Palladium Oxide Nanoparticles: Preparation, Characterization and Catalytic Activity Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ζ-Potential
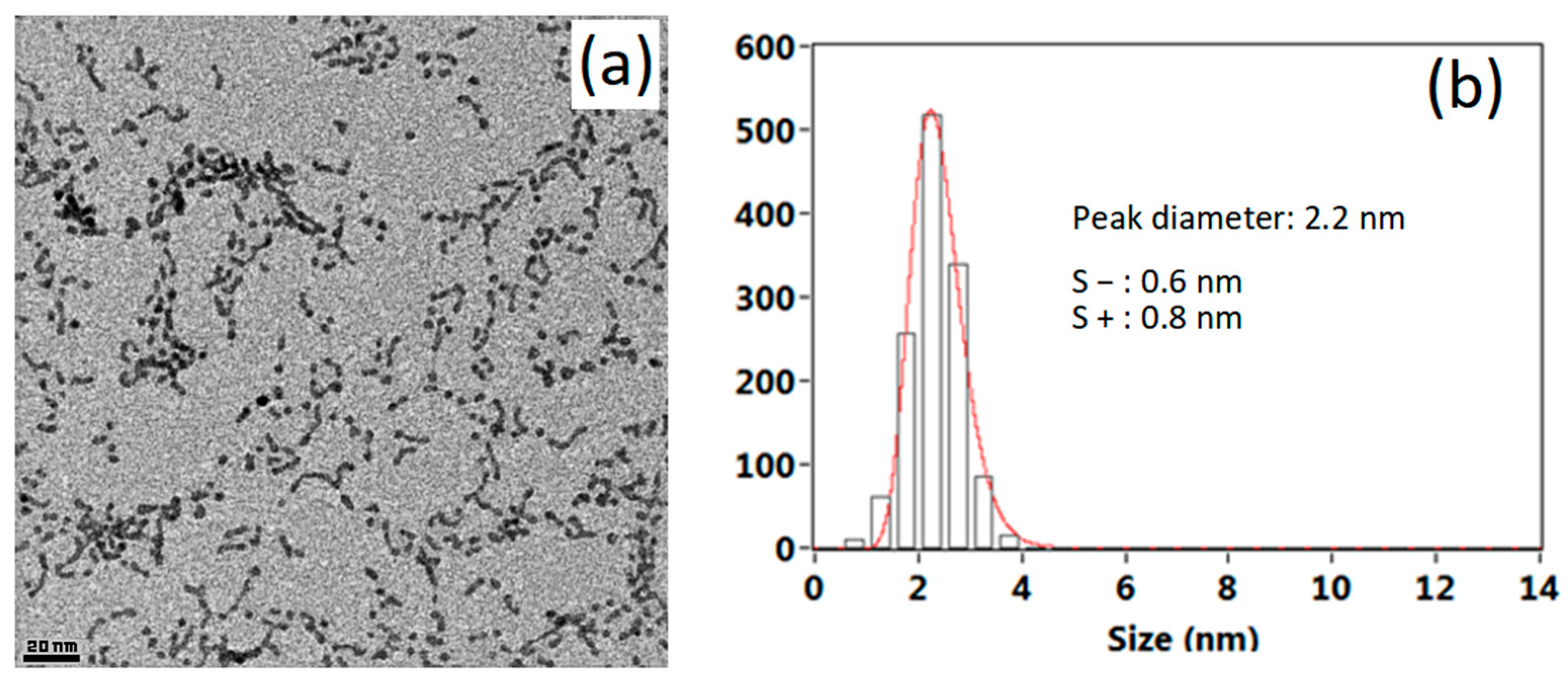
2.3. Microscopy
2.4. Absorption
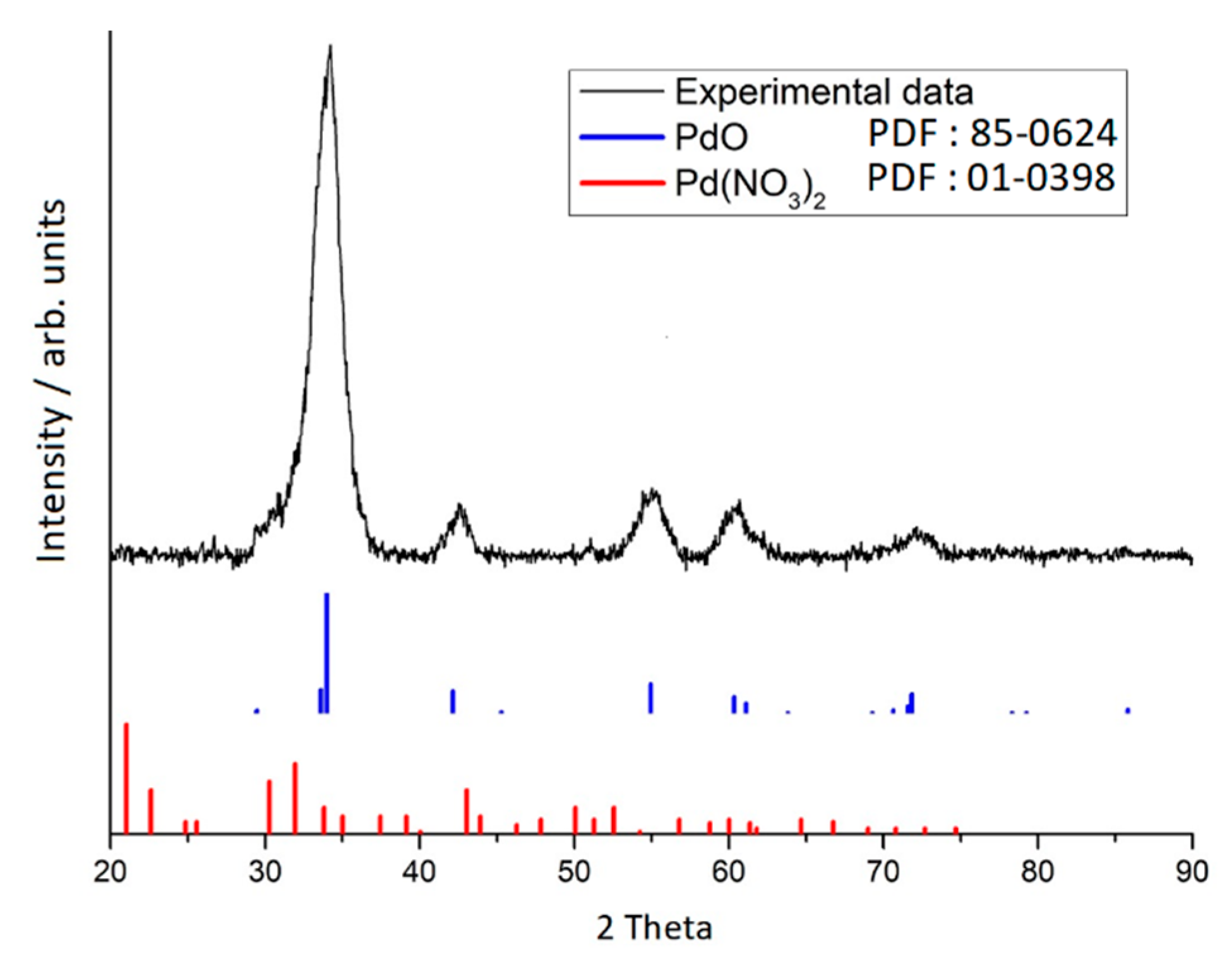
2.5. X-ray Diffraction
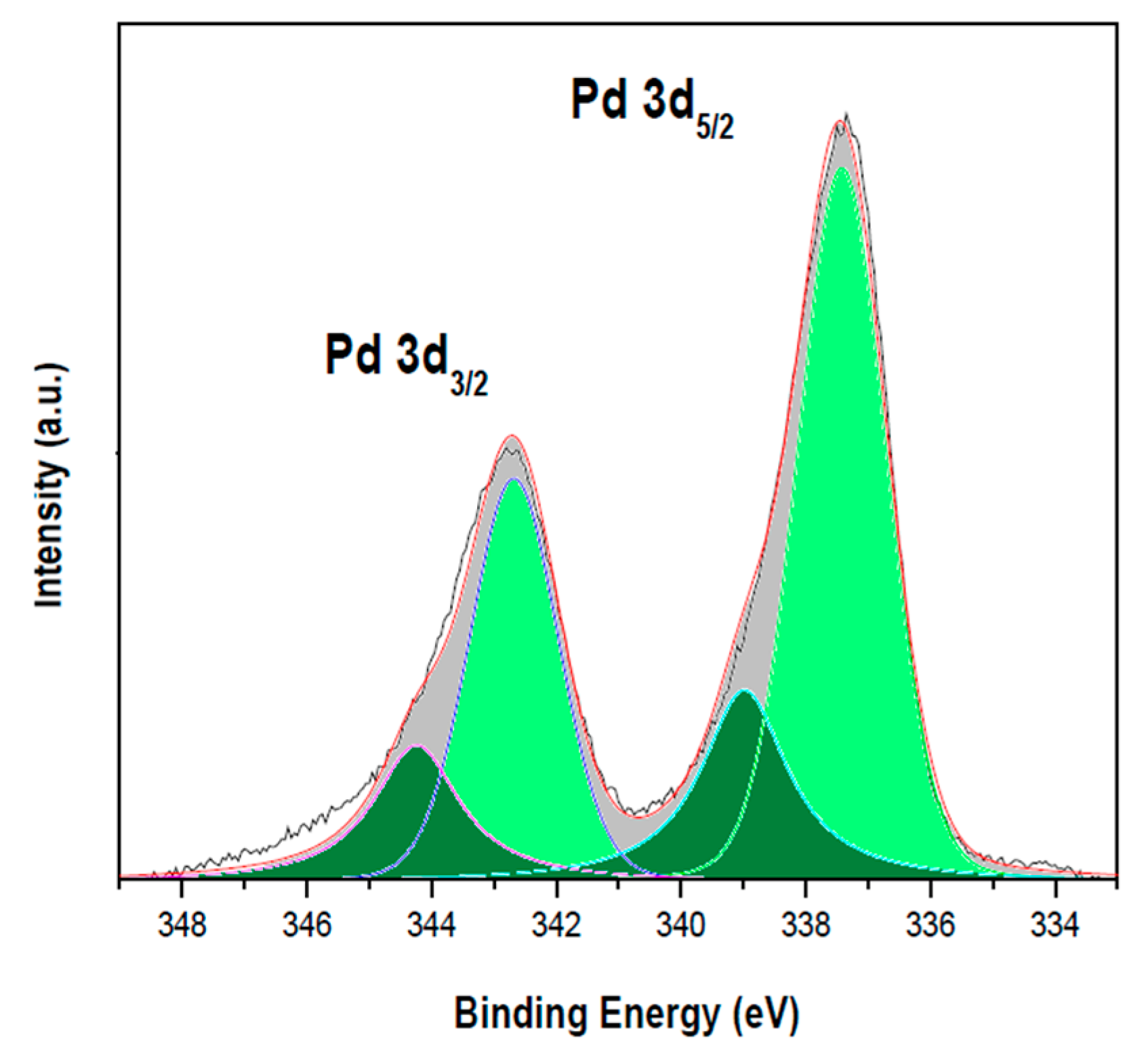
2.6. X-ray Photoelectron Spectroscopy
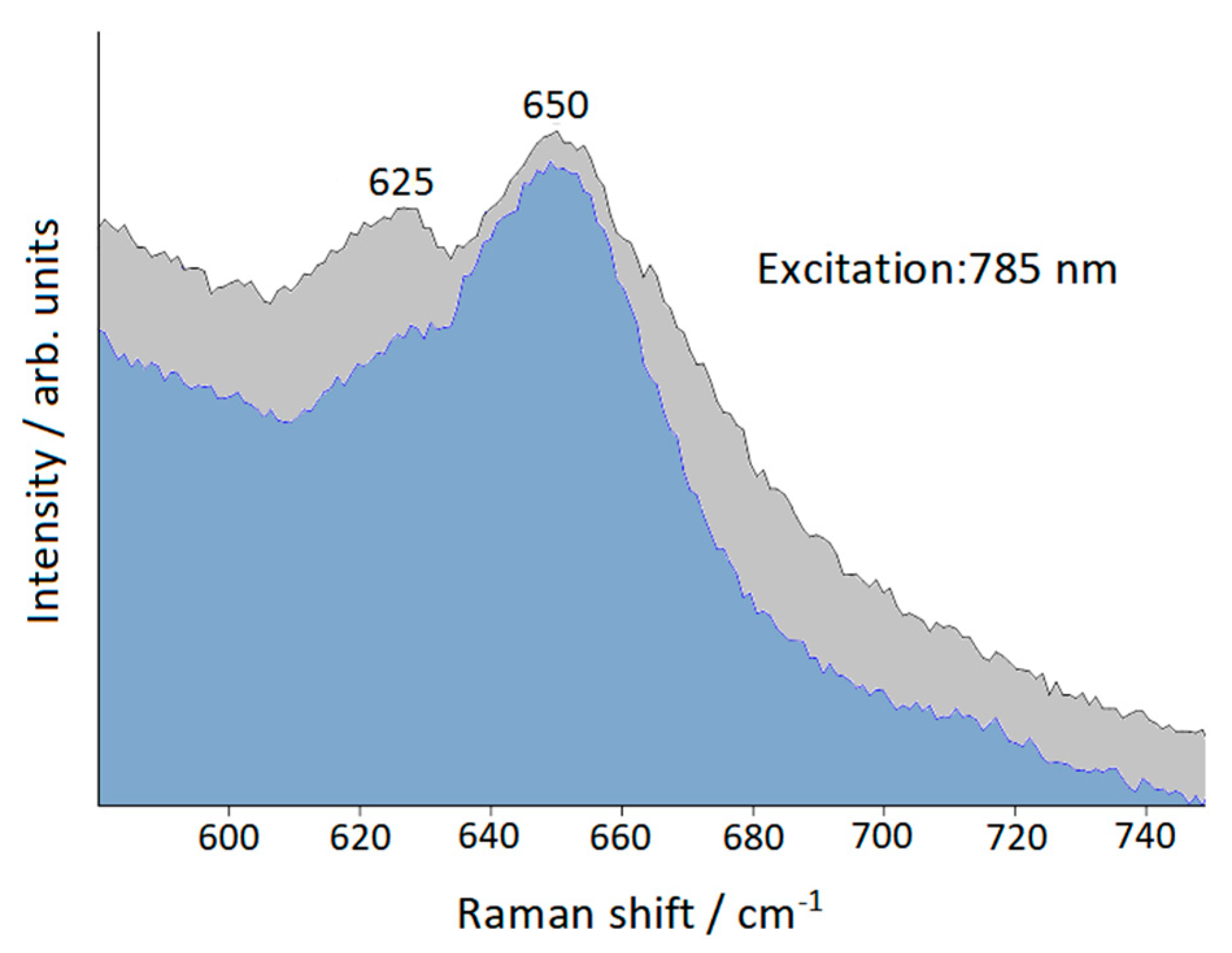
2.7. Raman Scattering
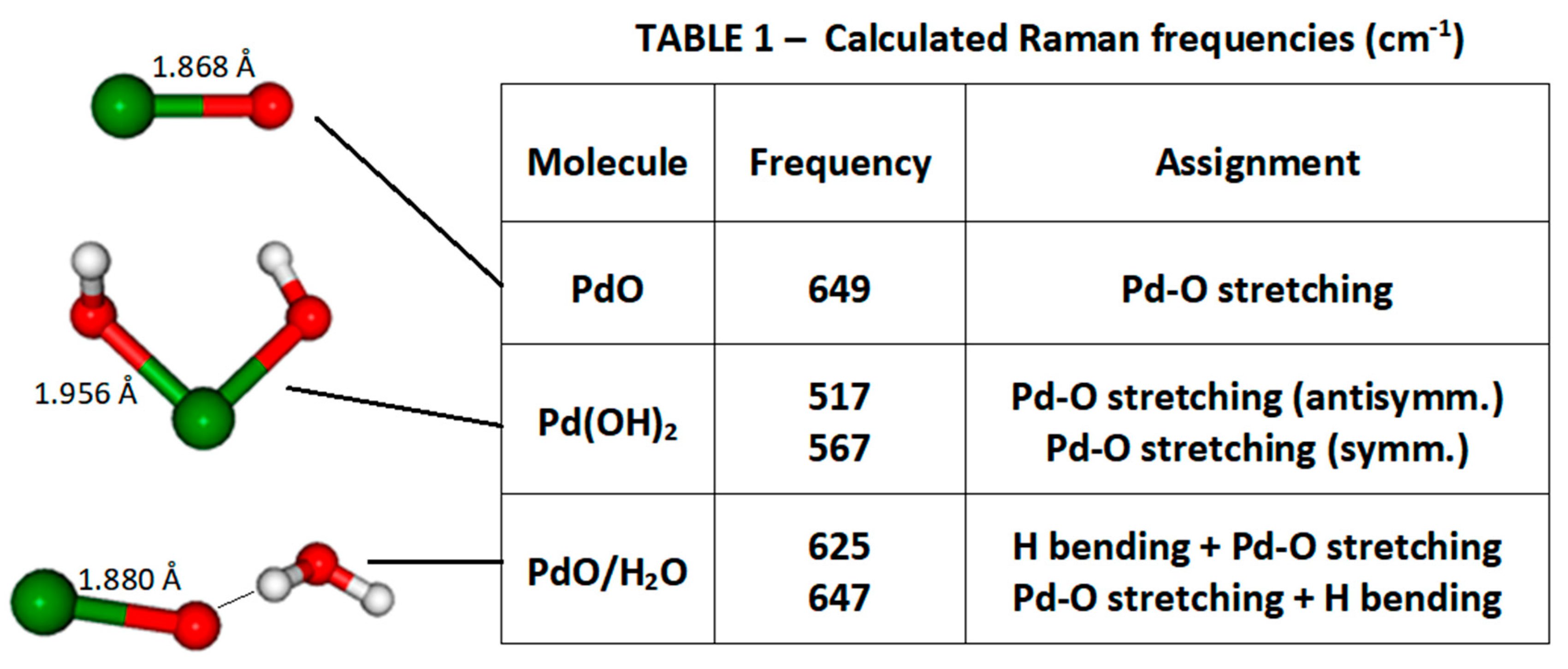
2.8. Density Functional Theory (DFT) Calculations
2.9. Catalysis Tests
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blazer, H.-U.; Indolese, A.; Schnyder, A.; Steiner, H.; Studer, M. Supported palladium catalysts for fine chemicals synthesis. J. Mol. Catal. A Chem. 2001, 173, 3–18. [Google Scholar] [CrossRef]
- Liu, X.; Astruc, D. Development of the Applications of Palladium on Charcoal in Organic Synthesis. Adv. Synth. Catal. 2018, 360, 3426–3459. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Grunes, J.; Zhu, A.; Somorjai, G.A. Catalysis and nanoscience. Chem. Commun. 2003, 2257–2260. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Bräse, S.; Kirchhoff, J.H.; Köbberling, J. Palladium-catalyzed reaction in solid phase organic synthesis. Tetrahedron 2003, 59, 885–939. [Google Scholar]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M.; Drechsler, M. Catalytic activity of palladium nanoparticles encapsulated in spherical polyelectrolyte brushes and core-shell microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Jv, X.; Sun, S.; Zhang, Q.; Du, M.; Wang, L.; Wang, B. Efficient and Mild Reductive Amination of Carbonyl Compounds Catalyzed by Dual-Function Palladium Nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 1618–1626. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Augustyniak, A.W. The role of palladium nanoparticles in catalytic C–C cross-coupling reactions. Co-ord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Bej, A.; Ghosh, K.; Sarkar, A.; Knight, D.W. Palladium nanoparticles in the catalysis of coupling reactions. RSC Adv. 2016, 6, 11446–11453. [Google Scholar] [CrossRef]
- Favier, I.; Pla, D.; Gómez, M. Palladium Nanoparticles in Polyols: Synthesis, Catalytic Couplings, and Hydrogenations. Chem. Rev. 2020, 120, 1146–1183. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lorenzo, M. Palladium Nanoparticles as Efficient Catalysts for Suzuki Cross-Coupling Reactions. J. Phys. Chem. Lett. 2012, 3, 167–174. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki–Miyaura coupling in water. J. Mol. Catal. A Chem. 2015, 396, 297–303. [Google Scholar] [CrossRef]
- Kalaiselvi, A.; Mohana Roopan, S.; Madhumitha, G.; Ramalingam, C.; Elango, G. Synthesis and characterization of palladium nanoparticles using Catharanthus roseus leaf extract and its application in the photo-catalytic degradation. Spectrochim. Acta A 2015, 135, 116–119. [Google Scholar] [CrossRef]
- Zhu, G.H.; Han, J.Y.; Zernlyanov, D.Y.; Ribeiro, F.H. Temperature dependence of the kinetics for the complete oxidation of methane on palladium and palladium oxide. J. Phys. Chem. B 2005, 109, 2331–2337. [Google Scholar] [CrossRef]
- Oh, S.H.; Hoflund, G.B. Low-temperature catalytic carbon monoxide oxidation over hydrous and anhydrous palladium oxide powders. J. Catal. 2007, 245, 35–44. [Google Scholar] [CrossRef]
- Lichtenberger, J.; Lee, D.; Iglesia, E. Catalytic oxidation of methanol on Pd metal and oxide clusters at near-ambient temperatures. Phys. Chem. Chem. Phys. 2007, 9, 4902–4906. [Google Scholar] [CrossRef]
- Fujimoto, K.-I.; Ribeiro, F.H.; Avalos-Borja, M.; Iglesia, E. Structure and Reactivity of PdOx/ZrO2 Catalysts for Methane Oxidation at Low Temperatures. J. Catal. 1998, 179, 431–442. [Google Scholar] [CrossRef]
- Wang, K.M.; Huang, T.; Liu, H.F.; Zhao, Y.X.; Liu, H.M.; Sun, C.T. Size control synthesis of palladium oxide nanoparticles by microwave irradiation. Colloids Surf. A 2008, 325, 21–25. [Google Scholar] [CrossRef]
- Bagchi, V.; Bandyopadhyay, D. In situ generation of palladium oxide nano-crystals. J. Organomet. Chem. 2009, 694, 1259–1262. [Google Scholar] [CrossRef]
- Meher, S.; Rana, R.K. A rational design of a Pd-based catalyst with a metal-metal oxide interface influencing molecular oxygen in the aerobic oxidation of alcohols. Green Chem. 2019, 21, 2494–2503. [Google Scholar] [CrossRef]
- Wu, Q.; Rao, Z.; Yuan, L.; Jiang, L.; Sun, G.; Ruan, J.; Zhou, Z.; Sang, S. Carbon supported PdO with improved activity and stability for oxygen reduction reaction in alkaline solution. Electrochim. Acta 2014, 150, 157–166. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Gellini, C.; Deepak, F.L.; Muniz-Miranda, M.; Caporali, S.; Muniz-Miranda, F.; Pedone, A.; Innocenti, C.; Sangregorio, C. Magneto-plasmonic colloidal nanoparticles obtained by laser ablation of nickel and silver targets in water. J. Phys. Chem. C 2017, 121, 3597–3606. [Google Scholar] [CrossRef]
- Zoppi, A.; Caporali, S.; Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Adsorption of Trans-Zeatin on Laser-Ablated Gold Nanoparticles for Transport into Plant Cells and Growth Stimulation. ACS Appl. Nano Mater. 2019, 2, 7319–7327. [Google Scholar] [CrossRef]
- López-Tocón, I.; Valdivia, S.; Soto, J.; Otero, J.C.; Muniz-Miranda, F.; Menziani, M.C.; Muniz-Miranda, M. A DFT Approach to the Surface-Enhanced Raman Scattering of 4-Cyanopyridine Adsorbed on Silver Nanoparticles. Nanomaterials 2019, 9, 1211. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Pagliai, M.; Muniz-Miranda, F.; Schettino, V. Raman and computational study of solvation and chemisorption of thiazole in silver hydrosol. Chem. Commun. 2011, 47, 3138–3140. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, M.; Muniz-Miranda, F.; Schettino, V.; Muniz-Miranda, M. Competitive Solvation and Chemisorption in Silver Colloidal Suspensions. Prog. Colloid Polym. Sci. 2012, 139, 39–44. [Google Scholar]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Caporali, S. SERS and DFT study of copper surfaces coated with corrosion inhibitor. Beilstein J. Nanotechnol. 2014, 5, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Spectroscopic and DFT investigation on the photo-chemical properties of a push-pull chromophore: 4-Dimethylamino-4′-nitrostilbene. Spectrochim. Acta A 2018, 190, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Gellini, C.; Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. SERS active Ag-SiO2 nanoparticles obtained by laser ablation of silver in colloidal silica. Beilstein J. Nanotechnol. 2018, 9, 2396–2404. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Fuentes, S.; Bogdanchikova, N.; Barrera, A. Influence of modifying additives on the electronic state of supported palladium. Chem. Phys. Lett. 2003, 367, 102–108. [Google Scholar] [CrossRef]
- Kravchuk, L.S.; Stel’mak, E.I.; Ivashchenko, N.I.; Valieva, S.V.; Molod’yanova, V.S. Formation and physicochemical properties of the system palladium oxide (PdO)/ZrO2. Kinetika i Kataliz 1992, 33, 672–677. [Google Scholar]
- Martínez, J.M.; Torrico, F.; Pappalardo, R.R.; Sánchez Marcos, E. Understanding the Hydration Structure of Square-Planar Aquaions: The [Pd(H2O)4]2+ Case. J. Phys. Chem. B 2004, 108, 15851–15855. [Google Scholar] [CrossRef]
- Adnan Ali Shah, S.; Hofer, T.S.; Qaiser Fatmi, M.; Randolf, B.R.; Rode, B.M. A QM/MM MD simulation study of hydrated Pd2+. Chem. Phys. Lett. 2006, 426, 301–305. [Google Scholar] [CrossRef]
- Torrico, F.; Pappalardo, R.R.; Sánchez Marcos, E.; Martínez, J.M. Hydration Structure and Dynamic Properties of the Square Planar Pt(II) Aquaion Compared to the Pd(II) Case. Theor. Chem. Accounts 2006, 115, 196–203. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Muniz-Miranda, F.; Caporali, S.; Calisi, N.; Pedone, A. SERS, XPS and DFT investigation on palladium surfaces coated with 2, 2′-bipyridine monolayers. Appl. Surf. Sci. 2018, 457, 98–103. [Google Scholar] [CrossRef]
- Jürgensen, A.; Heutz, N.; Raschke, H.; Merz, K.; Hergenröder, R. Behavior of Supported Palladium Oxide Nanoparticles under Reaction Conditions, Studied with near Ambient Pressure XPS. Anal. Chem. 2015, 87, 7848–7856. [Google Scholar] [CrossRef] [PubMed]
- Remillard, J.T. Optical studies of PdO thin films. J. Appl. Phys. 1992, 71, 4515–4522. [Google Scholar] [CrossRef]
- Weber, W.H.; Baird, R.J.; Graham, G.W. Raman Investigation of Palladium Oxide, Rhodium Sesquioxide and Palladium Rhodium Dioxide. J. Raman Spectrosc. 1988, 19, 239–244. [Google Scholar] [CrossRef]
- Pozun, Z.D.; Rodenbusch, S.E.; Keller, E.; Tran, K.; Tang, W.; Stevenson, K.J.; Henkelman, G. A Systematic Investigation of p-Nitrophenol Reduction by Bimetallic Dendrimer Encapsulated Nanoparticles. J. Phys. Chem. C 2013, 117, 7598–7604. [Google Scholar] [CrossRef]
- Krishna, R.; Fernandes, D.M.; Dias, C.; Ventura, J.; Venkata Ramana, E.; Freire, C.; Titus, E. Novel synthesis of Ag@Co/RGO nanocomposite and its high catalytic activity towards hydrogenation of 4-nitrophenol to 4-aminophenol. Int. J. Hydrogen Energy 2015, 40, 4996–5005. [Google Scholar] [CrossRef]
- Rocha, M.; Fernandes, C.; Pereira, C.; Rebelo, S.L.H.; Pereira, M.F.R.; Freire, C. Gold-supported magnetically recyclable nanocatalysts: A sustainable solution for the reduction of 4-nitrophenol in water. RSC Adv. 2015, 5, 5131–5141. [Google Scholar] [CrossRef]
- Ayán-Varela, M.; Fernández-Merino, M.J.; Paredes, J.I.; Villar-Rodil, S.; Fernández-Sánchez, C.; Guardia, L.; Martínez-Alonso, A.; Tascón, J.M.D. Highly efficient silver-assisted reduction of graphene oxide dispersions at room temperature: Mechanism, and catalytic and electrochemical performance of the resulting hybrids. J. Mater. Chem. A 2014, 2, 7295–7305. [Google Scholar] [CrossRef]
- Barman, B.K.; Nanda, K.K. Rapid reduction of GO by hydrogen spill-over mechanism by in situ generated nanoparticles at room temperature and their catalytic performance towards 4-nitrophenol reduction and ethanol oxidation. Appl. Catal. A Gen. 2015, 491, 45–51. [Google Scholar] [CrossRef]
- Ren, R.; Li, S.; Li, J.; Ma, J.; Liu, H.; Ma, J. Enhanced catalytic activity of Au nanoparticles self-assembled on thiophenol functionalized graphene. Catal. Sci. Technol. 2015, 5, 2149–2156. [Google Scholar] [CrossRef]

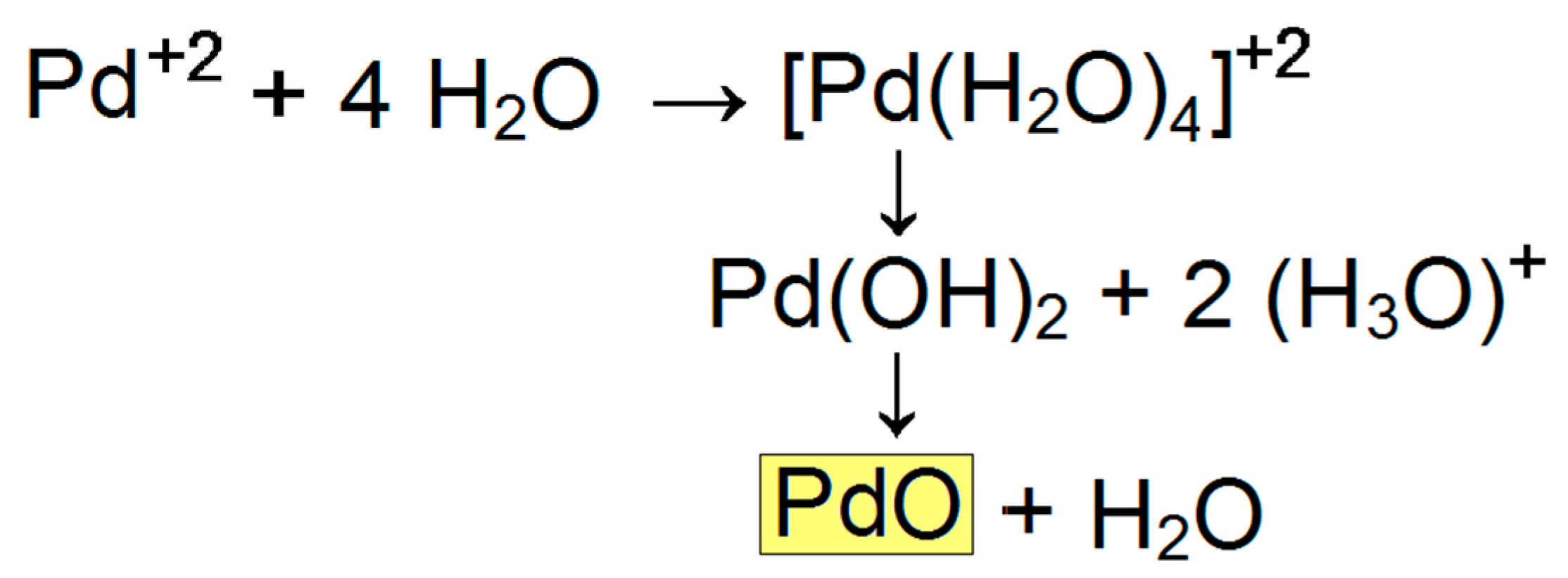

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muniz-Miranda, M.; Zoppi, A.; Muniz-Miranda, F.; Calisi, N. Palladium Oxide Nanoparticles: Preparation, Characterization and Catalytic Activity Evaluation. Coatings 2020, 10, 207. https://doi.org/10.3390/coatings10030207
Muniz-Miranda M, Zoppi A, Muniz-Miranda F, Calisi N. Palladium Oxide Nanoparticles: Preparation, Characterization and Catalytic Activity Evaluation. Coatings. 2020; 10(3):207. https://doi.org/10.3390/coatings10030207
Chicago/Turabian StyleMuniz-Miranda, Maurizio, Angela Zoppi, Francesco Muniz-Miranda, and Nicola Calisi. 2020. "Palladium Oxide Nanoparticles: Preparation, Characterization and Catalytic Activity Evaluation" Coatings 10, no. 3: 207. https://doi.org/10.3390/coatings10030207
APA StyleMuniz-Miranda, M., Zoppi, A., Muniz-Miranda, F., & Calisi, N. (2020). Palladium Oxide Nanoparticles: Preparation, Characterization and Catalytic Activity Evaluation. Coatings, 10(3), 207. https://doi.org/10.3390/coatings10030207






