Technical Characteristics and Wear-Resistant Mechanism of Nano Coatings: A Review
Abstract
1. Introduction
2. Preparation Technology of Nano-Coatings
2.1. Chemical Vapor Deposition
2.1.1. Principles and Characteristics of Chemical Vapor Deposition
- Ability to produce high density and pure materials;
- Uniform films with good reproducibility and adhesion are produced at a rather high deposition rate;
- CVD is a non-line-of-sight process with fine ejection capability. It can be used to uniformly coat components with complex shapes and deposit thin films with good conformal coverage. This unique feature surpasses the PVD process;
- It takes on the capability to control the crystallographic texture, surface morphology and tropism of CVD products pass through controlling the technological parameter of CVD;
- The deposition rate can be easily adjusted, and the low deposition rate facilitates the growth of epitaxial films for microelectronic applications;
- Traditional CVD technology has low processing costs.
- Toxic, corrosive, flammable and/or explosive precursor gases are used in CVD processes, which can cause chemical and safety hazards;
- Because different precursors have different evaporation rates, it is difficult to use stoichiometric multi-component materials with well-controlled multi-source precursor deposition;
- CVD variants use more complex reactors and vacuum systems, which are manufactured by low pressure or ultra-high vacuum CVD, plasma-assisted CVD and photo-assisted CVD, etc., resulting in increased manufacturing costs.
2.1.2. Application of Chemical Vapor Deposition in Nano-Coating
2.2. Physical Vapor Deposition
2.2.1. Principles and Characteristics of Physical Vapor Deposition
- Very uniform coatings can be obtained, with thicknesses ranging from a few nanometers to thousands of nanometers;
- High repeatability.
- Good coiled parts, suitable for coating various complex shapes of workpieces;
- Possibility of selective deposition in choose sections;
- Material selection with almost no restrictions on the substrate;
- Sufficient flexibility in temperature requirements for substrates;
- A wide range of coating materials, including metals, alloys and compounds;
- Environmental protection and no pollution.
- The complexity and high cost of technology and monitoring equipment;
- High requirements for the work of operators;
- The productivity is relatively low;
- High-precision chemical composition is required;
- Special preparation of the coating surface is required.
2.2.2. Application of Physical Vapor Deposition in Nano-Coating
2.3. Sol-Gel Method
2.3.1. Principles and Characteristics of Sol-Gel Method
- The reaction can be carried out at a low temperature;
- Capable of preparing high-purity, homogeneous coatings;
- It is suitable for large-area film formation, and the composition of the film is relatively easy to control, and materials can be designed and prepared from the molecular level;
- The process is simple and the equipment requirements are low.
- Raw materials are expensive and some organics are harmful to health;
- The preparation cycle is long, which usually takes days or weeks;
- There are a lot of micropores in the gel, and many gases and organics will escape during the drying process, and shrinkage will occur.
2.3.2. Application of Sol-Gel Method in Nano-Coating
2.4. Laser Cladding Method
2.4.1. Principle and Characteristics of Laser Cladding Method
- The metallurgical quality of the laser cladding layer is poor;
- Porosity, cracks and other problems will occur, affecting the performance of the coating;
- Uneven composition and structure during laser cladding;
- Repeatability check.
2.4.2. Application of Laser Cladding in Nano-Coating
2.5. Thermal Spraying
2.5.1. Principle and Characteristics of Thermal Spraying
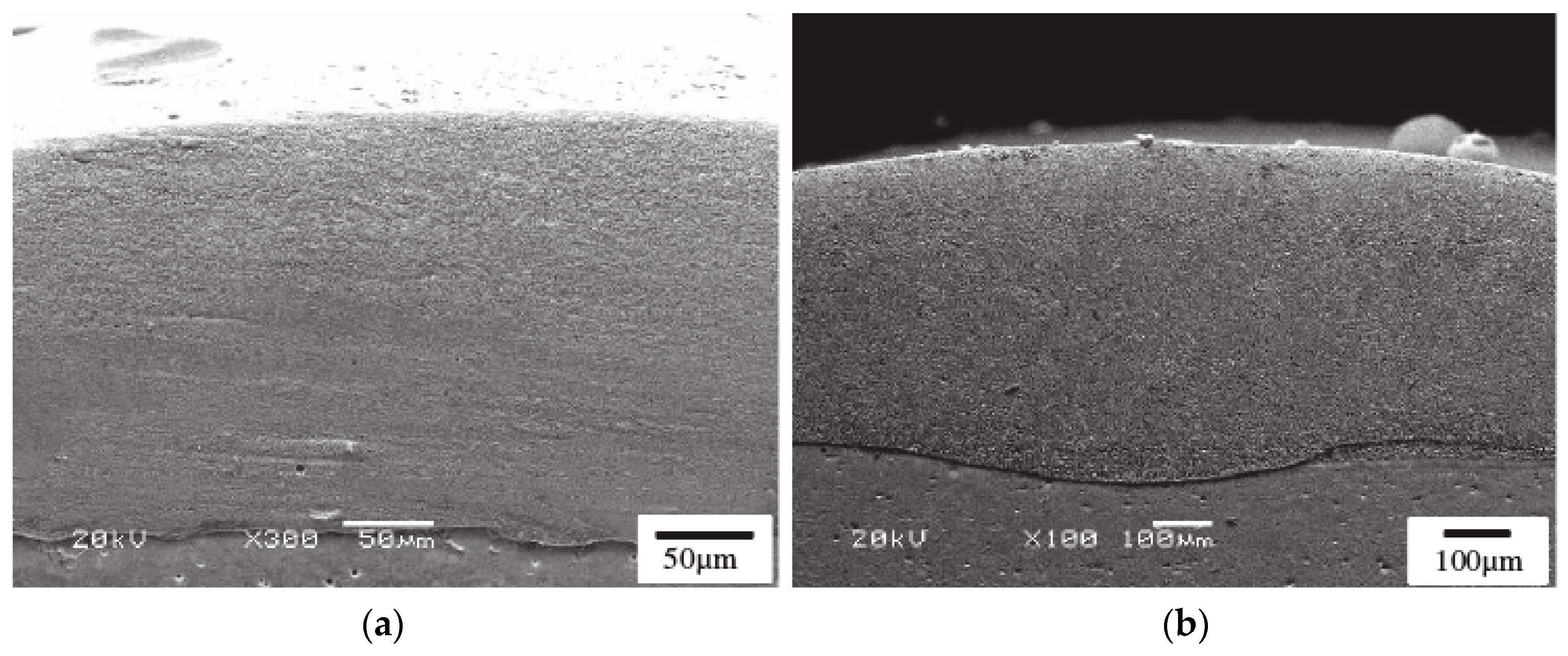
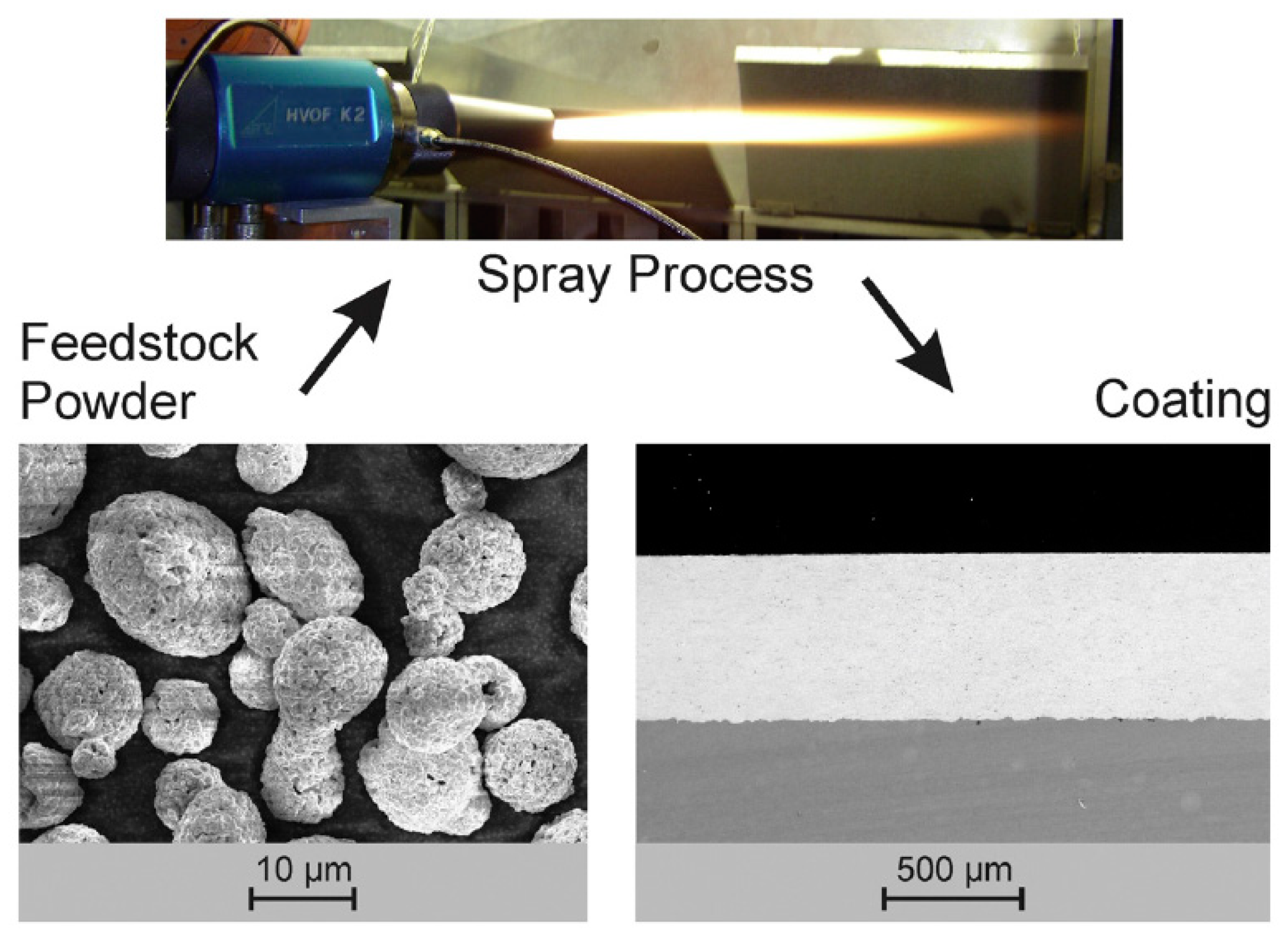
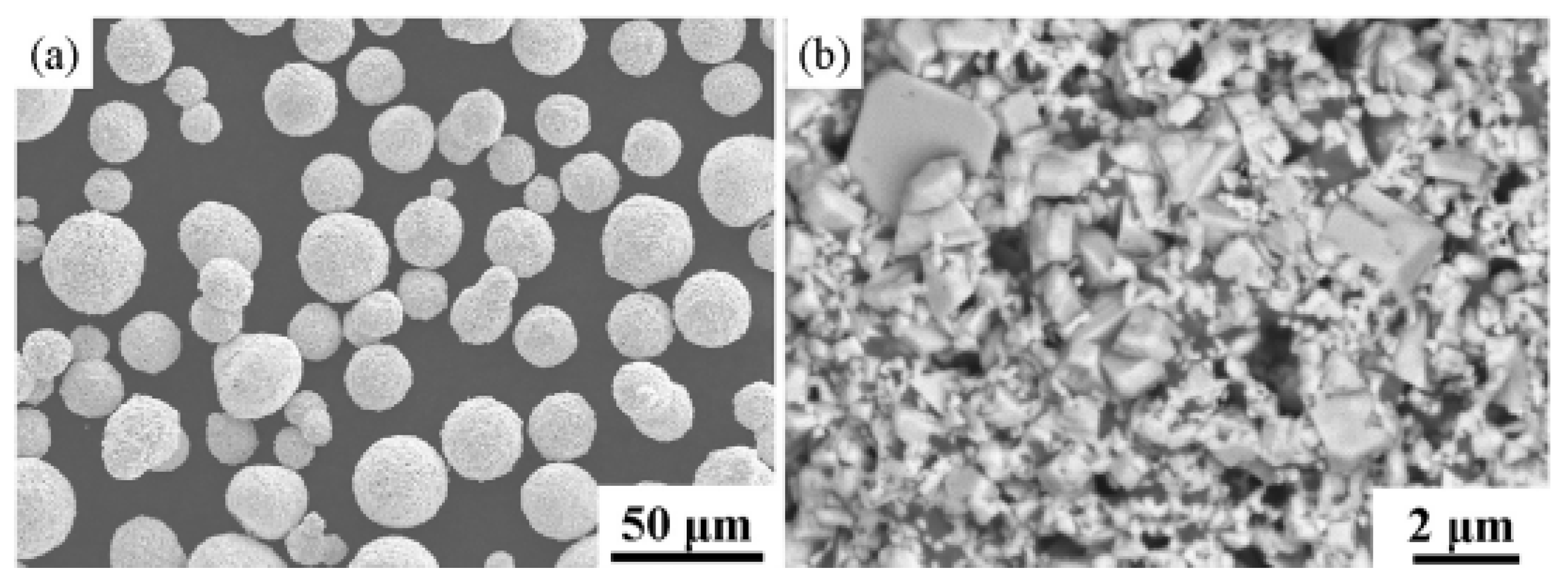
2.5.2. Application of Thermal Spraying in Nano-Coating
2.6. Summary of Each Preparation Technology
3. Type of Nano-Coating Materials
3.1. Inorganic/Inorganic Nanomaterial Coatings
3.1.1. Ceramic/ceramic Nano-Coating Materials
3.1.2. Metal/ceramic Nano-Coating Materials
3.1.3. Metal/non-Ceramic Nano-Coating Materials
3.2. Organic/Inorganic Nanomaterial Coatings
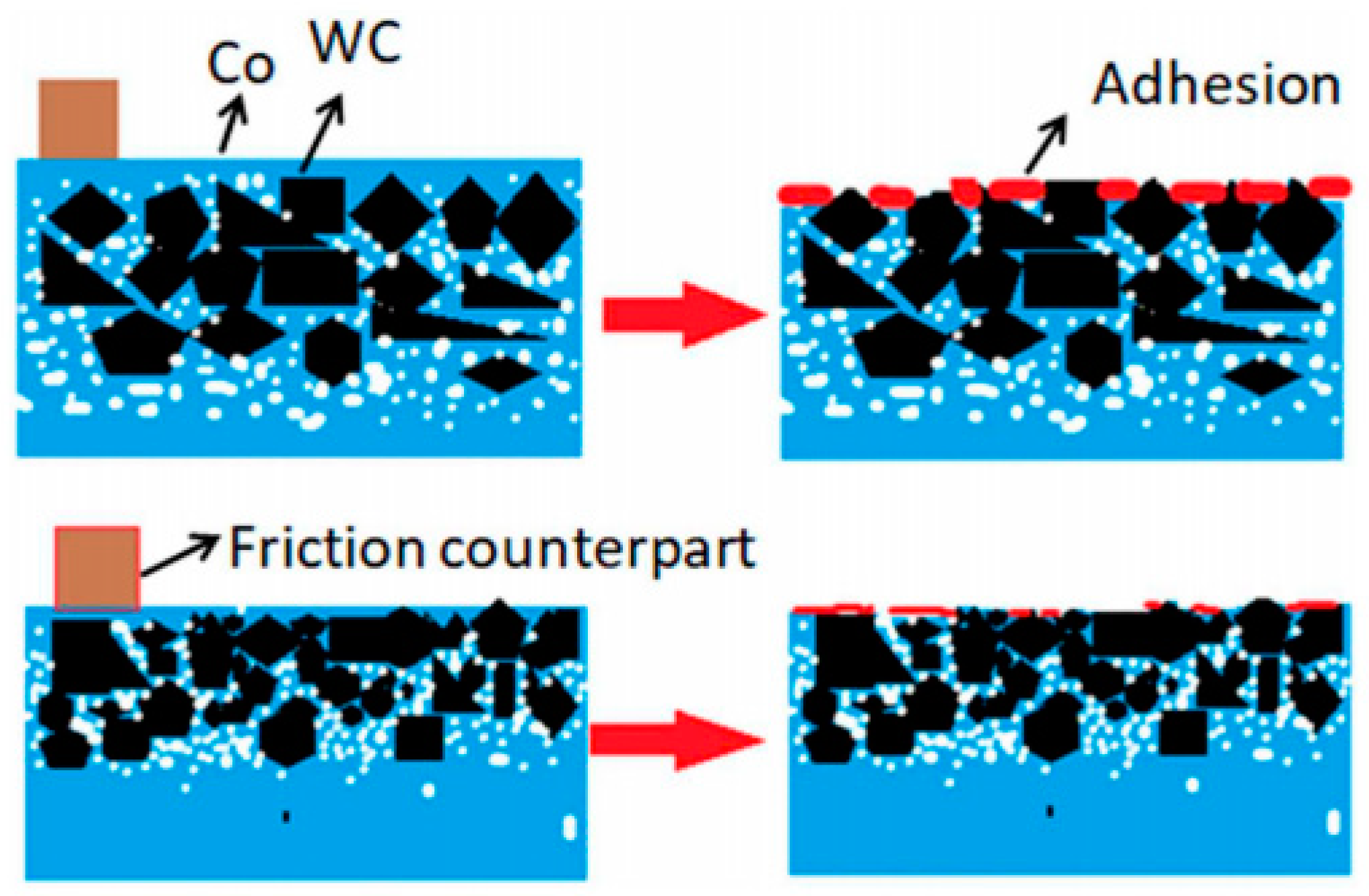
4. Mechanism of Nano-Coating Wear-Resistant
4.1. Grain Refinement
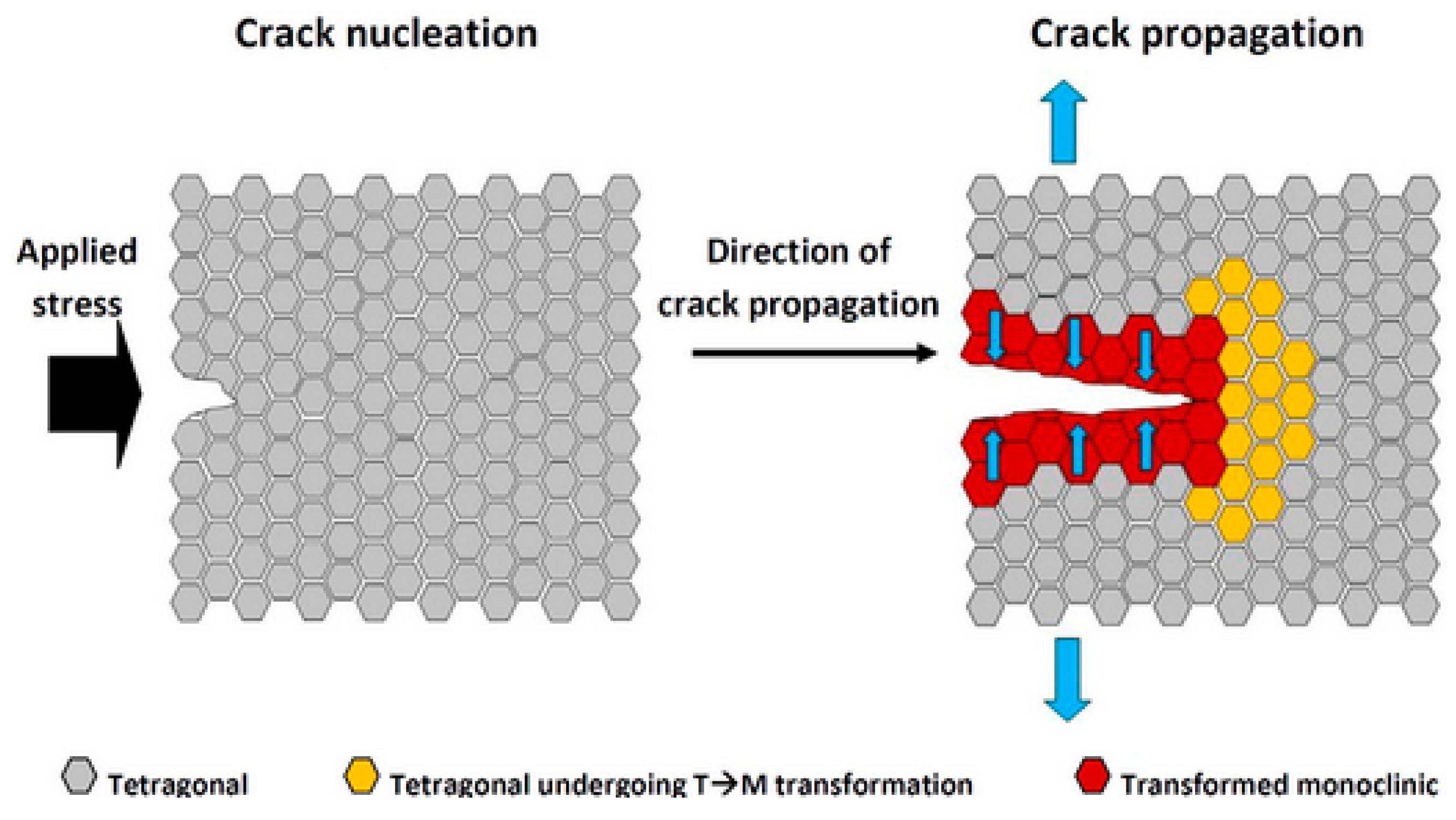
4.2. Phase Transformation Toughening Mechanism
4.3. Nano-Effects
5. Future Scope and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gleiter, H. Nanocrystalline materials. Prog. Mater Sci. 1989, 33, 223–315. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Chung, S.J. Biogenic nanomaterials: Synthesis, characterization, growth mechanism, and biomedical applications. J. Microbiol. Methods 2019, 157, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.J. Progress in Chinese tribology research and application. Sci. Technol. Rev. 2008, 23, 3. [Google Scholar]
- Koga, G.Y.; Wolf, W.; Schulz, R.; Savoie, S.; Bolfarini, C.; Kiminami, C.S.; Botta, W.J. Corrosion and wear properties of FeCrMnCoSi HVOF coatings. Surf. Coat. Technol. 2019, 357, 993–1003. [Google Scholar] [CrossRef]
- Kajikawa, Y. Roughness evolution during chemical vapor deposition. Mater. Chem. Phys. 2008, 112, 311–318. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Abbasi, H.; Antunes, M.; Velasco, J.I. Effects of Graphene Nanoplatelets and Cellular Structure on the Thermal Conductivity of Polysulfone Nanocomposite Foams. Polymers 2020, 12, 25. [Google Scholar] [CrossRef]
- Bundy, F.P.; Hall, H.T.; Strong, H.M.; Wentorf Jun, R.H. Man-made diamonds. Nature 1955, 176, 51–55. [Google Scholar] [CrossRef]
- He, Q.; Paiva, J.M.; Kohlscheen, J.; Beake, B.D.; Veldhuis, S.C. An integrative approach to coating/carbide substrate design of CVD and PVD coated cutting tools during the machining of austenitic stainless steel. Ceram. Int. 2020, 46, 5149–5158. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Arunachalam, N.; Ramachandra Rao, M.S. Wear performance of nano-engineered boron doped graded layer CVD diamond coated cutting tool for machining of Al-SiC MMC. Wear 2019, 426, 1536–1547. [Google Scholar] [CrossRef]
- Ishigaki, T.; Tatsuoka, S.; Sato, K.; Yanagisawa, K.; Yamaguchi, K.; Nishida, S. Influence of the Al content on mechanical properties of CVD aluminum titanium nitride coatings. Int. J. Refract. Met. Hard Mater. 2018, 71, 227–231. [Google Scholar] [CrossRef]
- Zhao, X.; Gou, L. Comparative analysis of graphene grown on copper and nickel sheet by microwave plasma chemical vapor deposition. Vacuum 2018, 153, 48–52. [Google Scholar] [CrossRef]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 1–24. [Google Scholar]
- Baptista, A.; Silva, F.J.G.; Porteiro, J.; Míguez, J.L.; Pinto, G.; Fernandes, L. On the physical vapour deposition (PVD): Evolution of magnetron sputtering processes for industrial applications. Procedia Manuf. 2018, 17, 746–757. [Google Scholar] [CrossRef]
- Helmersson, U.; Lattemann, M.; Bohlmark, J.; Ehiasarian, A.P.; Gudmundsson, J.T. Ionized physical vapor deposition (IPVD): A review of technology and applications. Thin Solid Film. 2006, 513, 1–24. [Google Scholar] [CrossRef]
- Shafyei, H.; Ashiri, R. Electron beam assisted physical vapor deposition of very hard TiCN coating with nanoscale characters. Ceram. Int. 2019, 45, 14821–14828. [Google Scholar] [CrossRef]
- Wolfe, D.E.; Singh, J.; Narasimhan, K. Synthesis and characterization of multilayered TiC/TiB2 coatings deposited by ion beam assisted, electron beam-physical vapor deposition (EB-PVD). Surf. Coat. Technol. 2003, 165, 8–25. [Google Scholar] [CrossRef]
- Tessier, P.Y.; Issaoui, R.; Luais, E.; Boujtita, M.; Granier, A.; Angleraud, B. Ionized Physical Vapour Deposition combined with PECVD, for synthesis of carbon–metal nanocomposite thin films. Solid State Sci. 2009, 11, 1824–1827. [Google Scholar] [CrossRef]
- Wang, P.; Deng, S.J.; He, Y.D.; Liu, C.X.; Zhang, J. Oxidation and hot corrosion behavior of Al2O3/YSZ coatings prepared by cathode plasma electrolytic deposition. Corros. Sci. 2016, 109, 13–21. [Google Scholar] [CrossRef]
- Kazmanli, K.; Daryal, B.; Urgen, M. Characterization of nano-composite TiN-Sb coating produced with hybrid physical vapor deposition system. Thin Solid Film. 2007, 515, 3675–3680. [Google Scholar] [CrossRef]
- Biksa, A.; Yamamoto, K.; Dosbaeva, G.; Veldhuis, S.C.; Fox-Rabinovich, G.S.; Elfizy, A.; Wagg, T.; Shuster, L.S. Wear behavior of adaptive nano-multilayered AlTiN/MexN PVD coatings during machining of aerospace alloys. Tribol. Int. 2010, 43, 1491–1499. [Google Scholar] [CrossRef]
- Li, M.; Su, B.; Zhou, B.; Wang, H.; Meng, J. One-pot synthesis and self-assembly of anti-wear octadecyltrichlorosilane/silica nanoparticles composite films on silicon. Appl. Surf. Sci. 2020, 508, 145187. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Yang, L.; Zhang, D.; Song, W.L. High performance, flexible and renewable nano-biocomposite artificial muscle based on mesoporous cellulose/ionic liquid electrolyte membrane. Sens. Actuators B 2019, 283, 579–589. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U. Sol-Gel processing of silica nanoparticles and their applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef]
- Kim, S.; Nam, G.; Yoon, H.; Park, H.; Choi, H. Structural, optical, and electrical properties of ZnO thin films deposited by sol-gel dip-coating process at low temperature. Electron. Mater. Lett. 2014, 10, 869–878. [Google Scholar] [CrossRef]
- Znaidi, L.; Touam, T.; Vrel, D.; Souded, N.; Yahia, S. AZO Thin Films by Sol-Gel Process for Integrated Optics. Coatings 2013, 3, 126–139. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Song, W.L.; Zhao, G. Chitosan-based polymer gel paper actuators coated with multi-wall carbon nanotubes and MnO2 composite electrode. Cellulose 2017, 24, 4383–4392. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, Y.; Yin, T.; Tian, X.; Qi, L. Building the silicon carbide nanowire network on the surface of carbon fibers: Enhanced interfacial adhesion and high-performance wear resistance. Ceram. Int. 2019, 45, 22571–22577. [Google Scholar] [CrossRef]
- Wang, D.H.; Bierwagen, G.P. Sol–gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, K.X.; Tang, H.; Wang, Y.L. Enhanced wettability and wear resistance on TiO2/PDA thin films prepared by sol-gel dip coating. Surf. Coat. Technol. 2019, 375, 334–340. [Google Scholar] [CrossRef]
- Claire, L.; Marie, G.; Julien, G. New architectured hybrid sol-gel coatings for wear and corrosion protection of low-carbon steel. Prog. Org. Coat. 2016, 99, 337–345. [Google Scholar] [CrossRef]
- Wang, H.J.; Chen, T.; Cong, W.L.; Liu, D.F. Laser cladding of Ti-based ceramic coatings on Ti6Al4V alloy: Effects of CeO2 nanoparticles additive on wear performance. Coatings 2019, 9, 109. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, B.H.; Cui, H.C.; Lu, F.G.; Xu, P.Q. Microstructure, wear, and oxidation resistance of nanostructured carbide-strengthened cobalt-based composite coatings on Invar alloys by laser cladding. Surf. Coat. Technol. 2020, 381, 125188. [Google Scholar] [CrossRef]
- Rahman Rashid, R.A.; Nazari, K.A.; Barr, C.; Palanisamy, S.; Orchowski, N.; Matthews, N.; Dargusch, M.S. Effect of laser reheat post-treatment on the microstructural characteristics of laser-cladded ultra-high strength steel. Surf. Coat. Technol. 2019, 372, 93–102. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Yu, S.W.; Mou, J.G.; Wu, D.H.; Zheng, S.H. Research Progress on the Collaborative Drag Reduction Effect of Polymers and Surfactants. Materials 2020, 13, 444. [Google Scholar] [CrossRef]
- More, S.R.; Bhatt, D.V.; Menghani, J.V. Resent Research Status on Laser Cladding as Erosion Resistance Technique - An Overview. Mater. Today Proc. 2017, 4, 9902–9908. [Google Scholar] [CrossRef]
- Tran, V.N.; Yang, S.; Phung, T.A. Microstructure and properties of Cu/TiB2 wear resistance composite coating on H13 steel prepared by in-situ laser cladding. Opt. Laser Eng. 2018, 108, 480–486. [Google Scholar] [CrossRef]
- Haldar, B.; Saha, P. Identifying defects and problems in laser cladding and suggestions of some remedies for the same. Mater. Today Proc. 2018, 5, 13090–13101. [Google Scholar] [CrossRef]
- Liu, Y.N.; Sun, R.L.; Niu, W.; Zhang, T.G.; Lei, Y.W. Effects of CeO2 on microstructure and properties of TiC/Ti2Ni reinforced Ti-based laser cladding composite coatings. Opt. Laser Eng. 2019, 120, 84–94. [Google Scholar]
- Gao, W.Y.; Chang, C.; Li, G.; Xue, Y.F.; Wang, J.L.; Zhang, Z.Y.; Lin, X.C. Study on the laser cladding of FeCrNi coating. Optik 2019, 178, 950–957. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, P.L.; Gao, Q.S.; Qin, Y.; Li, R.D. Laser cladding Ni-based alloy/nano-Ni encapsulated h-BN self-lubricating composite coatings. Surf. Coat. Technol. 2017, 332, 422–427. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, K.; Yao, C.W.; Nie, P.L.; Huang, J.; Li, Z.G. Microstructure and tribological properties of laser cladded self-lubricating nickel-base composite coatings containing nano-Cu and h-BN solid lubricants. Surf. Coat. Technol. 2019, 359, 485–494. [Google Scholar] [CrossRef]
- Nazari, K.A.; Rahman Rashid, R.A.; Palanisamy, S.; Xia, K.; Dargusch, M.S. A novel Ti-Fe composite coating deposited using laser cladding of low cost recycled nano-crystalline titanium powder. Mater. Lett. 2018, 229, 301–304. [Google Scholar] [CrossRef]
- Berger, L.M. Application of hardmetals as thermal spray coatings. Int. J. Refract. Met. Hard Mater. 2015, 49, 350–364. [Google Scholar] [CrossRef]
- Berger, L.M. Hard but slippery-titanium hardmetal coatings have industrial potential. Met. Powder Rep. 2005, 60, 28–31. [Google Scholar] [CrossRef]
- Li, T.C.; Liu, Y.; Liu, B.; Guo, W.M.; Xu, L.Y. Microstructure and wear behavior of FeCoCrNiMo0.2 high entropy coatings prepared by air plasma spray and the high velocity Oxy-fuel spray processes. Coatings 2017, 7, 151. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, C.; Zhang, G.; Liao, H.L. Wear and corrosion resistant performance of thermal-sprayed Fe-based amorphous coatings: A review. Surf. Coat. Technol. 2019, 377, 124896. [Google Scholar] [CrossRef]
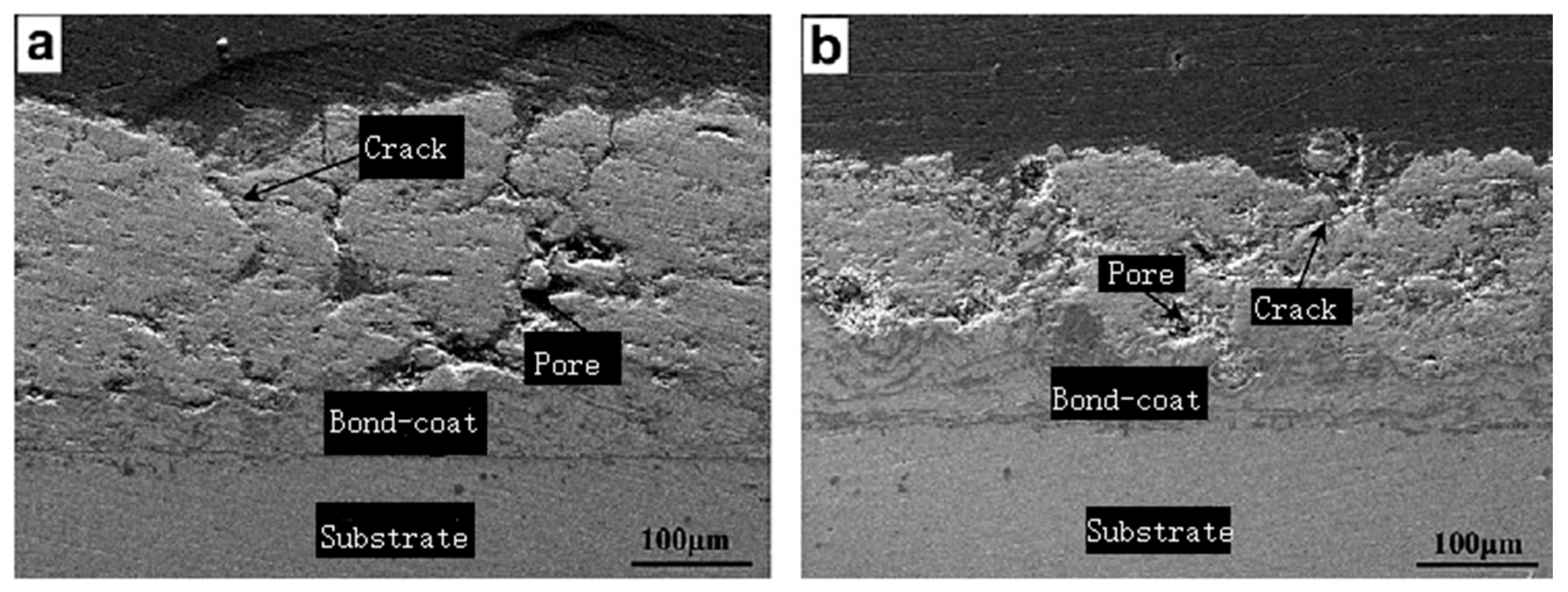
- Psyllaki, P.; Mourlas, A.; Vourlias, G.; Pavlidou, E.; Vardavoulias, M. Influence of microstructure flaws on the tribological performance of Cr-based thermal-sprayed ceramic coatings. Ceram. Int. 2019, 45, 19360–19369. [Google Scholar] [CrossRef]
- Matikainen, V.; Rubio Peregrina, S.; Ojala, N.; Koivuluoto, H.; Schubert, J. Erosion wear performance of WC-10Co4Cr and Cr3C2-25NiCr coatings sprayed with high-velocity thermal spray processes. Surf. Coat. Technol. 2019, 370, 196–212. [Google Scholar] [CrossRef]
- Amanov, A. Wear resistance and adhesive failure of thermal spray ceramic coatings deposited onto graphite in response to ultrasonic nanocrystal surface modification technique. Appl. Surf. Sci. 2019, 477, 184–197. [Google Scholar] [CrossRef]
- Jackson, G.A.; Bai, M.; Pala, Z.; Hussain, T.; Sun, W. Small punch creep testing of thermally sprayed Stellite 6 coating: A comparative study of as-received vs post-heat treatment. Mater. Sci. Eng. A 2019, 749, 137–147. [Google Scholar] [CrossRef]
- Kiilakoski, J.; Langlade, C.; Koivuluoto, H.; Vuoristo, P. Characterizing the micro-impact fatigue behavior of APS and HVOF-sprayed ceramic coatings. Surf. Coat. Technol. 2019, 371, 245–254. [Google Scholar] [CrossRef]
- Kear, B.H. High pressure synthesis of nanophase WC/Co/Dlamond powders: Implications for thermal spraying. J. Therm. Spray Technol. 1999, 7, 412. [Google Scholar]
- Gao, P.H.; Chen, B.Y.; Wang, W.; Jia, H. Simultaneous increase of friction coefficient and wear resistance through HVOF sprayed WC-(nano WC-Co). Surf. Coat. Technol. 2019, 363, 379–389. [Google Scholar] [CrossRef]
- Wu, Y.M.; Chen, X.M.; Zhou, X.L.; Mao, P.Z.; Fang, Y. Surface Engineering and Remanufacturing Technology-New Surface Technology for Hydraulic Machinery and Hydraulic Metal Structures; The Yellow River Water Conservancy Press: Zhengzhou, China, 2017; pp. 20–30. [Google Scholar]
- Wu, Y.M.; Zhao, J.; Chen, X.M.; Fu, L.; Mao, P.Z.; Zhou, X.L. Effect of high temperature and oxidation on microstructure and properties of WC-10Co4Cr coatings. Chin. J. Nonferrous Metals 2017, 27, 1395–1402. [Google Scholar]
- Myalska, H.; Lusvarghi, L.; Bolelli, G.; Sassatelli, P.; Moskal, G. Tribological behavior of WC-Co HVAF-sprayed composite coatings modified by nano-sized TiC addition. Surf. Coat. Technol. 2019, 371, 401–416. [Google Scholar] [CrossRef]
- Mi, P.B.; Zhao, H.J.; Wang, T.; Ye, F.X. Sliding wear behavior of HVOF sprayed WC-(nano-WC-Co) coating at elevated temperatures. Mater. Chem. Phys. 2018, 206, 1–6. [Google Scholar] [CrossRef]
- Suresh Babu, P.; Srinivasa Rao, D.; Rama Krishna, L.; Sundararajan, G. Weibull analysis of hardness distribution in detonation sprayed nano-structured WC-12Co coatings. Surf. Coat. Technol. 2017, 319, 394–402. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Sun, X.G.; He, J.Q.; Wang, C.H. Microstructure and indentation mechanical properties of plasma sprayed nano-bimodal and conventional ZrO2-8wt%Y2O3 thermal barrier coatings. Vacuum 2012, 86, 1174–1185. [Google Scholar] [CrossRef]
- Yin, B.; Liu, G.; Zhou, H.D.; Chen, J.M.; Yan, F.Y. Microstructures and properties of plasma sprayed FeAl/CeO2/ZrO2 nano-composite coating. Appl. Surf. Sci. 2010, 256, 4176–4184. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Ren, X.X.; Yang, Z.L.; Gao, P.Y.; Zhao, C.C.; Chu, Z.H.; Wang, L.; Li, J.; Dong, Y.C. Microstructure and properties of composite coatings prepared by plasma spraying ZrO2-B2O3-Al composite powders. J. Alloy. Compd. 2018, 740, 124–131. [Google Scholar] [CrossRef]
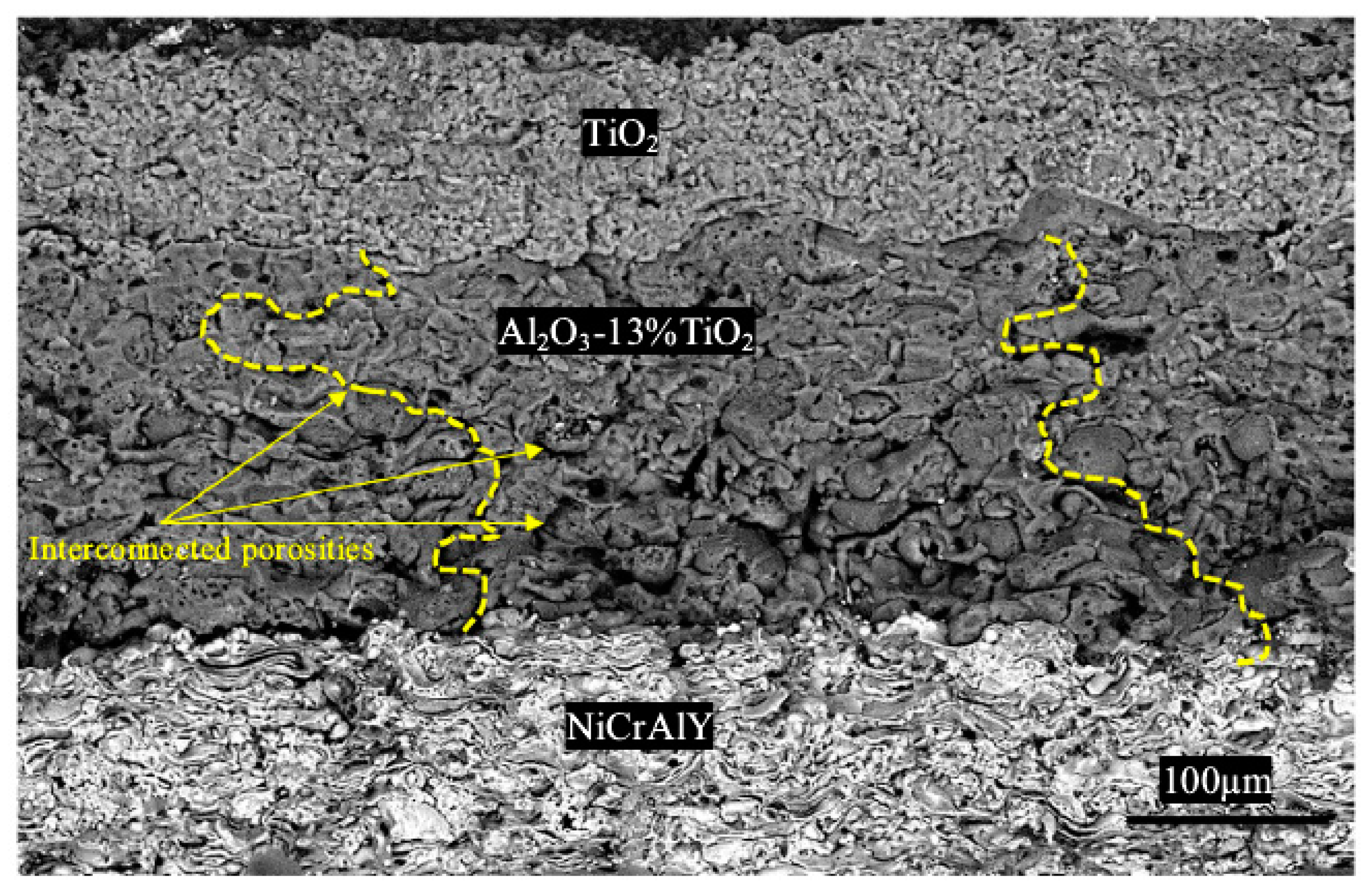
- Daroonparvar, M.; Yajid, M.A.M.; Yusof, N.M.; Bakhsheshi-Rad, H.R. Fabrication and properties of triplex NiCrAlY/nano Al2O3·13%TiO2/nano TiO2 coatings on a magnesium alloy by atmospheric plasma spraying method. J. Alloy. Compd. 2015, 645, 450–466. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, Y.H.; Miao, L.L.; Wang, Y.; Tian, W.; Ma, Y.D.; Zhang, X.; Zhang, C.; Chen, X.G.; Wang, L.; et al. Effects of treatment process and nano-additives on the microstructure and properties of Al2O3-TiO2 nanocomposite powders used for plasma spraying. Podwer Technol. 2018, 338, 304–312. [Google Scholar] [CrossRef]
- Ajdelsztajn, L.; Picas, J.A.; Kim, G.E. Oxidation behavior of HVOF sprayed nanocrystalline NiCrAlY powder. Mater. Sci. Eng. A 2002, 338, 33–43. [Google Scholar] [CrossRef]
- Matthews, S.; Asadov, A.; Ruddell, S.; Berger, L.M. Thermally induced metallurgical processes in Cr3C2-NiCr thermal spray coatings as a function of carbide dissolution. J. Alloy. Compd. 2017, 728, 445–463. [Google Scholar] [CrossRef]
- Ren, X.Y. Developing status of nanocoaters and coating technology. Nonferrous Metals. 2004, 56, 31–34. [Google Scholar]
- Tang, C.; Li, T.H.; Bai, Y.H.; Gao, J.J.; Yang, W.B.; Jiao, S.S.; Zhao, T.K. Two-step method to deposit ZrO2 coating on carbon fiber: Preparation, characterization, and performance in SiC composites. Ceram. Int. 2018, 44, 11812–11819. [Google Scholar] [CrossRef]
- Reghu, V.R.; Shankar, V.; Ramaswamy, P. Challenges in plasma spraying of 8% Y2O3-ZrO2 thermal barrier coatings on Al alloy automotive piston and influence of vibration and thermal fatigue on coating characteristics. Mater. Today Proc. 2018, 5, 23927–23936. [Google Scholar] [CrossRef]
- Li, Q.L.; Song, P.; Lü, K.Y.; Huang, W.F.; Duan, W.H.; Huang, T.H.; Lu, J.S. Enhanced interface adhesion by in-situ oxidation within metal-ceramic coatings. Ceram. Int. 2018, 44, 23273–23278. [Google Scholar] [CrossRef]
- Bigelow, S.; Shen, Y.L. Parametric computational analysis of indentation-induced shear band formation in metal-ceramic multilayer coatings. Surf. Coat. Technol. 2018, 350, 779–787. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Müller, A.H.E. One-dimensional organic–inorganic hybrid nanomaterials. Polymer 2010, 51, 4015–4036. [Google Scholar] [CrossRef]
- Khodair, Z.T.; Khadom, A.A.; Jasim, H.A. Corrosion protection of mild steel in different aqueous media via epoxy/nanomaterial coating: Preparation, characterization and mathematical views. J. Mater. Res. Technol. 2019, 8, 424–435. [Google Scholar] [CrossRef]
- Lee, W.; Kim, D.; Lee, S.; Park, J. Stimuli-responsive switchable organic-inorganic nanocomposite materials. Nano Today 2018, 23, 97–123. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhang, Y.G.; Chen, Q.H.; Liu, J.F. Numerical prediction of thermo-hydrodynamics on the gas-lubricated film in wedge-shaped microchannel. J. Drain. Irrig. Mach. Eng. 2019, 37, 692–698. [Google Scholar]
- Wang, X.; Liu, Z.J.; Hill, E.H.; Zheng, Y.B.; Guo, G.Q. Organic-Inorganic Hybrid Pillarene-Based Nanomaterial for Label-Free Sensing and Catalysis. Matter 2019, 1, 848–861. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Jiang, Y.; Yi, X.; Guo, W.F. Influence of liquid water content on wind turbine blade icing by numerical simulation. J. Drain. Irrig. Mach. Eng. 2019, 37, 513–520. [Google Scholar]
- Wang, Y.N.; Dai, X.Y.; Xu, T.L.; Qu, L.J.; Zhang, C.L. Preparation and anticorrosion properties of silane grafted nano-silica/epoxy composite coating. Chem. J. Chin. Univ. 2018, 39, 1564–1572. [Google Scholar]
- Atta, A.M.; Ezzat, A.O.; El-Saeed, A.M.; Wahby, M.H.; Abdallah, M.M.S. Superhydrophobic organic and inorganic clay nanocomposites for epoxy steel coatings. Prog. Org. Coat. 2020, 140, 105502. [Google Scholar] [CrossRef]
- Quazi, M.M.; Fazal, M.A.; Haseeb, A.S.M.A.; Yusof, F.; Masjuki, H.H.; Arslan, A. Effect of rare earth elements and their oxides on tribo-mechanical performance of laser claddings: A review. J. Rare Earths. 2016, 34, 549–564. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Parvin, N.; Valefi, Z. Effect of microstructure and mechanical properties on wear behavior of plasma-sprayed Cr2O3-YSZ-SiC coatings. Ceram. Int. 2019, 45, 5284–5296. [Google Scholar] [CrossRef]
- Zamani, P.; Valefi, Z. Microstructure, phase composition and mechanical properties of plasma sprayed Al2O3, Cr2O3 and Cr2O3-Al2O3 composite coatings. Surf. Coat. Technol. 2017, 316, 138–145. [Google Scholar] [CrossRef]
- Chen, W.; Lin, T.J.; Dai, Y.Y.; An, Y.L.; Yu, F. Recent advances in the investigation of nanoeffects of Fischer-Tropsch catalysts. Catal. Today 2018, 311, 8–22. [Google Scholar] [CrossRef]
- Emarati, S.M.; Mozammel, M. Efficient one-step fabrication of superhydrophobic nano-TiO2/TMPSi ceramic composite coating with enhanced corrosion resistance on 316L. Ceram. Int. 2020, 46, 1652–1661. [Google Scholar] [CrossRef]
- Liang, X.B.; Shang, J.C.; Chen, Y.X.; Zhou, Z.D.; Zhang, Z.B.; Xu, B.S. Influence of ceramic particles and process parameters on residual stress of flame-sprayed Fe-based coatings. Surf. Coat. Technol. 2018, 354, 10–17. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zuo, D.W.; Sun, Y.L.; Xu, F.; Zhang, D. Microstructure of nanometer Al2O3 dispersion strengthened Ni-based high-temperature protective coatings by laser cladding. Trans. Nonferrous Met. Soc. China 2009, 19, 586–591. [Google Scholar] [CrossRef]
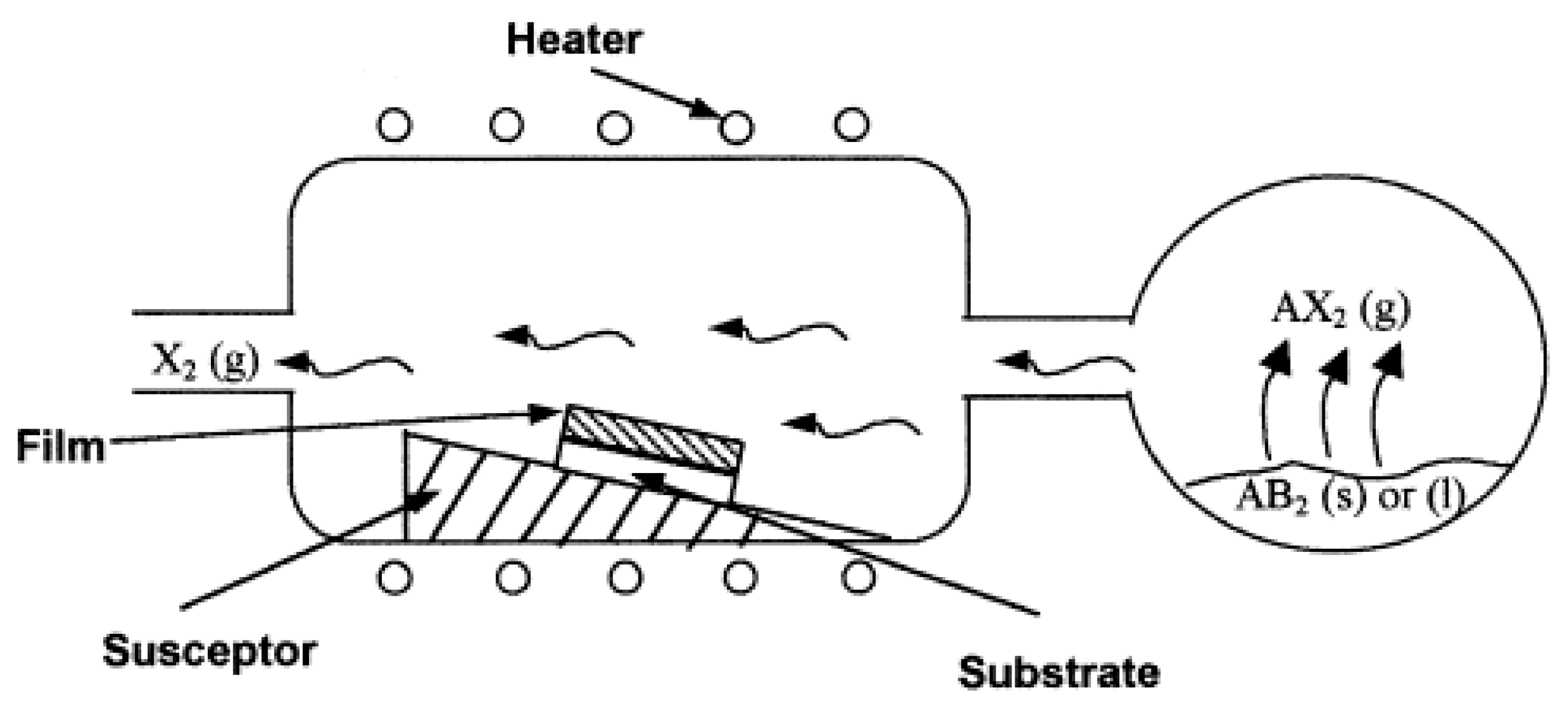
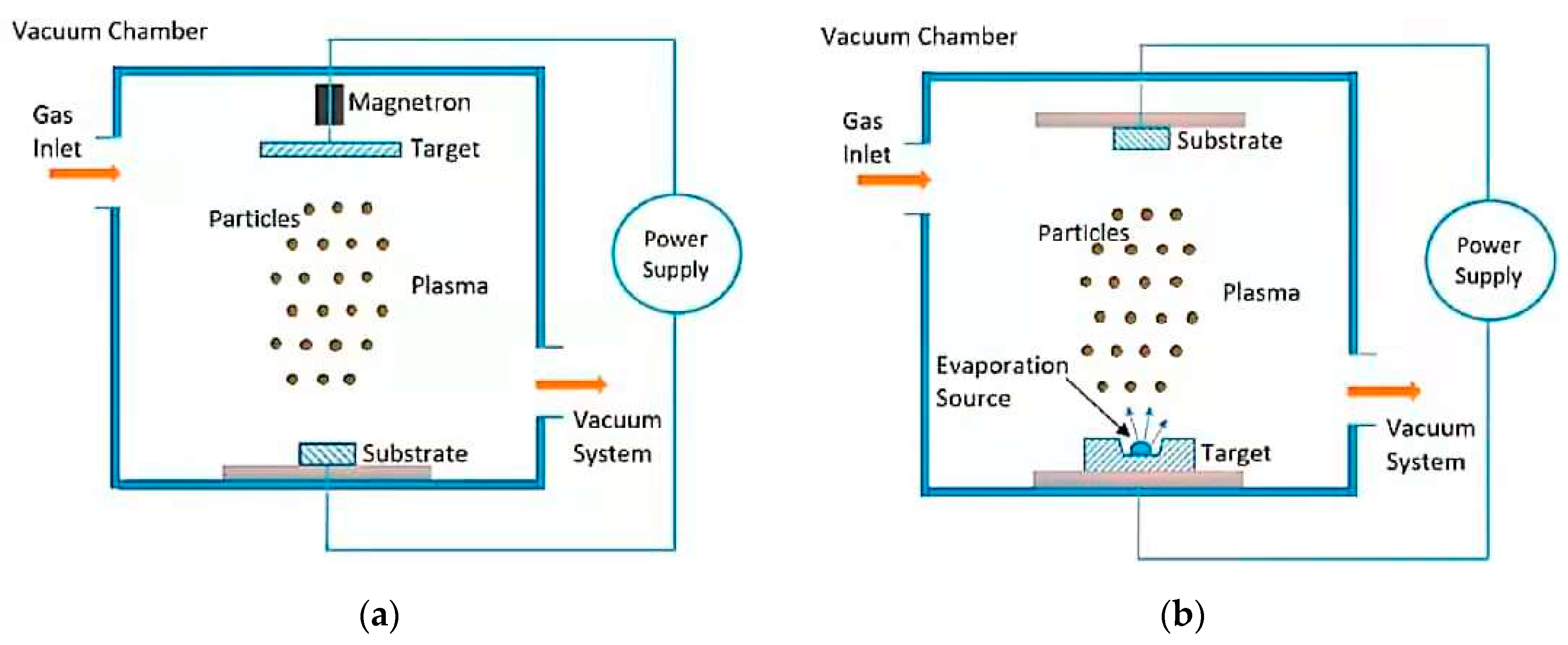

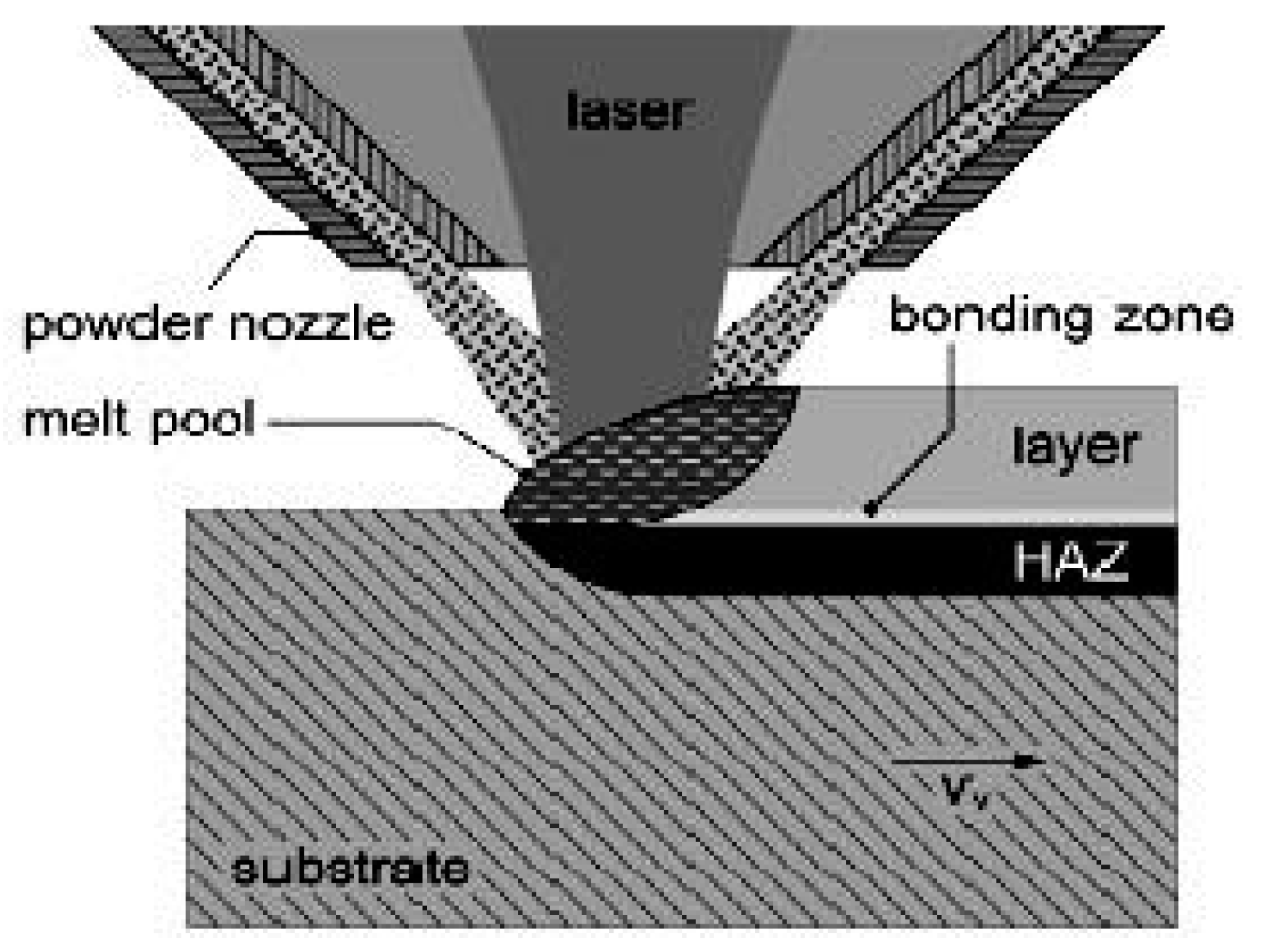
| Preparation Technology | Principle | Advantages | Disadvantages | Materials |
|---|---|---|---|---|
| CVD | (1)Formation of volatile substances; (2)Transfer the above substances to the deposition area; (3)Produce chemical reactions on solids and produce solid substances [6]. | (1)Material purity; (2)High production efficiency; (3)Excellent performance; (4)Easy to control; (5)Processing costs [7]. | (1)Harmful gas will be generated; (2)Difficult to produce multi-component materials. | Refractory borides, carbides, nitrides and oxides (TiC, TiN, Al2O3, TaC, HfN and TiB2) [9,10,11]. |
| PVD | (1)Generation of gas-phase substances; (2)Transportation; (3)Deposition [14]. | (1)The coating is uniform; (2)High repeatability; (3)Good coiled parts; (4)Local deposition; (5)Almost unlimited material selection for the substrate; (6)Have sufficient flexibility for the temperature requirements of the substrate; (7)Wide selection of coating materials; (8)Environmental protection without pollution [15]. | (1)The complexity and high cost of technology and monitoring equipment; (2)High work requirements for operators; (3)Relatively low productivity; (4)Chemical components with high precision are required; (5)Special preparation is required for the coating surface. | Metal, alloy, compound [16,17]. |
| Sol-gel Method | (1)Hydrolysis reaction; (2)Coating; (3)Heat treatment [22,23,24]. | (1)The reaction can be performed at low temperature; (2)Can prepare high purity and homogeneous coatings; (3)Suitable for large area film formation; (4)The process is simple [38,39]. | (1)Raw materials are expensive and some are harmful; (2)Long preparation period; (3)There are a lot of micropores in the gel. | Metal alkoxide or inorganic salt [22,23,24]. |
| Laser Cladding Method | (1)Single-step method: material is continuously fed in a laser-generated molten bath; (2)Two-step method: deposition and melting [37]. | (1)Fast cooling speed; (2)Low coating dilution rate; (3)Less heat input and distortion; (4)Powder selection is almost unlimited; (5)The thickness of the cladding layer is large; (6)High cost performance; (7)Easy to implement automation [38,39]. | (1)The metallurgical quality of the laser cladding layer is poor; (2)Porosity, cracks and other problems will occur, affecting the performance of the coating; (3)Uneven composition and structure during laser cladding; (4)Poor repeatability. | Preparation of Fe-based, Ni-based, Co-based, Al-based, Ti-based, Mg-based metal-based composite materials [40]. |
| Thermal Spraying | (1)Heat the material to the melting state; (2)Atomize materials with airflow; (3)Deposited on the substrate [45]. | (1)The process is simple; (2)Wide selection of coatings and substrates; (3)Large range of coating thickness variation; (4)High deposition efficiency; (5)Easy to form composite coating [47]. | Inherent high temperature will cause oxide inclusions, affecting the hardness and abrasion resistance of the coating. | Oxides, carbides and their composites and nickel-based alloys [53]. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Xia, K.; Wu, D.; Mou, J.; Zheng, S. Technical Characteristics and Wear-Resistant Mechanism of Nano Coatings: A Review. Coatings 2020, 10, 233. https://doi.org/10.3390/coatings10030233
Gu Y, Xia K, Wu D, Mou J, Zheng S. Technical Characteristics and Wear-Resistant Mechanism of Nano Coatings: A Review. Coatings. 2020; 10(3):233. https://doi.org/10.3390/coatings10030233
Chicago/Turabian StyleGu, Yunqing, Ke Xia, Denghao Wu, Jiegang Mou, and Shuihua Zheng. 2020. "Technical Characteristics and Wear-Resistant Mechanism of Nano Coatings: A Review" Coatings 10, no. 3: 233. https://doi.org/10.3390/coatings10030233
APA StyleGu, Y., Xia, K., Wu, D., Mou, J., & Zheng, S. (2020). Technical Characteristics and Wear-Resistant Mechanism of Nano Coatings: A Review. Coatings, 10(3), 233. https://doi.org/10.3390/coatings10030233





