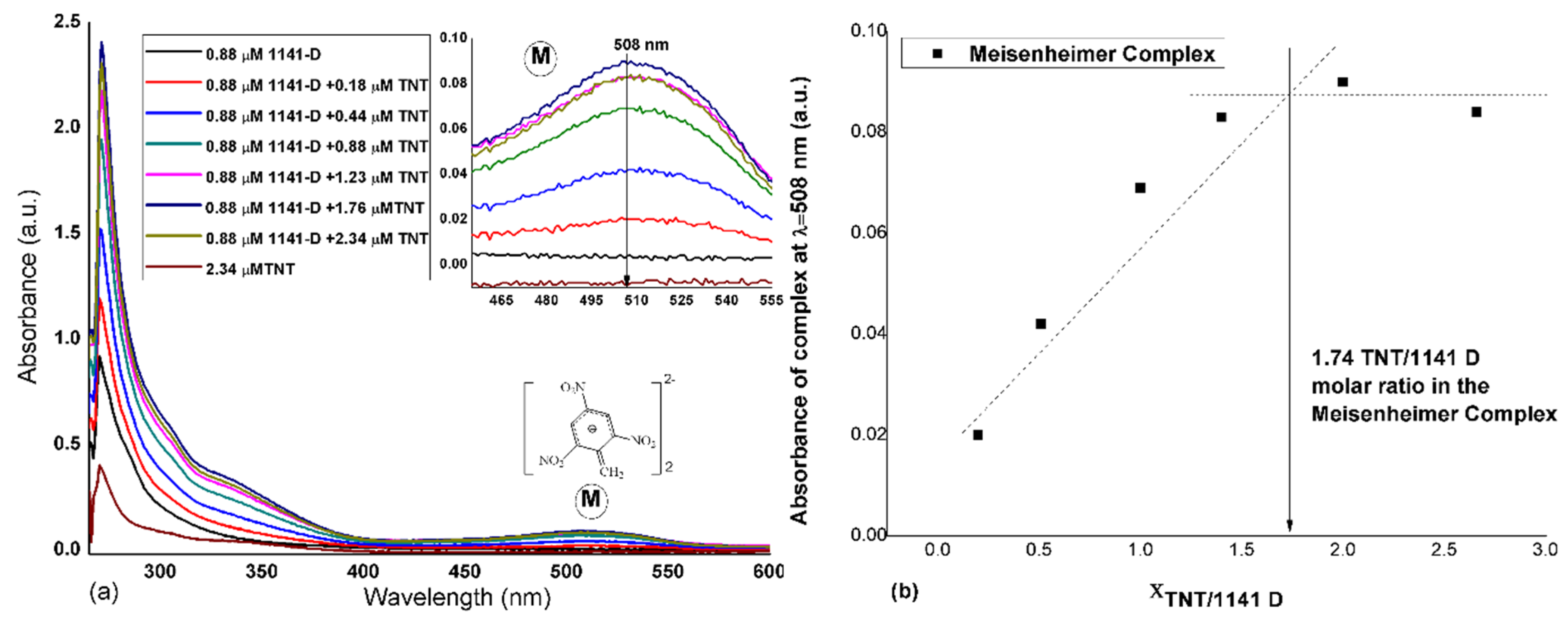
3.1. Double Meisenheimer Complex Formation
3.2. Spectroscopy Results for the Films Deposited on Glass Slides
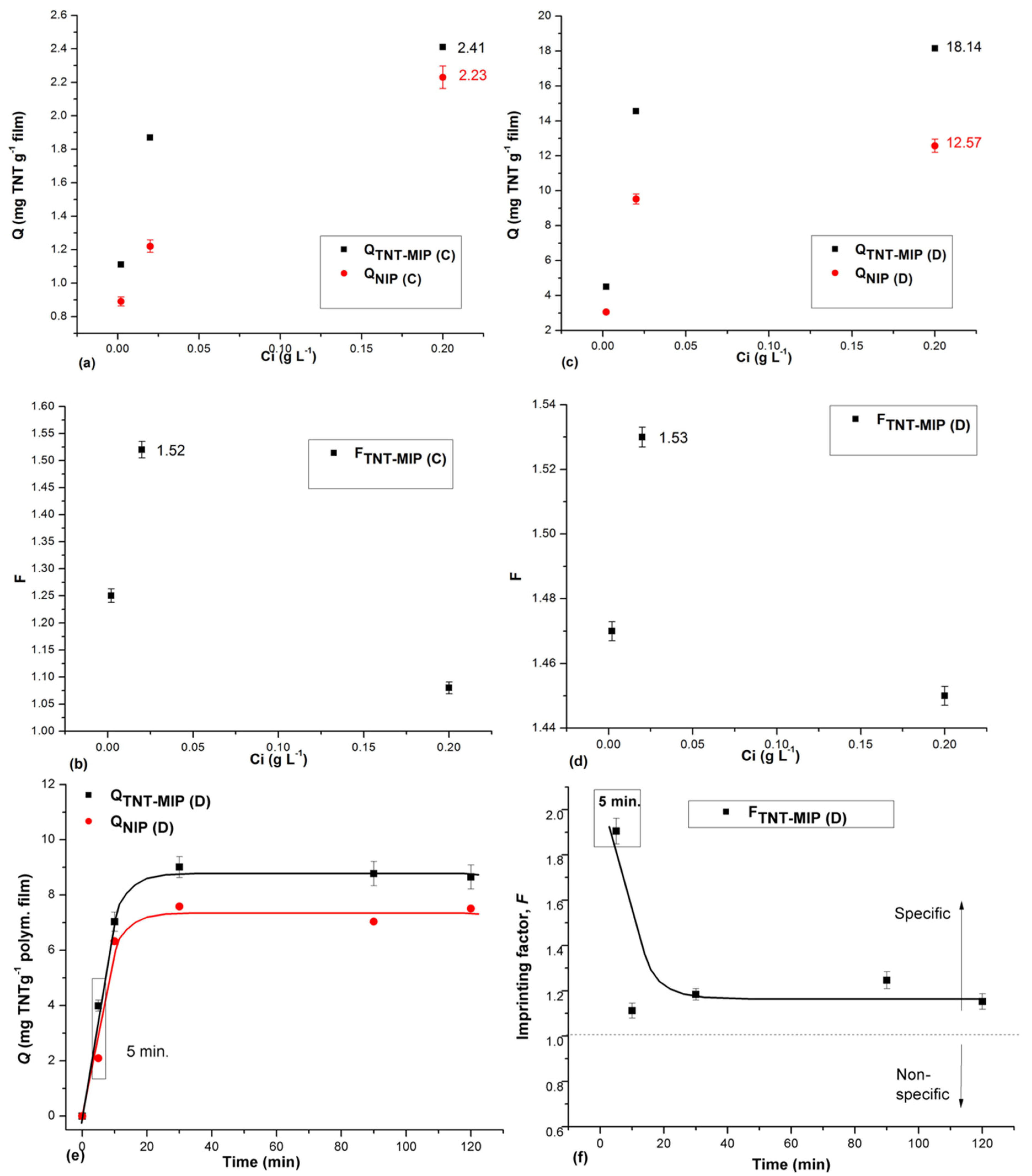
3.3. Batch Rebinding Studies for Films Deposited on Glass Slides
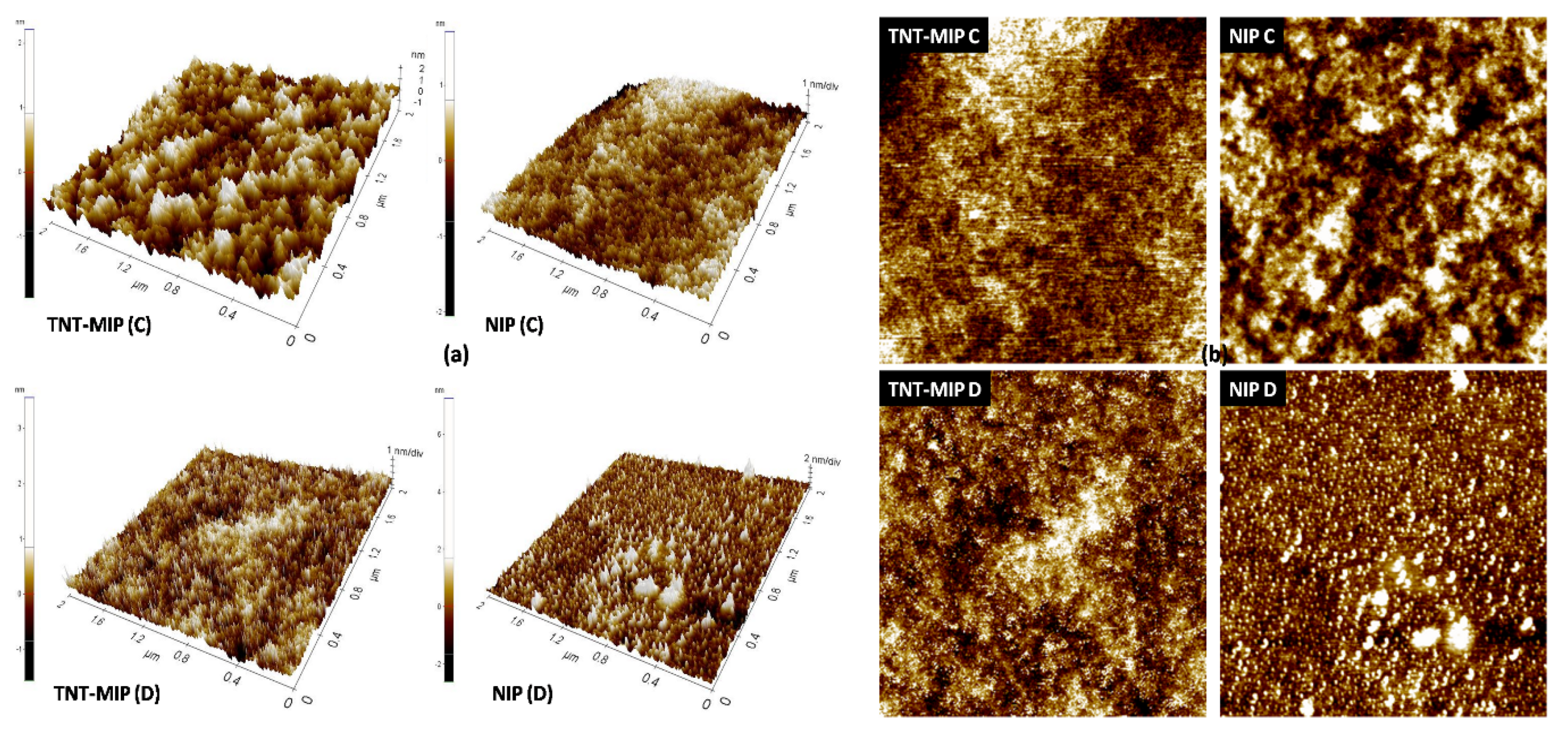
3.4. Microscopy of Films Deposited on Glass Slides
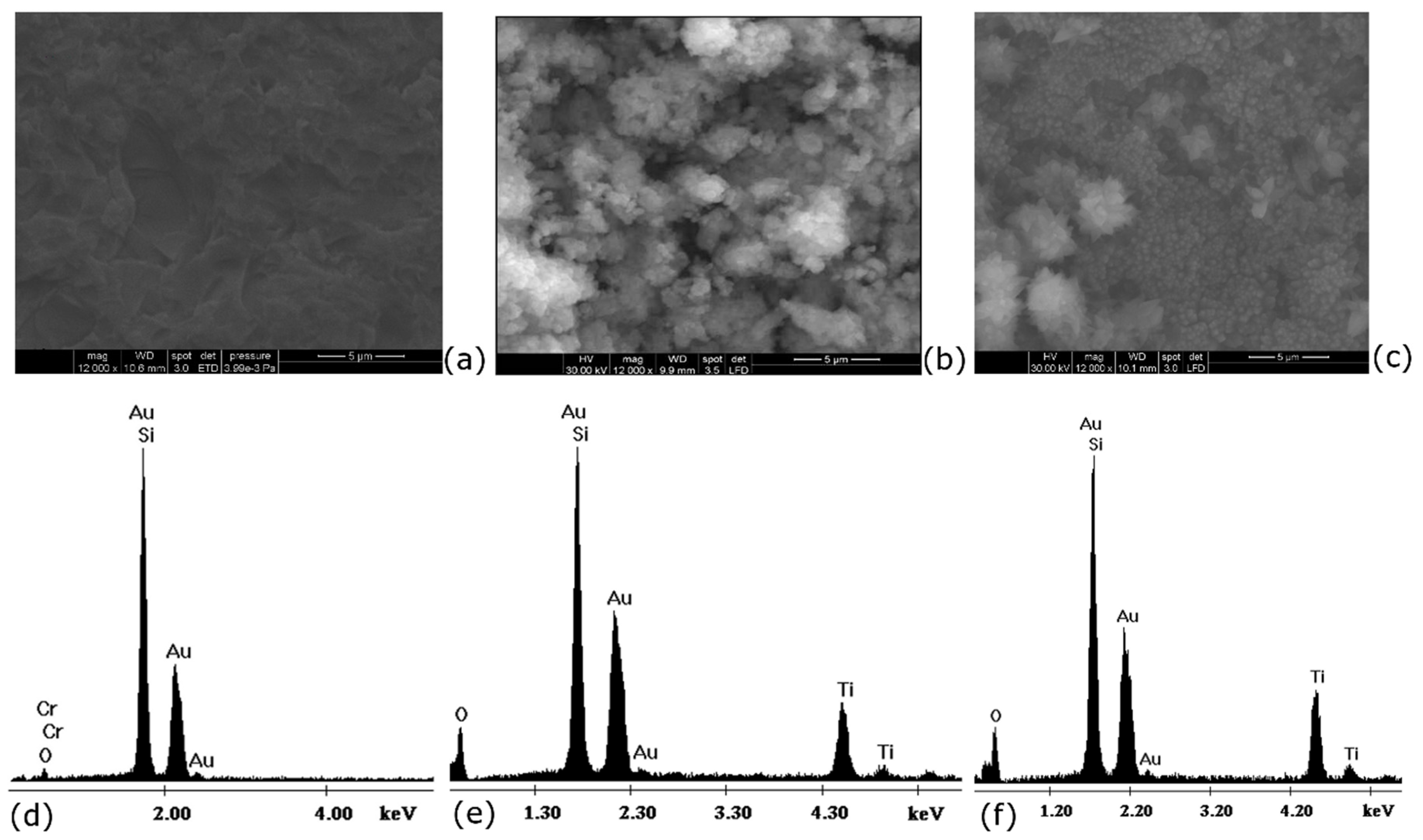
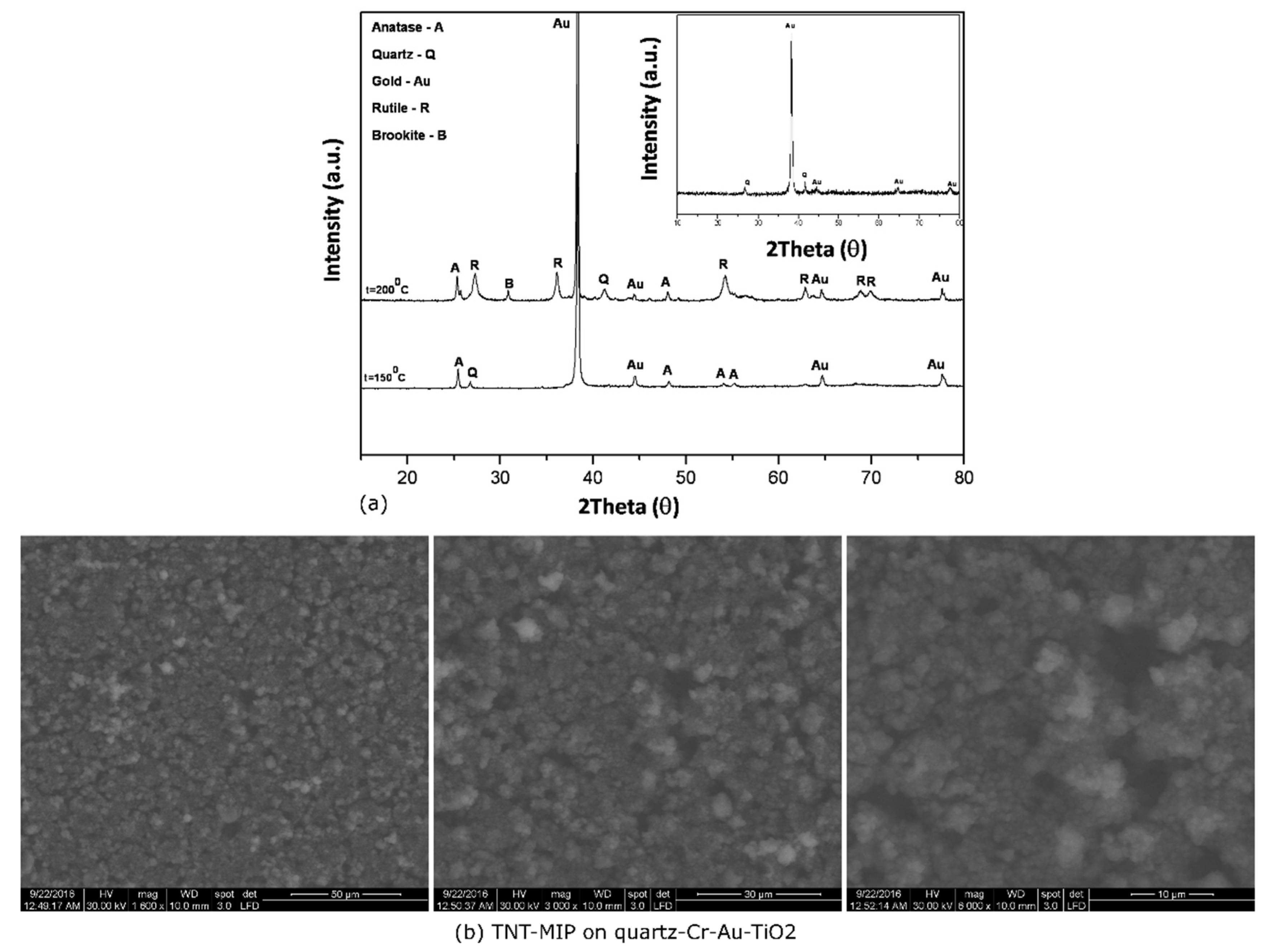
3.5. Characterization of Quartz-Cr-Au-TiO2-MIP Sensors
3.6. TNT Detection in Vapour State Using EM-Quartz-Cr-Au-TiO2-MIP Sensors
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, L.Q.; Zheng, Y.; Qu, W.G.; Xu, A.W. Convenient and sensitive synchronous fluorescence detection of trace TNT based on FRET using FITC–PAH as a probe. Anal. Methods 2013, 5, 603–607. [Google Scholar] [CrossRef]
- Ban, R.; Zheng, F.; Zhang, J. A highly sensitive fluorescence assay for 2,4,6-trinitrotoluene using amine-capped silicon quantum dots as a probe. Anal. Methods 2015, 7, 1732–1737. [Google Scholar] [CrossRef]
- Tu, R.Y.; Liu, B.H.; Wang, Z.Y. Amine-capped ZnS-Mn2+nanocrystals for fluorescence detection of trace TNT explosive. Anal. Chem. 2008, 80, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Lü, F.T.; Zhang, S.J. Preparation of monolayer-assembled fluorescent film and its sensing performances to hidden nitroaromatic explosives. Chin. Sci. Bull. 2008, 53, 1644–1650. [Google Scholar]
- Sohn, H.; Sailor, M.J.; Magde, D. Detection of nitroaromatic explosives based on photoluminescent polymers containing metalloles. J. Am. Chem. Soc. 2003, 125, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Dasary, S.S.R.; Singh, A.K.; Senapati, D.; Yu, H.T.; Ray, P.C. Gold nanoparticle based label-free SERS probe for ultrasensitive and selective detection of trinitrotoluene. J. Am. Chem. Soc. 2009, 131, 13806–13812. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Dasary, S.S.; Begum, S.; Williams, N.; Yu, H. Meisenheimer complex between 2,4,6-trinitrotoluene and 3-aminopropyltriethoxysilane and its use for a paper-based sensor. Sens. Biosensing Res. 2015, 5, 37–41. [Google Scholar] [CrossRef]
- Altstein, M.; Bronshtein, A.; Glattstein, B.; Zeichner, A.; Tamiri, T.; Almog, J. Immunochemical approaches for purification and detection of TNT traces by antibodies entrapped in a sol-gel matrix. Anal. Chem. 2001, 73, 2461–2467. [Google Scholar] [CrossRef]
- Ewing, R.G.; Atkinson, D.A.; Eiceman, G.A.; Ewing, G.J. A critical review of ion mobility spectrometry for the detection of explosives and explosive related compounds. Talanta 2001, 54, 515–529. [Google Scholar] [CrossRef]
- Asbury, G.R.J.; Klasmeier, H.H.; Hill, J. Analysis of explosives using electrospray ionization/ ion mobility spectrometry (ESI/IMS). Talanta 2000, 50, 1291–1298. [Google Scholar] [CrossRef]
- Zhou, H.B.; Zhang, Z.P.; Jiang, C.L.; Guan, G.J.; Zhang, K.; Mei, Q.S.; Liu, R.Y.; Wang, S.H. Trinitrotoluene explosive lights up ultrahigh Raman scattering of nonresonant molecule on a top-closed silver nanotube array. Anal. Chem. 2011, 6913–6917. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.A.; Edwards, H.G.M.; Scowen, I.J. Raman spectroscopy and security applications: The detection of explosives and precursors on clothing. J. Raman Spectrosc. 2009, 40, 2009–2014. [Google Scholar] [CrossRef]
- He, X.; Wang, H.; Li, Z.; Chen, D.; Zhang, Q. ZnO–Ag hybrids for ultrasensitive detection of trinitrotoluene by surface-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 14706–14712. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.G.; Deng, B.; Zhong, S.L.; Shi, H.Y.; Wang, S.S.; Xu, A.W. Plasmonic resonance energy transfer-based nanospectroscopy for sensitive and selective detection of 2,4,6-trinitrotoluene (TNT). Chem. Commun. 2011, 47, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Riskin, M.; Tel-Vered, R.; Lioubashevski, O.; Willner, I. Ultrasensitive surface plasmon resonance detection of trinitrotoluene by a bis-aniline-cross-linked Au nanoparticles composite. J. Am. Chem. Soc. 2009, 131, 7368–7378. [Google Scholar] [CrossRef] [PubMed]
- Giustina, G.D.; Sonato, A.; Gazzola, E.; Ruffato, G.; Brusa, S.; Romanato, F. SPR Enhanced molecular imprinted sol–gel film: A promising tool for gas-phase TNT detection. Mat. Let. 2016, 162, 44–47. [Google Scholar] [CrossRef]
- Ekberg, B.; Mosbach, K. Molecular imprinting: A technique for producing specific separation materials. Trends Biotech. 1989, 7, 92–96. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. Molecular imprinting science and technology: A survey of the literature for the years 2004-2011. J. Mol. Recognit. 2014, 27, 297–401. [Google Scholar]
- Bird, L.; Herdes, C. The porogen effect on the complexation step of trinitrotoluene–methacrylic acid: Towards efficient imprinted polymer sensors. Mol. Syst. Des. Eng. 2018, 3, 73–88. [Google Scholar] [CrossRef]
- Herdes, C.; Sarkisov, L. Computer simulation of volatile organic compound adsorption in atomistic models of molecularly imprinted polymers. Langmuir 2009, 25, 5352–5359. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Molecular imprinting techniques used for the preparation of biosensors. Sensors (Basel) 2017, 17, pii: E288. [Google Scholar]
- Chen, L.; Xua, S.; Li, J. Recent advances in molecular imprinting technology: Current status, challenges and highlighted applications. Chem. Soc. Rev. 2011, 40, 2922–2942. [Google Scholar] [CrossRef] [PubMed]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polymer Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Sarbu, A.; Iordache, T.V.; Florea, A.M.; Apostol, S.; Sandu, T.; Lazau, C.; Radu, A.L.; Nita, G. Trinitrotoluene Molecularly Imprinted Polymer films deposited onto TiO2 support and obtaining process. Patent RO 131692 B1 2018. [Google Scholar]
- Kalivretenos, A.G.; Van Houten, K.A.; Gluckman, J.P.; Hardy, F.M.; Dorovskoy, I.P.; Trower, R. Molecularly-imprinted polymeric materials for visual detection of explosives. Patent WO2010078426A9 18 November 2010. [Google Scholar]
- Sarbu, A.; Iordache, T.V.; Florea, A.M.; Apostol, S.; Sandu, T.; Lazau, C.; Bandas, C.E.; Orha, C.I.; Rotariu, T.; Voicu, E.A.; et al. Trinitrotoluene molecularly imprinted films by sol-gel method and process for their obtaining. Patent Application OSIM A00144 2016. [Google Scholar]
- Lazau, C.; Iordache, T.V.; Florea, A.M.; Orha, C.; Bandas, C.; Radu, A.L.; Sarbu, A.; Rotariu, T. Towards developing an efficient sensitive element for trinitrotoluene detection: TiO2 thin films functionalized with molecularly imprinted copolymer films. Appl. Surf. Sci. 2016, 384, 449–458. [Google Scholar] [CrossRef]
- Lazau, C.; Bandas, C.; Orha, C. Process for obtaining titanium dioxide films deposited on noble metal substrates, using the classical hydrothermal method. Patent RO132256 2019. [Google Scholar]
- Florea, A.M.; Iordache, T.V.; Branger, C.; Ghiurea, M.; Avramescu, S.; Hubca, G.; Sarbu, A. An innovative approach to prepare hypericin molecularly imprinted pearls using a phyto-template. Talanta 2016, 148, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Udomsap, D.; Branger, C.; Culiol, G.; Dollet, P.; Brisset, H. A versatile electrochemical sensing receptor based on a molecularly imprinted polymer. Chem. Commun. 2014, 50, 7488–7491. [Google Scholar] [CrossRef]
- Li, D.Y.; He, X.W.; Chen, Y.; Li, W.Y.; Zhang, Y.K. Novel hybrid structure silica/CdTe/molecularly imprinted polymer: Synthesis, specific recognition, and quantitative fluorescence detection of bovine hemoglobin. ACS Appl. Mater. Interfaces 2013, 23, 12609–12616. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitayama, Y.; Sasao, R.; Yamada, T.; Toh, K.; Matsumoto, Y.; Kataoka, K. Molecularly imprinted nanogels acquire stealth in situ by cloaking themselves with native dysopsonic proteins. Angew. Chem. Int. Ed. 2017, 56, 7088–7092. [Google Scholar] [CrossRef]
- Bertolla, M.; Cenci, L.; Anesi, A.; Ambrosi, E.; Tagliaro, F.; Vanzetti, L.; Guella, G.; Bossi, A.M. Solvent-responsive molecularly imprinted nanogels for targeted protein analysis in MALDI-TOF Mass Spectrometry. ACS Appl. Mater. Interfaces 2017, 9, 6908–6915. [Google Scholar] [CrossRef]
- Boysen, R.I.; Schwarz, L.J.; Nicolau, D.V.; Hearn, M.T. Molecularly imprinted polymer membranes and thin films for the separation and sensing of biomacromolecules. J. Sep. Sci. 2017, 40, 314–335. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, A.; Lieberzeit, P.A.; Dickert, F.L. Chemical sensors based on molecularly imprinted sol-gel materials. Materials 2010, 3, 2196–2217. [Google Scholar] [CrossRef]
- Florea, A.M.; Iordache, T.V.; Branger, C.; Brisset, H.; Zaharia, A.; Radu, A.L.; Hubca, G.; Sarbu, A. One-step preparation of molecularly imprinted hollow beads for pseudohypericin separation from Hypericum perforatum L. Extracts. Eur. Polym. J. 2018, 100, 48–56. [Google Scholar] [CrossRef]
- Nakai, S.; Sunayama, H.; Kitayama, Y.; Nishijima, M.; Wada, T.; Inoue, Y.; Takeuchi, T. Regioselective molecularly imprinted reaction field for [4 + 4] photocyclodimerization of 2-anthracenecarboxylic acid. Langmuir 2017, 33, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.A.; Whitcombe, M.J. Designing Receptors for the Next Generation of Biosensors, 1st ed.; Springer Series on Chemical Sensors and Biosensors: Berlin/Heidelberg, Germany, 2013; pp. 1–256. [Google Scholar]
- Di Bello, M.P.; Lazzoi, M.R.; Mele, G.; Scorrano, S.; Mergola, L.; Del Sole, R. A New Ion-Imprinted Chitosan-Based Membrane with an Azo-Derivative Ligand for the Efficient Removal of Pd(II). Materials 2017, 10, 1133. [Google Scholar] [CrossRef]
- Du, X.; Guan, G.; Li, X.; Jagadale, A.D.; Ma, X.; Wang, Z.; Hao, X.; Abudul, A. A novel electroactive λ-MnO2/PPy/PSS core–shell nanorod coated electrode for selective recovery of lithium ions at low concentration. J. Mater. Chem. A 2016, 4, 13989–13996. [Google Scholar] [CrossRef]
- Öncel, P.; Çetin, K.; Topçu, A.A.; Yavuz, H.; Denizli, A. Molecularly imprinted cryogel membranes for mitomycin C delivery. J. Biomater. Sci. Polym. Ed. 2017, 28, 519–531. [Google Scholar] [CrossRef]
- Székely, G.; Valtcheva, I.B.; Kim, J.F.; Livingston, A.G. Molecularly imprinted organic solvent nanofiltration membranes—Revealing molecular recognition and solute rejection behavior. React. Funct. Polym. 2015, 86, 215–224. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Z.; Wu, M.; Xie, C.; Guan, G.; Wang, D. A surface functional monomer-directing strategy for highly dense imprinting of TNT at surface of silica nanoparticles. J. Am. Chem. Soc. 2007, 129, 7859–7866. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, Z.; Wang, D.; Guan, G.; Gao, D.; Liu, J. Surface molecular self-assembly strategy for TNT imprinting of polymer nanowire/nanotube arrays. Anal. Chem. 2006, 78, 8339–8346. [Google Scholar] [CrossRef]
- Janata, J. Principles of Chemical Sensors, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 99–266. [Google Scholar]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Terzic, E.; Terzic, J.; Nagarajah, R.; Alamgir, M. (Eds.) Capacitive Sensing Technology. In A Neural Network Approach to Fluid Quantity Measurement in Dynamic Environments; Springer: Berlin/Heidelberg, Germany, 2012; pp. 11–37. [Google Scholar]
- Xu, B.; Wu, X.; Li, H.; Tong, H.; Wang, L. Selective Detection of TNT and Picric Acid by Conjugated Polymer Film Sensors with Donor–Acceptor Architecture. Macromolecules 2011, 44, 5089–5092. [Google Scholar] [CrossRef]
- Stoica, E.B.; Gavrila, A.M.; Branger, C.; Brisset, H.; Dyshlyuk, A.V.; Vitrik, O.B.; Iovu, H.; Sarbu, A.; Iordache, T.V. Evaluation of Molecularly Imprinted Thin Films for Ephedrine recognition. Mater. Plast. 2019, 4, 865–874. [Google Scholar] [CrossRef]
- Martelo, L.M.; Marques, L.F.; Burrows, H.D.; Berberan-Santos, M.N. Fluorescence in Industry. In Explosives Detection: From Sensing to Response; Bruno, P., Ed.; Springer: Cham, Switzerland, 2019; Volume 18, pp. 293–320. [Google Scholar]
- Fang, Y.; Furniss, D.; Jayasuriya, D.; Parnell, H.; Tang, Z.Q.; Gibson, D.; Bayya, S.; Sanghera, J.; Seddon, A.B.; Benson, T.M. Methods for determining the refractive indices and thermo-optic coefficients of chalcogenide glasses at MIR wavelengths. Opt. Mater. X 2019, 2, 100030. [Google Scholar] [CrossRef]
- El Kirat, K.; Bartkowski, M.; Haupt, K. Probing the recognition specificity of a protein molecularly imprinted polymer using force spectroscopy. Biosens. Bioelectron. 2009, 24, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Iatsunskyi, I.; Jancelewicz, M.; Nowaczyk, G.; Kempiński, M.; Peplińska, B.; Jarek, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Atomic layer deposition TiO2 coated porous silicon surface: Structural characterization and morphological features. Thin Solid Films 2015, 589, 303–308. [Google Scholar] [CrossRef]
- Ragazzon, D.; Farstad, M.H.; Schaefer, A.; Walle, L.E.; Uvdal, P.; Borg, A.; Sandell, A. Growth of TiO2(B)(001) on Au(111) by chemical vapor deposition. Surf. Sci. 2015, 633, 102–108. [Google Scholar] [CrossRef]
- Grunwaldt, J.D.; Göbel, U.; Baiker, A. Preparation and characterization of thin TiO2-films on gold/mica. Fresenius J. Anal. Chem. 1997, 358, 96–100. [Google Scholar] [CrossRef]
- Allen, N.S.; Mahdjoub, N.; Vishnyakov, V.; Kelly, P.J.; Kriek, R.J. The effect of crystalline phase (anatase, brookite and rutile) and size on the photocatalytic activity of calcined polymorphic titanium dioxide (TiO2). Polym. Degrad. Stab. 2018, 150, 31–36. [Google Scholar] [CrossRef]
- Folger, A.; Kalb, J.; Schmidt-Mende, L.; Scheu, C. Tuning the Electronic Conductivity in Hydrothermally Grown Rutile TiO2 Nanowires: Effect of Heat Treatment in Different Environments. Nanomaterials (Basel) 2017, 7, 289. [Google Scholar] [CrossRef]
- Kavalenka, M.N.; Striemera, C.C.; DesOrmeaux, J.P.S.; McGrath, J.L.; Fauchet, P.M. Chemical capacitive sensing using ultrathin flexible nanoporous electrodes. Sens. Actuators B Chem. 2012, 162, 22–26. [Google Scholar] [CrossRef]
- Scherz, P. Practical Electronics for Inventors, 4th ed.; McGraw-Hill Professional Publishing: New York, NY, USA, 2000; Chapter 2. [Google Scholar]


| Polymer Film | 1141-D (mmoles) | TNT (mmoles) | 1141-D:TNT (M/M) |
|---|---|---|---|
| TNT-MIP (C) | 0.726 | 0.073 | 10:1 |
| NIP (C) | 0.726 | 0 | 0 |
| TNT-MIP (D) | 0.363 | 0.036 | 10:1 |
| NIP (D) | 0.363 | 0 | 0 |
| Film | d layer (nm) | Roughness (nm) | MSE |
|---|---|---|---|
| NIP (C) | 77.32 | 0.00 | 0.52 |
| NIP (D) | 24.24 | 2.45 | 0.31 |
| TNT-MIP (C) | 2174.19 | 0.03 | 0.98 |
| TNT-MIP (D) | 231.57 | 0.77 | 1.24 |
| N 1 | R | C | PA | I | Rt | r | H |
|---|---|---|---|---|---|---|---|
| 1 | 192.2 | 163.3 | −78.90 | −974.5 | 21.5 | 0/0 | 5 |
| 2 | 198.5 | 166.9 | −78.24 | 953.4 | 24.0 | 0/0 | 31 |
| 3 | 234.0 | 329.8 | −64.14 | −482.6 | 23.5 | 2/0 | 5 |
| 4 | 234.0 | 329.8 | −64.14 | −482.6 | 25.0 | 2/2 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
GAVRILA, A.M.; IORDACHE, T.V.; LAZAU, C.; ROTARIU, T.; CERNICA, I.; STROESCU, H.; STOICA, M.; ORHA, C.; BANDAS, C.E.; SARBU, A. Biomimetic Sensitive Elements for 2,4,6-Trinitrotoluene Tested on Multi-Layered Sensors. Coatings 2020, 10, 273. https://doi.org/10.3390/coatings10030273
GAVRILA AM, IORDACHE TV, LAZAU C, ROTARIU T, CERNICA I, STROESCU H, STOICA M, ORHA C, BANDAS CE, SARBU A. Biomimetic Sensitive Elements for 2,4,6-Trinitrotoluene Tested on Multi-Layered Sensors. Coatings. 2020; 10(3):273. https://doi.org/10.3390/coatings10030273
Chicago/Turabian StyleGAVRILA, Ana Mihaela, Tanta Verona IORDACHE, Carmen LAZAU, Traian ROTARIU, Ileana CERNICA, Hermine STROESCU, Mihai STOICA, Corina ORHA, Cornelia Elena BANDAS, and Andrei SARBU. 2020. "Biomimetic Sensitive Elements for 2,4,6-Trinitrotoluene Tested on Multi-Layered Sensors" Coatings 10, no. 3: 273. https://doi.org/10.3390/coatings10030273
APA StyleGAVRILA, A. M., IORDACHE, T. V., LAZAU, C., ROTARIU, T., CERNICA, I., STROESCU, H., STOICA, M., ORHA, C., BANDAS, C. E., & SARBU, A. (2020). Biomimetic Sensitive Elements for 2,4,6-Trinitrotoluene Tested on Multi-Layered Sensors. Coatings, 10(3), 273. https://doi.org/10.3390/coatings10030273












