The Impact of Curcumin on Bone Osteogenic Promotion of MC3T3 Cells under High Glucose Conditions and Enhanced Bone Formation in Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Analysis
2.1.1. Cell Culture
2.1.2. Cell Proliferation
2.1.3. Cell Apoptosis
2.1.4. Osteogenesis-Related Gene Expression of MC3T3 Cells
2.2. In Vivo Analysis
2.2.1. Experimental Animals and Grouping
2.2.2. Polyfluorochrome Sequential Labeling of Bone
2.2.3. Histological Analysis
2.3. Statistical Analysis
3. Results and Discussion
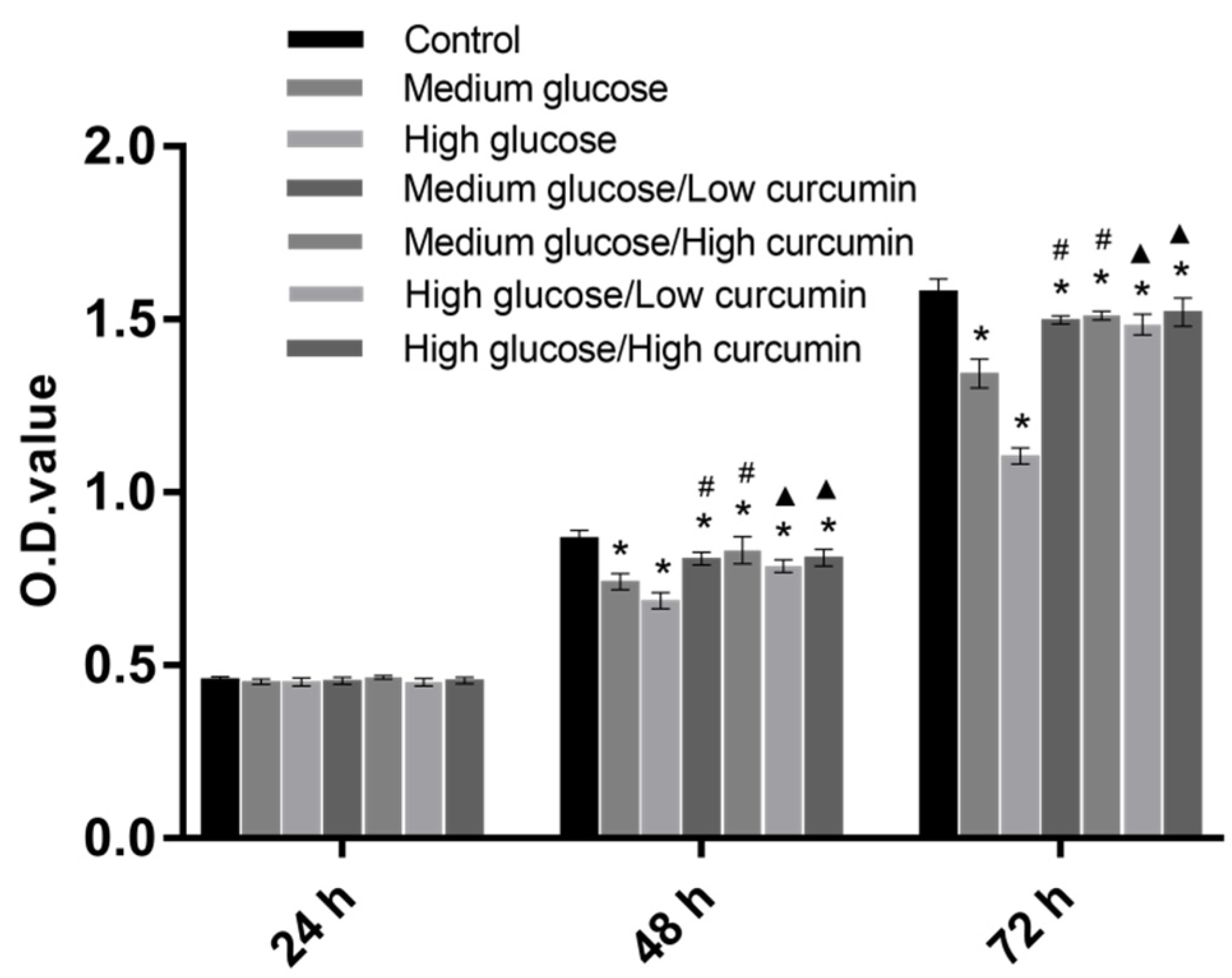
3.1. Effect of Curcumin on the Viability of MC3T3 Cells in High-Glucose Conditions
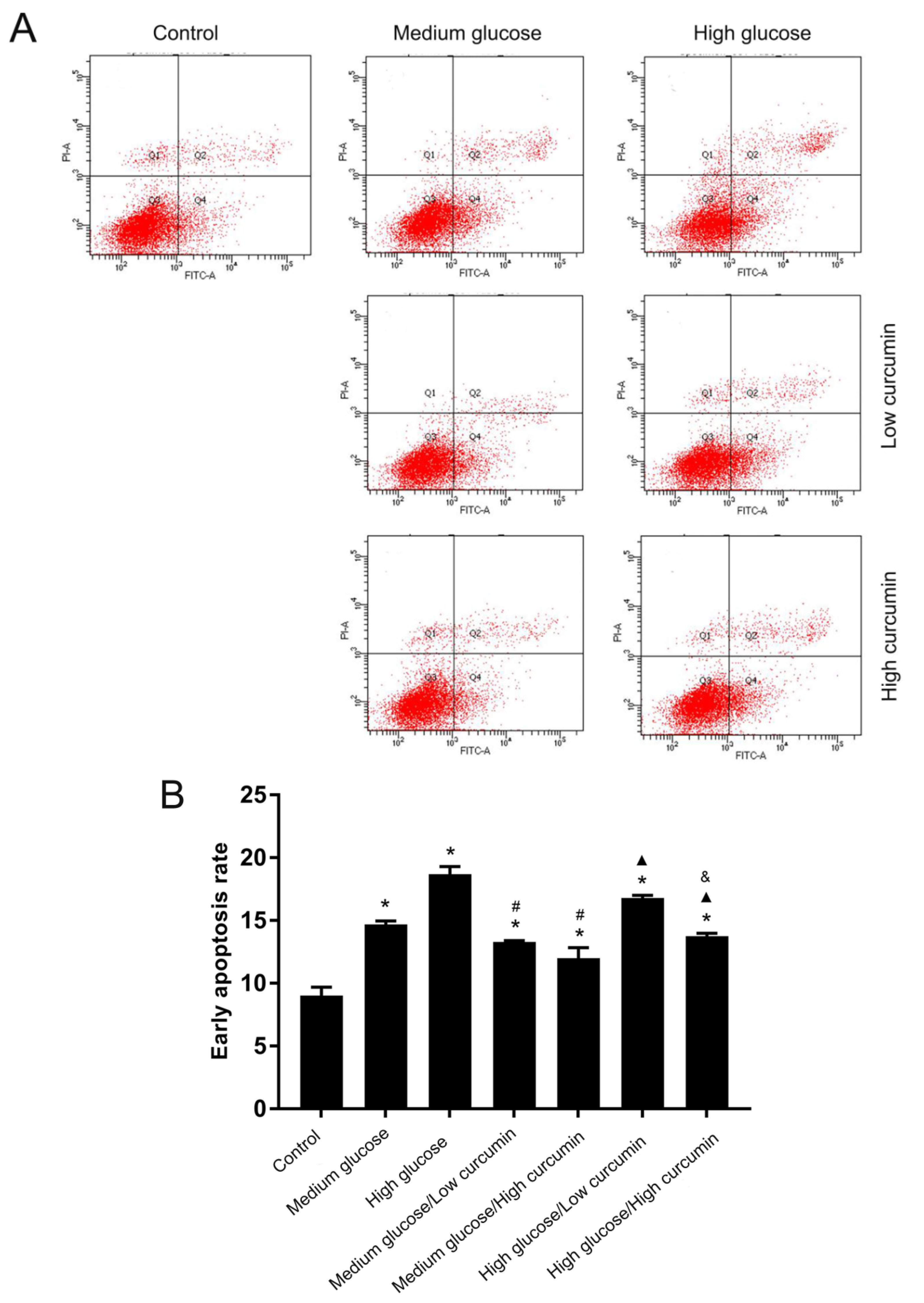
3.2. Effect of Curcumin on Apoptosis of MC3T3 Cells in High-Glucose Conditions
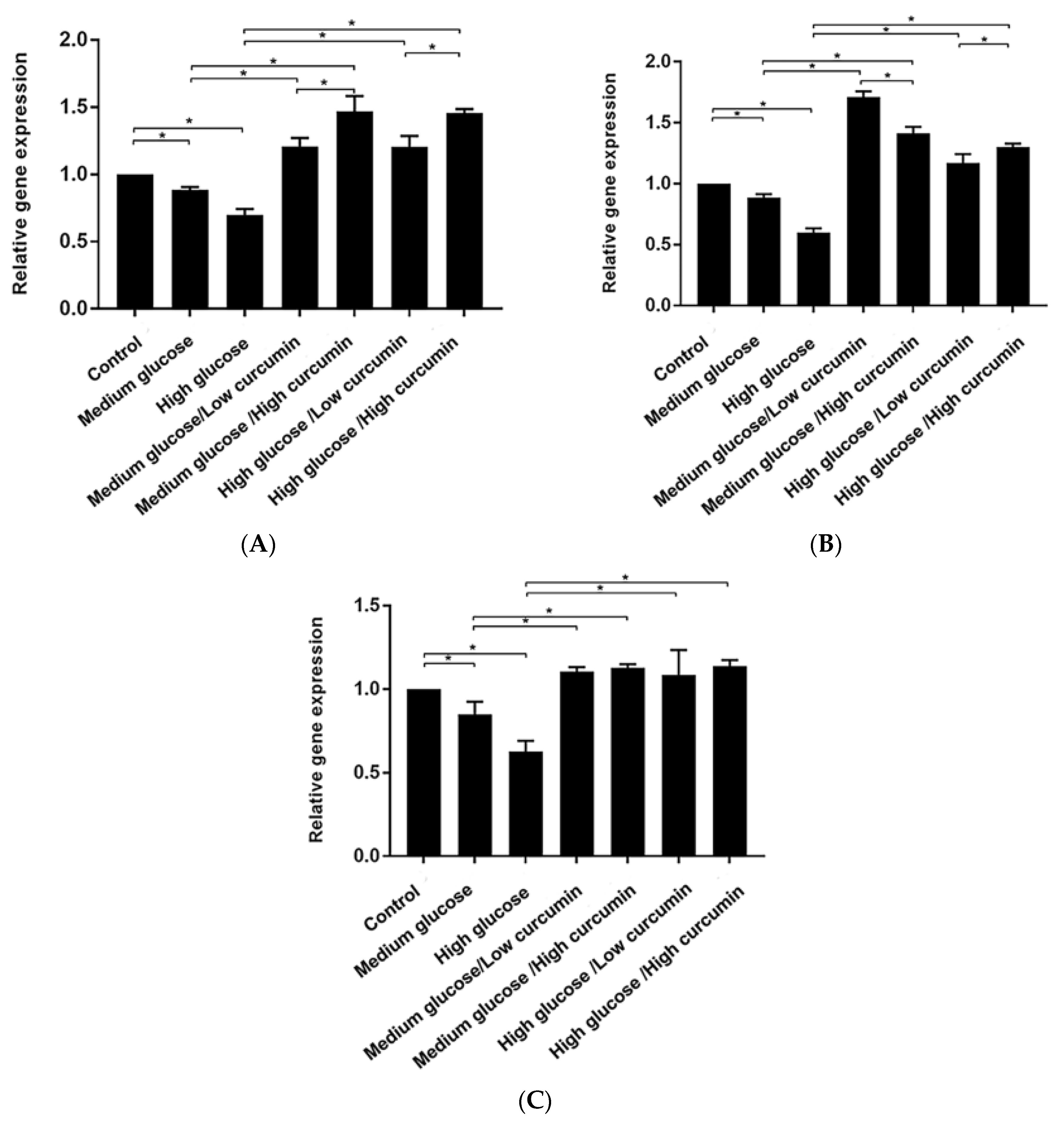
3.3. Effect of Curcumin on Osteogenic Differentiation of MC3T3 Cells in Glucose Conditions
3.4. Curcumin Treatment Improved Alveolar Bone Formation in Diabetic Mice
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, N.; Du, S.M.; Ma, G.S. Current lifestyle factors that increase risk of T2DM in China. Eur. J. Clin. Nutr. 2017, 71, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qiao, S.; Shi, C.; Wang, S.; Ji, G. Metabolomics window into diabetic complications. J. Diabetes Investig. 2018, 9, 244–255. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, L.M.N.; Vaz, S.R.; Cesário, G.; Coelho, A.S.G.; Botelho, P. Effect of green tea extract on bone mass and body composition in individuals with diabetes. J. Funct. Foods 2018, 40, 589–594. [Google Scholar] [CrossRef]
- Wang, T.; Cai, L.; Wang, Y.; Wang, Q.; Lu, D.; Chen, H.; Ying, X. The protective effects of silibinin in the treatment of streptozotocin-induced diabetic osteoporosis in rats. Biomed. Pharmacother. 2017, 89, 681–688. [Google Scholar] [CrossRef]
- Hamann, C.; Kirschner, S.; Günther, K.P.; Hofbauer, L.C. Bone, sweet bone-osteoporotic fractures in diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 297–305. [Google Scholar] [CrossRef]
- Schwartz, A.V. Efficacy of osteoporosis therapies in diabetic patients. Calcif. Tissue Int. 2017, 100, 165–173. [Google Scholar] [CrossRef]
- Onishi, Y.; Yamada, K.; Zacho, J.; Ekelund, J.; Iwamoto, Y. Insulin degludec/insulin aspart versus biphasic insulin aspart 30 twice daily in Japanese subjects with type 2 diabetes: A randomized controlled trial. J. Diabetes Investig. 2017, 8, 210–217. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Xiao, S.; Liu, S.; Li, X.; Yu, P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: A systematic review and meta-analysis. Diabetes Ther. 2017, 8, 727–738. [Google Scholar] [CrossRef]
- Eibich, P.; Green, A.; Hattersley, A.T.; Jennison, C.; Lonergan, M.; Pearson, E.R.; Gray, A.M. Costs and treatment pathways for type 2 diabetes in the UK: A mastermind cohort study. Diabetes Ther. 2017, 8, 1031–1045. [Google Scholar] [CrossRef]
- Xie, A.; Li, R.; Tao, J.; Jiang, T.; Yan, H.; Zhang, H.; Yang, Y.; Yang, L.; Yechoor, V.; Chan, L.; et al. Anti-TCRβ mAb in combination with Neurogenin3 gene therapy reverses established overt type 1 diabetes in female NOD mice. Endocrinology 2017, 158, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Elburki, M.S.; Moore, D.D.; Terezakis, N.G.; Zhang, Y.; Lee, H.M.; Johnson, F.; Golub, L.M. A novel chemically modified curcumin reduces inflammation-mediated connective tissue breakdown in a rat model of diabetes: Periodontal and systemic effects. J. Periodontal. Res. 2017, 52, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Alessandra, G.M.; Daniela, F.P.; Jossiele, W.L.; Tamiris, R.S.; Pedro, H.D.; Matheus, H.J.; Vania, L.L.; Daniela, B.R.L. Hyperlipidemia-induced lipotoxicity and immune activation in rats are prevented by curcumin and rutin. Int. Immunopharmacol. 2020, 81, 106217. [Google Scholar] [CrossRef]
- Busari, Z.A.; Dauda, K.A.; Morenikeji, O.A.; Afolayan, F.; Oyeyemi, O.T.; Meena, J.; Sahu, D.; Panda, A.K. Antiplasmodial activity and toxicological assessment of curcumin PLGA-encapsulated nanoparticles. Front. Pharmacol. 2017, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Ziwei, M.; Na, W.; Haibing, H.; Xing, T. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 31, 359–380. [Google Scholar] [CrossRef]
- Vincenzo, D.L.; Sante, D.G.; Francesco, M.; Paola, F.; Roberto, C.; Erminia, M.; Angela, A.; Massimo, C.; Lucia, C. Eudragit S100 entrapped liposome for curcumin delivery: Anti-oxidative effect in Caco-2 cells. Coatings 2020, 10, 114. [Google Scholar] [CrossRef]
- Anita, B.; Inese, M.; Simons, S.; Modra, M.; Martins, K. Curcumin effect on copper transport in HepG2 cells. Medicina 2018, 54, 14. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Watari, I.; Soma, K. The effect of diabetes mellitus on rat mandibular bone formation and microarchitecture. Eur. J. Oral. Sci. 2010, 118, 364–369. [Google Scholar] [CrossRef]
- Naijil, G.; Anju, T.R.; Jayanarayanan, S.; Paulose, C.S. Curcumin pretreatment mediates antidiabetogenesis via functional regulation of adrenergic receptor subtypes in the pancreas of multiple low-dose streptozotocin-induced diabetic rats. Nutr. Res. 2015, 35, 823–833. [Google Scholar] [CrossRef]
- Elburki, M.S.; Rossa, C.J.; Guimaraes-stabili, M.R.; Lee, H.M.; Curylofo, F.A.; Johnson, F.; Golub, L.M. A chemically modified curcumin (CMC 2.24) inhibits nuclear factor κB activation and inflammatory bone loss in murine models of LPS-induced experimental periodontitis and diabetes-associated natural periodontitis. Inflammation 2017, 40, 1436–1449. [Google Scholar] [CrossRef]
- Wu, T.J.; Lin, C.Y.; Tsai, C.H.; Huang, Y.L.; Tang, C.H. Glucose suppresses IL-1β-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ. Toxicol. 2018, 33, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, S.P.; Casati, M.Z.; Ribeiro, F.V.; Corrêa, M.G.; Franck, F.C.; Benatti, B.B.; Cirano, F.R. Impact of natural curcumin on the progression of experimental periodontitis in diabetic rats. J. Periodontal Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhanzhao, Z. Sustained curcumin release from PLGA microspheres improves bone formation under diabetic conditions by inhibiting the reactive oxygen species production. Drug Des. Dev. Ther. 2018, 12, 1453–1466. [Google Scholar] [CrossRef]
- Cirano, F.R.; Pimentel, S.P.; Casati, M.Z.; Corrêa, M.G.; Pino, D.S.; Messora, M.R.; Silva, P.H.F.; Ribeiro, F.V. Effect of curcumin on bone tissue in the diabetic rat: Repair of peri-implant and critical-sized defects. Int. J. Oral. Maxillofac. Surg. 2018, 47, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, J.; Shan, Q.; Geng, G.; Shao, P. High glucose inhibits proliferation and differentiation of osteoblast in alveolar bone by inducing pyroptosis. Biochem. Biophys. Res. Commun. 2020, 522, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Son, H.E.; Kim, E.J.; Jang, W.G. Curcumin induces osteoblast differentiation through mild-endoplasmic reticulum stress-mediated such as BMP2 on osteoblast cells. Life Sci. 2018, 193, 34–39. [Google Scholar] [CrossRef]
- Yang, H.; Gu, Q.; Huang, C.; Shi, Q.; Cai, Y. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn. Mag. 2012, 8, 202–208. [Google Scholar] [CrossRef]
- Chang, R.; Sun, L.; Webster, T.J. Short communication: Selective cytotoxicity of curcumin on osteosarcoma cells compared to healthy osteoblasts. Int. J. Nanomed. 2014, 9, 461–465. [Google Scholar] [CrossRef][Green Version]
- Moran, J.M.; Roncero-Martin, R.; Rodriguez-Velasco, F.J.; Calderon-Garcia, J.F.; Rey-Sanchez, P.; Vera, V.; Canal-Macias, M.L.; Pedrera-Zamorano, J.D. Effects of curcumin on the proliferation and mineralization of human osteoblast-like cells: Implications of nitric oxide. Int. J. Mol. Sci. 2012, 13, 16104–16118. [Google Scholar] [CrossRef]
- Notoya, M.; Nishimura, H.; Woo, J.T.; Nagai, K.; Ishihara, Y.; Hagiwara, H. Curcumin inhibits the proliferation and mineralization of cultured osteoblasts. Eur. J. Pharmacol. 2006, 534, 55–62. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.Z.; Casarin, R.C.; Cirano, F.R.; Tenenbaum, H.T.; Casati, M.Z. Systemic treatment with resveratrol and/or curcumin reduces the progression of experimental periodontitis in rats. J. Periodontal Res. 2017, 52, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Boșca, A.B.; Ilea, A.; Sorițău, O.; Tatomir, C.; Miklášová, N.; Pârvu, A.E.; Mihu, C.M.; Melincovici, C.S.; Fischer-Fodor, E. Modulatory effect of curcumin analogs on the activation of metalloproteinases in human periodontal stem cells. Eur. J. Oral. Sci. 2019, 127, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Jiao, G.; Liu, H.; Wu, W.; Li, S.; Wang, Q.; Xu, D.; Li, X.; Liu, H.; Chen, Y. Biological silicon stimulates collagen type 1 and osteocalcin synthesis in human osteoblast-like cells through the BMP-2/Smad/RUNX2 signaling pathway. Biol. Trace Elem. Res. 2016, 173, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.L.; Ao, M.; Salmon, C.R.; Chavez, M.B.; Kolli, T.N.; Tran, A.B.; Chu, E.Y.; Kantovitz, K.R.; Yadav, M.; Narisawa, S. Osteopontin regulates dentin and alveolar bone development and mineralization. Bone 2018, 107, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.S.; Balani, D.; Sloan, A.J.; Crean, S.J.; Okazaki, J.; Waddington, R.J. Delayed osteoblast differentiation and altered inflammatory response around implants placed in incisor sockets of type 2 diabetic rats. Clin. Oral. Implants Res. 2011, 22, 578–586. [Google Scholar] [CrossRef]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. J. Diabetes Res. 2017, 2017, 1–30. [Google Scholar] [CrossRef]

| Experiment Groups | Glucose | Curcumin | Abbreviation |
|---|---|---|---|
| Control | 5.5 mmol/L | 0 μmol/L | C |
| Medium glucose | 11 mmol/L | 0 μmol/L | Mg |
| High glucose | 16.5 mmol/L | 0 μmol/L | Hg |
| Medium glucose/Low curcumin | 11 mmol/L | 5 μmol/L | Mg-Lc |
| Medium glucose/High curcumin | 11 mmol/L | 10 μmol/L | Mg-Hc |
| High glucose/Low curcumin | 16.5 mmol/L | 5 μmol/L | Hg-Lc |
| High glucose/High curcumin | 16.5 mmol/L | 10 μmol/L | Hg-Hc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Yang, X.; Liu, F.; Li, D.; Zheng, B.; Abdullah, A.O.; Liu, Y. The Impact of Curcumin on Bone Osteogenic Promotion of MC3T3 Cells under High Glucose Conditions and Enhanced Bone Formation in Diabetic Mice. Coatings 2020, 10, 258. https://doi.org/10.3390/coatings10030258
He J, Yang X, Liu F, Li D, Zheng B, Abdullah AO, Liu Y. The Impact of Curcumin on Bone Osteogenic Promotion of MC3T3 Cells under High Glucose Conditions and Enhanced Bone Formation in Diabetic Mice. Coatings. 2020; 10(3):258. https://doi.org/10.3390/coatings10030258
Chicago/Turabian StyleHe, Jia, Xiaofeng Yang, Fan Liu, Duo Li, Bowen Zheng, Adil Othman Abdullah, and Yi Liu. 2020. "The Impact of Curcumin on Bone Osteogenic Promotion of MC3T3 Cells under High Glucose Conditions and Enhanced Bone Formation in Diabetic Mice" Coatings 10, no. 3: 258. https://doi.org/10.3390/coatings10030258
APA StyleHe, J., Yang, X., Liu, F., Li, D., Zheng, B., Abdullah, A. O., & Liu, Y. (2020). The Impact of Curcumin on Bone Osteogenic Promotion of MC3T3 Cells under High Glucose Conditions and Enhanced Bone Formation in Diabetic Mice. Coatings, 10(3), 258. https://doi.org/10.3390/coatings10030258






