Bioactivity Behavior Evaluation of PCL-Chitosan-Nanobaghdadite Coating on AZ91 Magnesium Alloy in Simulated Body Fluid
Abstract
1. Introduction
2. Materials and Methods
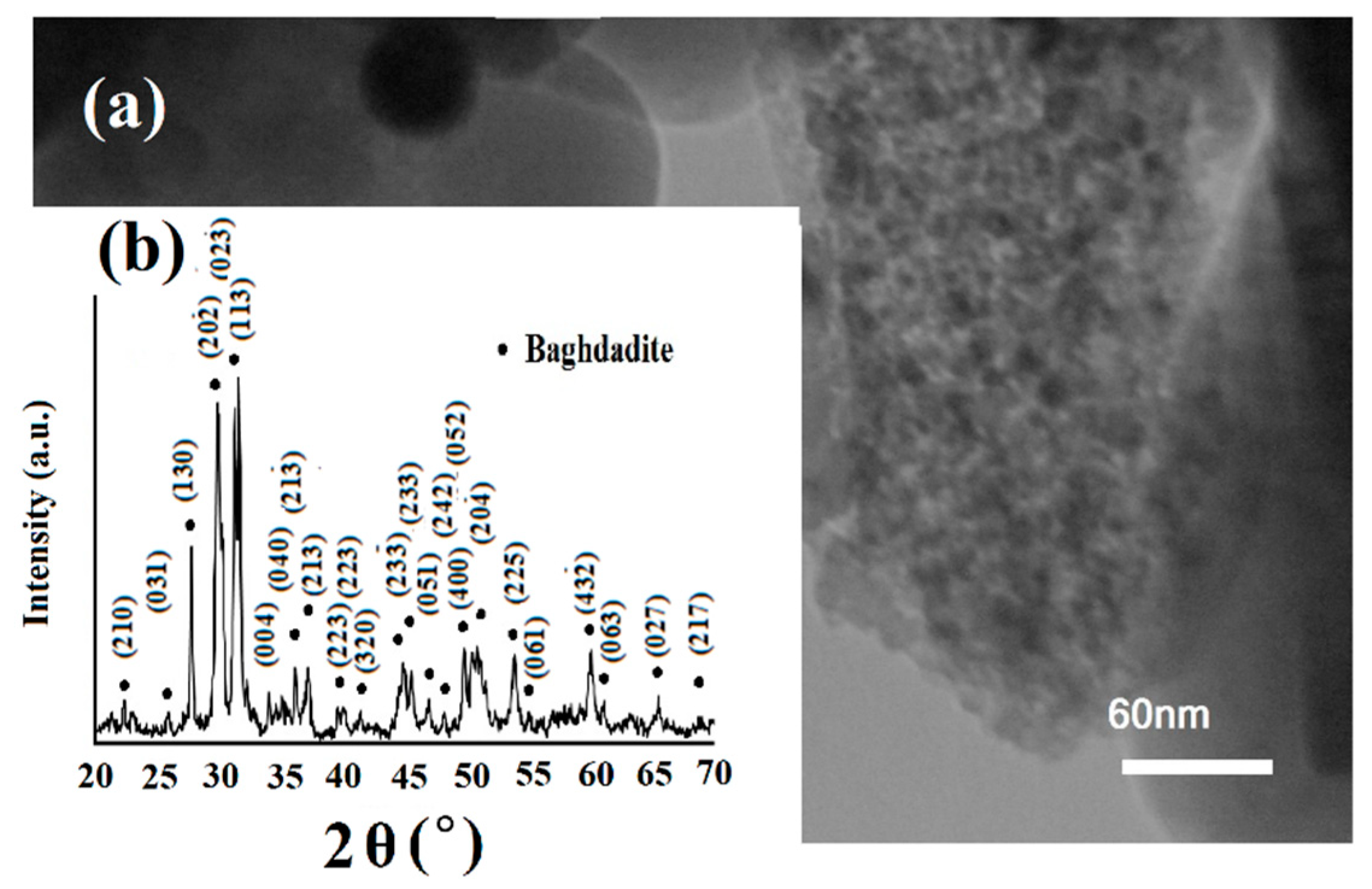
2.1. Nano-Baghdadite Fabrication
2.2. Polymeric Coating Process on AZ91
2.3. Evaluation of the Biocorrosion Behavior
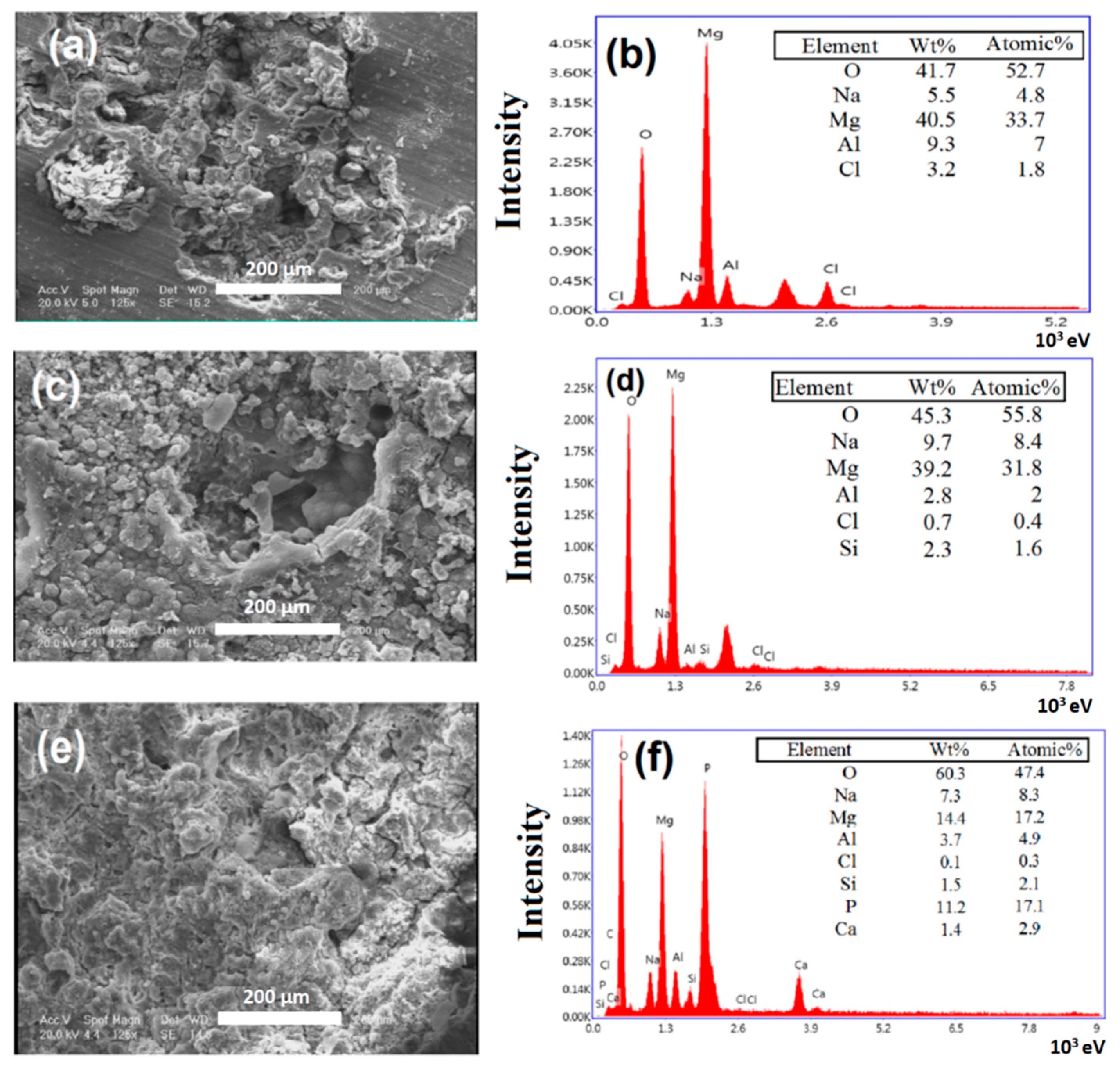
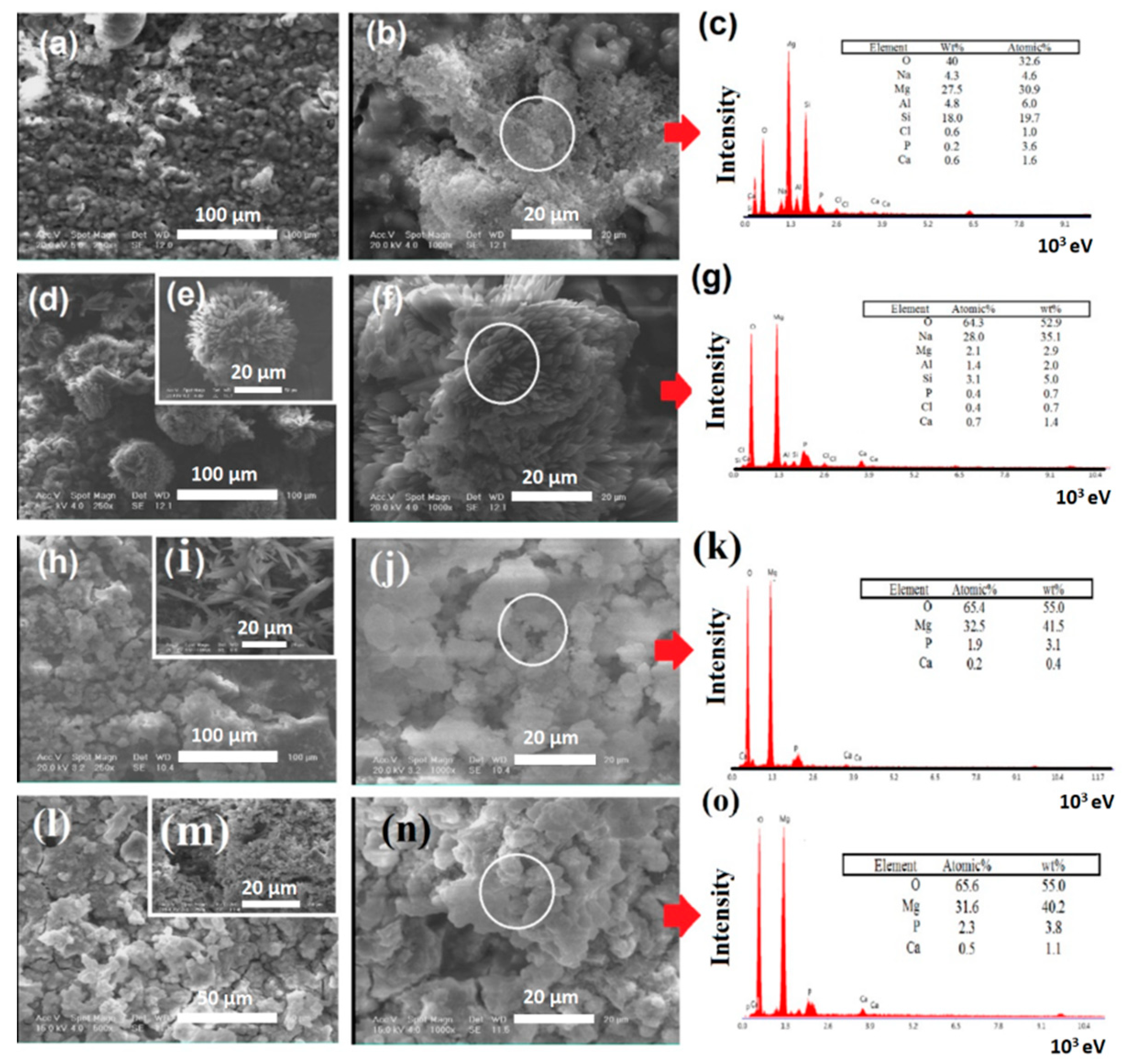
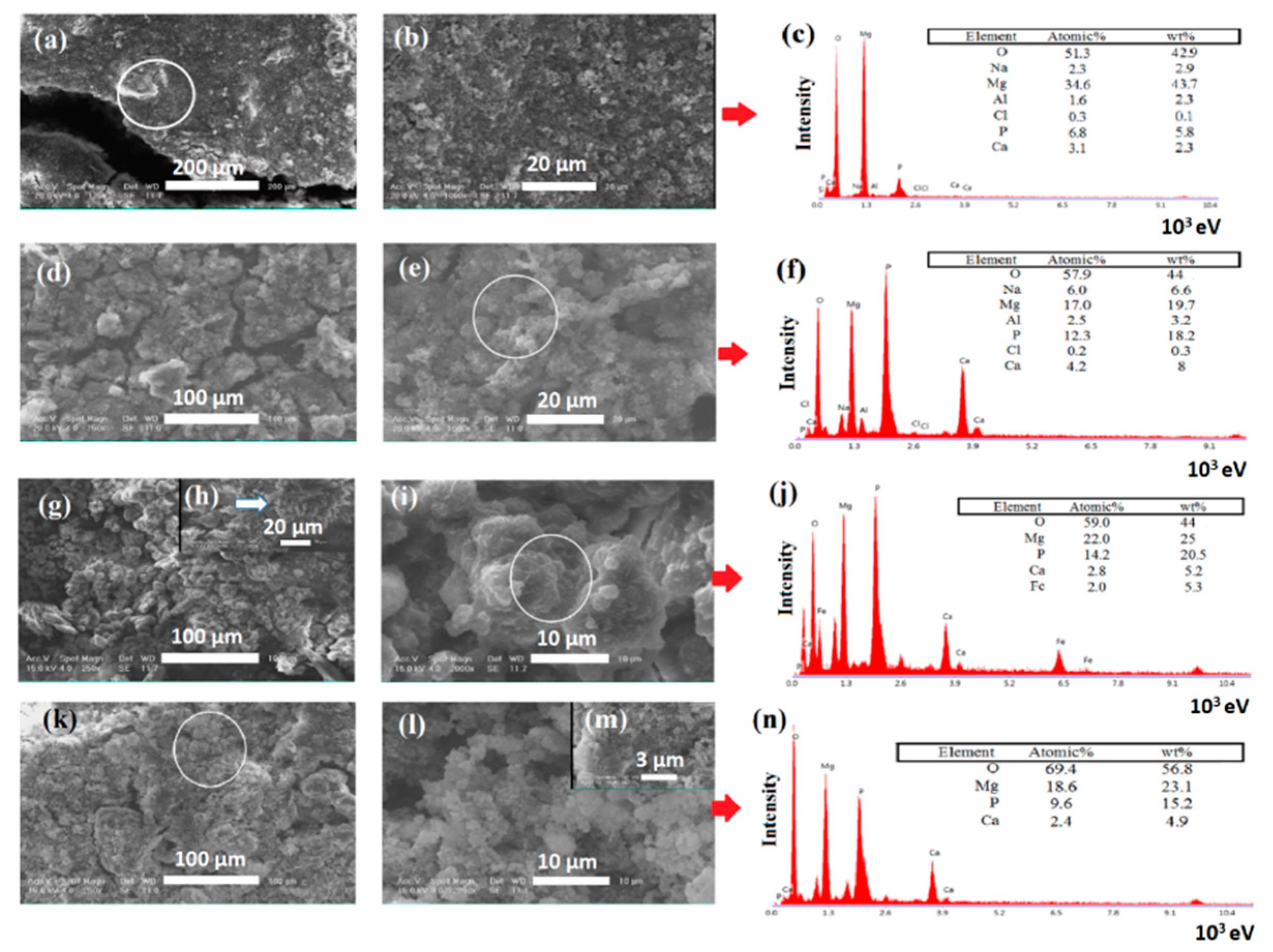
2.4. Characterization of Modified and Unmodified Samples
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buford, A.; Goswami, T. Review of wear mechanisms in hip implants: Paper I–General. Mater. Des. 2004, 25, 385–393. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases WJCC 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Katti, K.S. Biomaterials in total joint replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Babaie, E.; Bhaduri, S.B. Nanostructured amorphous magnesium phosphate/poly (lactic acid) composite coating for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy. Prog. Org. Coat. 2018, 118, 1–8. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, H.; Nabiyouni, M.; Bhaduri, S.B. Rapid coating of AZ31 magnesium alloy with calcium deficient hydroxyapatite using microwave energy. Mater. Sci. Eng. C 2015, 49, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-B.; Liu, H.-P.; Li, C.-Y.; Chen, Y.; Li, S.-Q.; Zeng, R.-C.; Wang, Z.-L. Corrosion resistance and adhesion strength of a spin-assisted layer-by-layer assembled coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2018, 434, 787–795. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Diba, M.; Goudouri, O.-M.; Tapia, F.; Boccaccini, A.R. Magnesium-containing bioactive polycrystalline silicate-based ceramics and glass-ceramics for biomedical applications. Curr. Opin. Solid State Mater. Sci. 2014, 18, 147–167. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Razavi, S.M.; Beni, B.H.; Vashaee, D.; Tayebi, L. Controlling the degradation rate of bioactive magnesium implants by electrophoretic deposition of akermanite coating. Ceram. Int. 2014, 40, 3865–3872. [Google Scholar] [CrossRef]
- Xu, W.; Yagoshi, K.; Koga, Y.; Sasaki, M.; Niidome, T. Optimized polymer coating for magnesium alloy-based bioresorbable scaffolds for long-lasting drug release and corrosion resistance. Colloids Surf. B Biointerfaces 2018, 163, 100–106. [Google Scholar] [CrossRef]
- Hu, R.-G.; Zhang, S.; Bu, J.-F.; Lin, C.-J.; Song, G.-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Jin, T.; Wang, Y.; Yin, H.; Hao, X. Corrosion protection properties and mechanism of epoxy/acetic acid-doped polyaniline coating on magnesium alloy. J. Nanosci. Nanotechnol. 2018, 18, 4992–5000. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Gao, S.-D.; Li, P.-P.; Zeng, R.-C.; Zhang, F.; Li, S.-Q.; Han, E.-H. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2017, 118, 84–95. [Google Scholar] [CrossRef]
- Sowmya, S.; Bumgardener, J.D.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Role of nanostructured biopolymers and bioceramics in enamel, dentin and periodontal tissue regeneration. Prog. Polym. Sci. 2013, 38, 1748–1772. [Google Scholar] [CrossRef]
- Pozzo, L.D.Y.; da Conceição, T.F.; Spinelli, A.; Scharnagl, N.; Pires, A.T.N. Chitosan coatings crosslinked with genipin for corrosion protection of AZ31 magnesium alloy sheets. Carbohydr. Polym. 2018, 181, 71–77. [Google Scholar] [CrossRef]
- Golshirazi, A.; Kharaziha, M.; Golozar, M.A. Polyethylenimine/kappa carrageenan: Micro-arc oxidation coating for passivation of magnesium alloy. Carbohydr. Polym. 2017, 167, 185–195. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Soleimani, B.; Tavangarian, F. Two-step modification process to improve mechanical properties and bioactivity of hydroxyfluorapatite scaffolds. Ceram. Int. 2018, 44, 19756–19763. [Google Scholar] [CrossRef]
- Jokar, M.; Darvishi, S.; Torkaman, R.; Kharaziha, M.; Karbasi, M. Corrosion and bioactivity evaluation of nanocomposite PCL-forsterite coating applied on 316L stainless steel. Surf. Coat. Technol. 2016, 307, 324–331. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, S.; Iqbal, T.; Bakhsheshi-Rad, H.R.; Alsakkaf, A.; Kamil, A.; Abdul kadir, M.R.; Idris, M.H.; Raghav, H.B. Zinc-doped hydroxyapatite—Zeolite/polycaprolactone composites coating on magnesium substrate for enhancing in-vitro corrosion and antibacterial performance. Trans. Nonferrous Met. Soc. China 2020, 30, 123–133. [Google Scholar] [CrossRef]
- Azarudeen, R.S.; Hassan, M.N.; Yassin, M.A.; Thirumarimurugan, M.; Muthukumarasamy, N.; Velauthapillai, D.; Mustafa, K. 3D printable Polycaprolactone-gelatin blends characterized for in vitro osteogenic potency. React. Funct. Polym. 2020, 146, 104445. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Doostmohammadi, A. In vitro evaluation of diopside/baghdadite bioceramic scaffolds modified by polycaprolactone fumarate polymer coating. Mater. Sci. Eng. C 2020, 106, 110176. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Li, L.; Yeh, C.-K.; Ren, X.; Sun, Y. Amphiphilic quaternary ammonium chitosan/sodium alginate multilayer coatings kill fungal cells and inhibit fungal biofilm on dental biomaterials. Mater. Sci. Eng. C 2019, 104, 109961. [Google Scholar] [CrossRef]
- Ramos Avilez, H.V.; Castilla Casadiego, D.A.; Vega Avila, A.L.; Perales Perez, O.J.; Almodovar, J. 11-Production of chitosan coatings on metal and ceramic biomaterials. In Chitosan Based Biomaterials Volume 1; Jennings, J., Bumgardner, J., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 255–293. [Google Scholar]
- Gao, F.; Hu, Y.; Gong, Z.; Liu, T.; Gong, T.; Liu, S.; Zhang, C.; Quan, L.; Kaveendran, B.; Pan, C. Fabrication of chitosan/heparinized graphene oxide multilayer coating to improve corrosion resistance and biocompatibility of magnesium alloys. Mater. Sci. Eng. C 2019, 104, 109947. [Google Scholar] [CrossRef]
- Soleymani, F.; Emadi, R.; Sadeghzade, S.; Tavangarian, F. Applying baghdadite/PCL/chitosan nanocomposite coating on AZ91 magnesium alloy to improve corrosion behavior, bioactivity, and biodegradability. Coatings 2019, 9, 789. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Shamoradi, F.; Emadi, R.; Tavangarian, F. Fabrication and characterization of baghdadite nanostructured scaffolds by space holder method. J. Mech. Behav. Biomed. Mater. 2017, 68, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, C. Development of anodic film on Mg alloy AZ91D. Surf. Coat. Technol. 2006, 201, 2381–2386. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Labbaf, S. Hardystonite-diopside nanocomposite scaffolds for bone tissue engineering applications. Mater. Chem. Phys. 2017, 202, 95–103. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F.; Naderi, M. Fabrication and evaluation of silica-based ceramic scaffolds for hard tissue engineering applications. Mater. Sci. Eng. C 2017, 71, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vitro study of nanostructured diopside coating on Mg alloy orthopedic implants. Mater. Sci. Eng. C 2014, 41, 168–177. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Nadaraia, K.V.; Kiryukhin, D.P.; Kichigina, G.A.; Kushch, P.P.; Buznik, V.M. Composite coatings formed on the PEO-layers with the use of solutions of tetrafluoroethylene telomers. Surf. Coat. Technol. 2018, 346, 53–62. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.I.; Dunstan, C.R.; Davies, B.; Pearce, S.; Williams, R.; Zreiqat, H. Repairing a critical-sized bone defect with highly porous modified and unmodified baghdadite scaffolds. Acta Biomater. 2012, 8, 4162–4172. [Google Scholar] [CrossRef]
- Ramaswamy, Y.; Wu, C.; Van Hummel, A.; Combes, V.; Grau, G.; Zreiqat, H. The responses of osteoblasts, osteoclasts and endothelial cells to zirconium modified calcium-silicate-based ceramic. Biomaterials 2008, 29, 4392–4402. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Labbaf, S. Formation mechanism of nano-hardystonite powder prepared by mechanochemical synthesis. Adv. Powder Technol. 2016, 27, 2238–2244. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Tavangarian, F. Combustion assisted synthesis of hardystonite nanopowder. Ceram. Int. 2016, 42, 14656–14660. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R.; Ghomi, H. Mechanical alloying synthesis of forsterite-diopside nanocomposite powder for using in tissue engineering. Ceram. Silikáty 2015, 59, 1–5. [Google Scholar]
- Sadeghzade, S.; Emadi, R.; Ahmadi, T.; Tavangarian, F. Synthesis, characterization and strengthening mechanism of modified and unmodified porous diopside/baghdadite scaffolds. Mater. Chem. Phys. 2019, 228, 89–97. [Google Scholar] [CrossRef]
- Sadeghzade, S.; Emadi, R. Improving the mechanical and bioactivity of hydroxyapatite porous scaffold ceramic with diopside/forstrite ceramic coating. Nanomed. J. 2019, 6, 50–54. [Google Scholar]
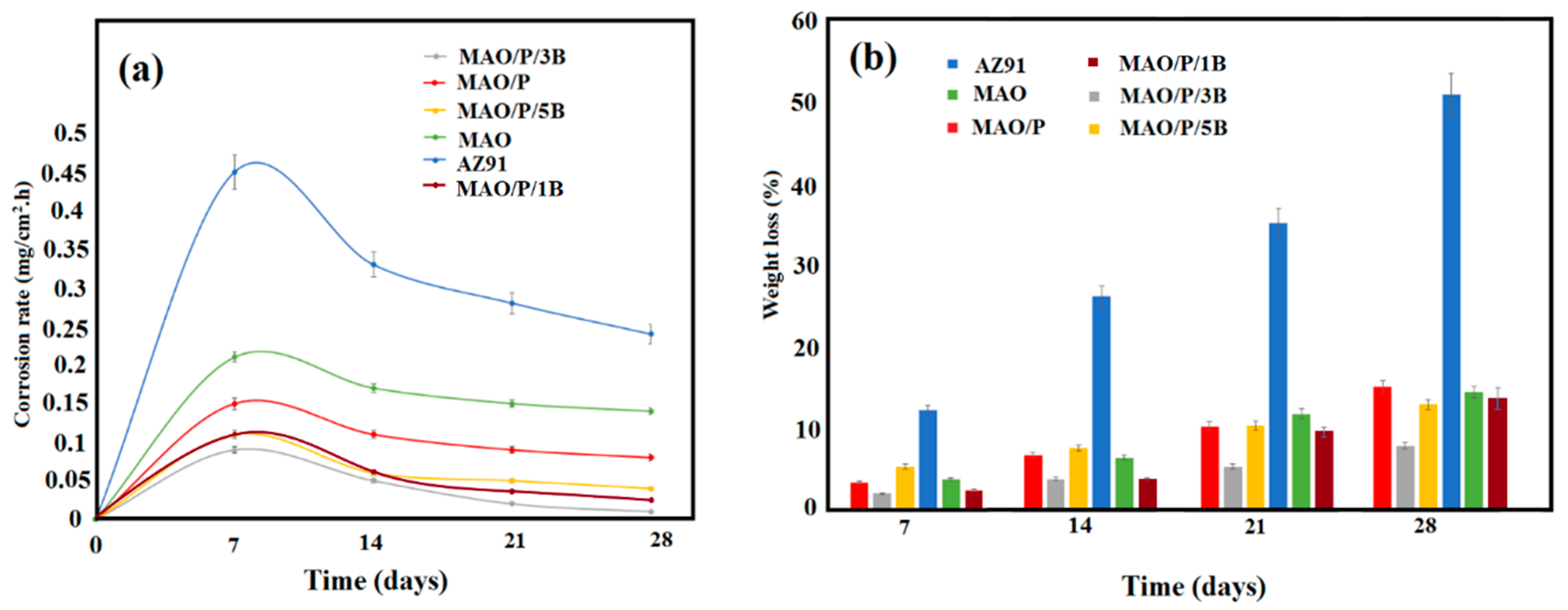
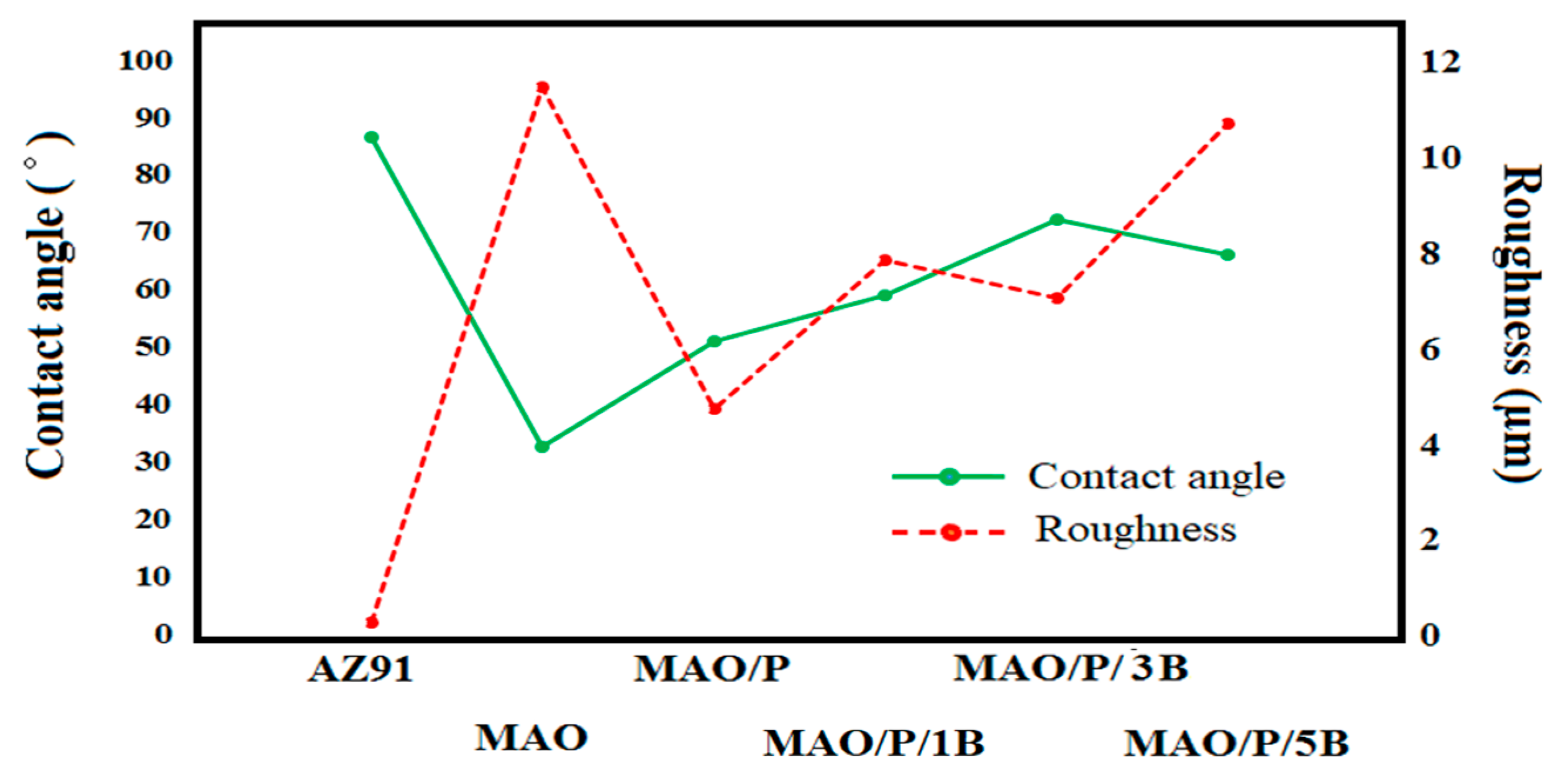
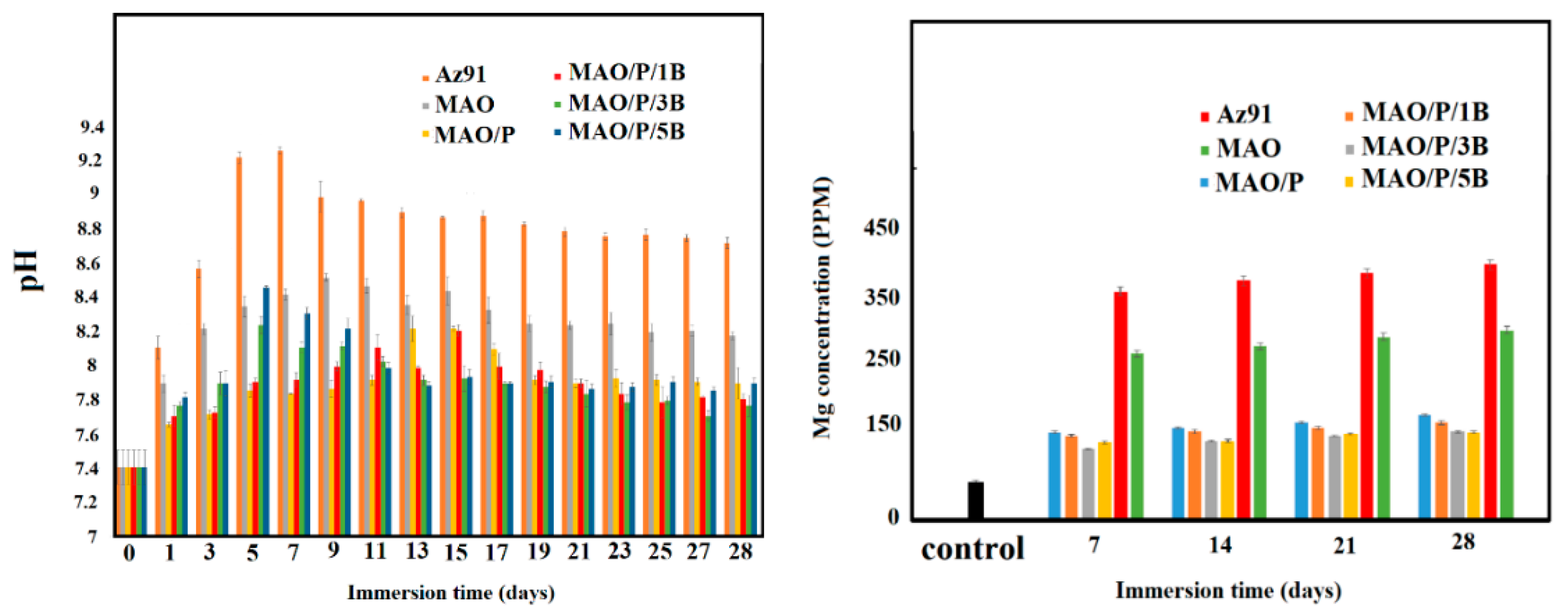
| Designation | Process |
|---|---|
| AZ91 MAO MAO/P MAO/P/1B MAO/P/3B MAO/P/5B | AZ91 Alloy AZ91 alloy subjected to MAO process MAO coated with PCL/Chitosan polymer MAO coated with PCL/Ch-1%Baghdadite MAO coated with PCL/Ch-3%Baghdadite MAO coated with PCL/Ch-5%Baghdadite |
| Ion | Ion Concentration (mM) |
|---|---|
| Na+ K+ Mg2+ Ca2+ Cl− pH | 142.0 5.0 1.5 2.5 147.8 4.2 1.0 0.5 7.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleymani, F.; Emadi, R.; Sadeghzade, S.; Tavangarian, F. Bioactivity Behavior Evaluation of PCL-Chitosan-Nanobaghdadite Coating on AZ91 Magnesium Alloy in Simulated Body Fluid. Coatings 2020, 10, 231. https://doi.org/10.3390/coatings10030231
Soleymani F, Emadi R, Sadeghzade S, Tavangarian F. Bioactivity Behavior Evaluation of PCL-Chitosan-Nanobaghdadite Coating on AZ91 Magnesium Alloy in Simulated Body Fluid. Coatings. 2020; 10(3):231. https://doi.org/10.3390/coatings10030231
Chicago/Turabian StyleSoleymani, Farzad, Rahmatollah Emadi, Sorour Sadeghzade, and Fariborz Tavangarian. 2020. "Bioactivity Behavior Evaluation of PCL-Chitosan-Nanobaghdadite Coating on AZ91 Magnesium Alloy in Simulated Body Fluid" Coatings 10, no. 3: 231. https://doi.org/10.3390/coatings10030231
APA StyleSoleymani, F., Emadi, R., Sadeghzade, S., & Tavangarian, F. (2020). Bioactivity Behavior Evaluation of PCL-Chitosan-Nanobaghdadite Coating on AZ91 Magnesium Alloy in Simulated Body Fluid. Coatings, 10(3), 231. https://doi.org/10.3390/coatings10030231






