Abstract
In an impressed current cathodic protection system (ICCP) to protect structures against corrosion, the efficient operation depends on the proper selection of the electrodes, particularly the anode, chosen considering the structure to be protected and the environment in which it is located. The nature and overpotential of the anodic reaction determine the operation costs of an ICCP system so that proper anode selection is critically important for an ICCP system to function efficiently. Commercial anodes based on titanium substrates coated with iridium–tantalum oxide mixtures (IrO2-Ta2O5/Ti) are frequently used for this purpose due to low operating overpotentials. However, the gradual passivation of its surface limits its useful life and increases its operating costs, so it is necessary to seek competitive alternatives for its replacement. This study aimed to determine the feasibility of using carbon steel substrates coated with nickel/cobalt/boron (NiCoB/CS) as a viable low-cost alternative to replace IrO2-Ta2O5/Ti anodes in ICCP systems. Comparison between the electrochemical behavior and the corrosion resistance of both types of electrodes shows that the NiCoB/CS anode shows a good electrocatalytic activity and a higher corrosion resistance than IrO2-Ta2O5/Ti coated anodes, indicating that the NiCoB/CS anodes are promising low-cost candidates for ICCP systems.
1. Introduction
The degradation of structures is originated in many cases by the effect of the corrosion. In the case of concrete structures, steel corrosion is a common problem and is the phenomenon responsible for numerous economic losses, in addition to the associated consequences of risk to human life and reduced lifetime of the physical infrastructure of buildings, monuments, and the adverse aesthetic effects, or even lead to its total deterioration and to take it out of service.
High reparation and reconstruction costs associated with steel corrosion in concrete structures are estimated at 2.5 trillion dollars, equivalent to 3.4% of the global Gross Domestic Product (GDP) [1]. Degradation of steel embedded in concrete structures is usually caused either by the effect of carbonation of concrete components with environmental carbon dioxide (CO2) or by the high concentration of chloride ions in marine environments. Several procedures have been developed and are focused on prevention and control [2,3] to minimize the effects of the corrosion phenomenon. Prevention methods are usually implemented during manufacturing (e.g., water/cement ratio), while control methods are employed as protection systems on the concrete structure. An effective way to prevent the corrosion of steel in metallic or concrete structures is the impressed current cathodic protection method (ICCP) [4]. This method is beneficial when applied to protect large surfaces or large structures, with the advantage of being easy to control. Since the ICCP method is of electrochemical nature, the electrochemical reactions involved when an ICCP system is operating are dependent both on the medium and on the nature of the employed electrodes. These electrochemical reactions are usually either the evolution of oxygen in neutral or alkaline medium or the evolution of chlorine in highly saline medium at the anode and the reduction of water at the cathode. Anodes with IrO2-Ta2O5/Ti coatings are currently the most efficient option for use in ICCP systems. These allow to evolve either oxygen or chlorine, depending on the environmental conditions, at low overpotentials [5]. These anodes have high electrocatalytic activity but surface passivation [6,7], and shortened service life [6,8,9,10,11,12,13,14] can show during its service life that leads to its loss of electrocatalytical activity and of mechanical properties. Other investigations of electrocatalytic materials less expensive than IrO2-Ta2O5/Ti anodes have been prompted to overcome the disadvantages and also the high manufacturing costs associated with IrO2-Ta2O5/Ti-coated anodes [15,16,17,18]. The goal is to obtain efficient anodes at lower manufacturing cost, with a high stability or resistance against corrosion, with similar or better properties than IrO2-Ta2O5/Ti-coated anodes to be considered as good candidates for ICCP applications.
Recent research on new materials for water electrolysis can be used as a basis to develop new anode materials for ICCP systems. For example, NiCoB coatings have been found to be good electrocatalytic materials for oxygen [19] and are used as anticorrosive coatings [19,20,21] in the gas and oil industry. NiCoB and IrO2-Ta2O5/Ti coatings have similar properties, such as resistance against corrosion and electrocatalytical activity for oxygen and hydrogen evolution. Still, the lower manufacturing cost and the higher availability of raw material to prepare NiCoB coatings allow the consideration of the feasibility of replacing the use of anodes of IrO2-Ta2O5/Ti for NiCoB anodes in ICCP systems. On this basis, in this work the behavior of an NiCoB coating as an anode in sodium chloride (NaCl) and calcium hydroxide (Ca(OH)2) solutions is investigated and results are compared with those observed with IrO2-Ta2O5/Ti to determine the advantages and limitations of each kind of anode.
2. Materials and Methods
Commercial samples of carbon steel 1018 substrates coated with a NiCoB coating (NiCoB/CS) (MAM Technologies) (5 cm × 5 cm) and of IrO2-Ta2O5/Ti (length = 3.9 cm; diameter = 0.3 cm) (LIDA electrodes, from Structural Technologies) were studied as anodes. Prior to their electrochemical study and physicochemical characterization, samples were cleaned and degreased, according to the procedure reported in [19].
2.1. Characterization
The studied samples were characterized by electrochemical techniques to evaluate their distinctive electrochemical parameters (exchange current density and values of Tafel slope), and also their electrocatalytic activity from the observed overpotentials for oxygen and chlorine evolution, and finally their corrosion rate in the selected studied solutions. Samples were also characterized by physicochemical methods to evaluate their chemical composition, crystallinity, morphology, and thickness.
2.1.1. Electrochemical Characterization
The electrochemical characterization of the studied samples was carried out by using a classical three electrode assembly: a working electrode of the material under investigation (geometric area = 1 cm2), an Ag/AgCl electrode as a reference electrode, and a platinum wire as auxiliary electrode. The studied solutions were a neutral medium containing chloride ions (MNC) (0.5 M NaCl solution) and an alkali medium (MAL) at pH 13.0 (0.01 M (Ca(OH)2 solution). All chemical reagents were analytical reactant grade. The assays were carried out in a Biologic potentiostat/galvanostat with EC-Lab V10.3 software.
Cyclic and linear voltammetry techniques cyclic were used to obtain the polarization curves associated with the electrochemical response of each anode material in each one of the studied media. From these obtained polarization curves, the overpotential for the oxygen and chlorine evolution were evaluated and also the exchange current density and the values of the Tafel slope corresponding to the electrochemical kinetic parameters associated with these reactions. The Tafel slopes and corrosion rates were evaluated according to the ASTM G59 [22] standard technique.
2.1.2. Physicochemical Characterization
The chemical composition of the samples was evaluated by energy-dispersive X-ray spectroscopy (EDS) in a scanning electron microscope (SEM) (Hitachi model S-3700N, Bruker Xflash 6, Bremen, Germany). The measured thickness was only conducted on the NiCoB/CS coating using SEM. The crystalline phases in the NiCoB/CS coating were analyzed using an X-ray diffractometer (XRD) (Bruker model D8 advance, Karlsrure, Germany).
3. Results
Physicochemical Characterization of the Anodes
3.1. Element Composition
The chemical compositions of the NiCoB/CS and IrO2-Ta2O5/Ti samples are shown in Table 1. The NiCoB/CS coating was rich in Ni and Co. The IrO2-Ta2O5/Ti coating showed high content in Ir and O, which is consistent with the expected composition for iridium oxide-based electrodes [23]. IrO2-Ta2O5/Ti anodes showed typically large amounts of oxygen at the titanium-metal oxide interface due to the presence of titanium oxide [23]. In fact, the percentage of Ti present provided insight into the influence of the substrate during the composition analysis testing.

Table 1.
Chemical composition of the NiCoB/CS and IrO2-Ta2O5/Ti coatings.
3.2. Coating Thickness
The micrograph used to measure the thickness of the NiCoB/CS coating is shown in Figure 1. The thickness was measured at six different positions, giving a mean thickness of 52 μm. The thickness of the coating was heterogenous, and a larger thickness was observed at the borders of the coating. In cases of heterogenous current and potential distribution this is a common phenomenon. The thickness of the samples in this study was larger than that reported by Campillo (20–40 μm). The IrO2-Ta2O5/Ti coating was much thicker than the NiCoB/CS coating, in the range of 630 μm to 1000 μm, as a different coating method was used (immersion and thermal decomposition) [10,11,24,25,26].
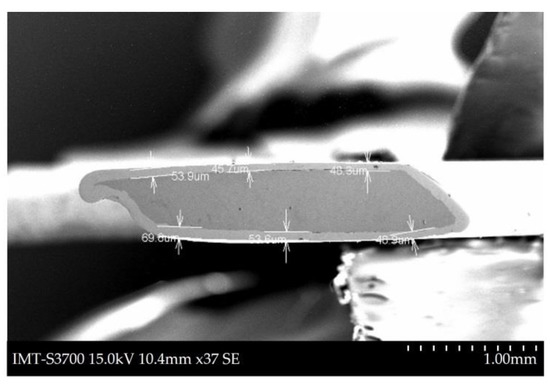
Figure 1.
Micrograph of the NiCoB/CS coating thickness obtained from scanning electron microscope (SEM) of a transverse cut sample.
The notorious difference in thickness between IrO2-Ta2O5/Ti and NiCoB/CS coating evidences the lower manufacturing cost of NiCoB/CS coatings being a real advantage of the use of NiCoB/CS as anode materials in ICCP systems.
3.3. Identification of the Phases in the Coating
The XRD spectrum indicated the presence of three crystalline compounds in the coating, as shown in Figure 2. The compounds present in Figure 2 correspond to Ni, Co, and Co2B, which is usually reported at 2 Theta = 44° in literature. The contributions of cobalt and cobalt borides were reported by Campillo and Bekish [20,27]. The presence of cobalt boride is related to increased hardness, which maintains the integrity of the coating/substrate interface.
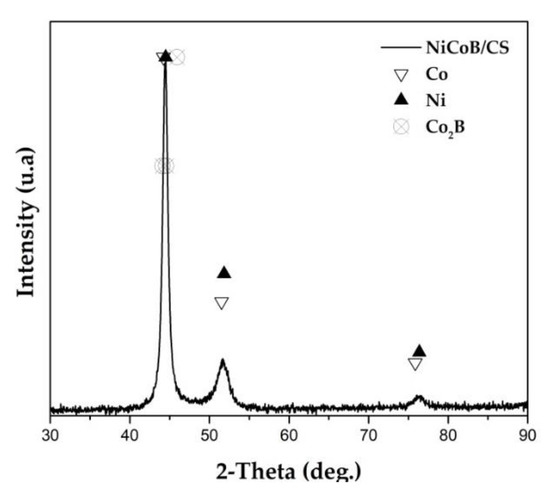
Figure 2.
X-ray spectrum of the NiCoB/CS coating obtained from a plaque type sample.
The confirmation of the proposed phases based on the position of the signals in 2 Theta was done by modeling the spectrum obtained with a Rietveld analysis using Bruker Topas 4.2 software. The results correspond to the presence of Co in 72.17%, Co2B in 10.88%, and Ni in 16.95%.
3.4. Resistance against Corrosion in MNC and MAL Media of the NiCoB/CS Coating
The resistance against corrosion of the NiCoB/CS coating was evaluated by triplicate, and the mean values shown in Table 2 Higher corrosion rates were observed in the MNC medium than the MAL medium, with values of 0.04 and 0.01 mm/year, respectively. The observed difference between these corrosion rates could be attributed to differences in the corrosion products (soluble nickel salts in neutral medium MNC and insoluble oxides or hydroxides in alkaline medium MAL) and then in the electrochemical involved reactions, as well as to imperfections on the surface of the coating, such as the 20.5 µm porous observed in Figure 3.

Table 2.
Corrosion rate for the NiCoB/CS and IrO2-Ta2O5/Ti coatings.
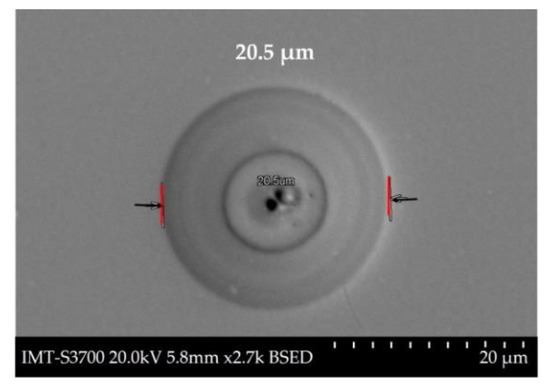
Figure 3.
Micrograph of the NiCoB/CS coating exposure to the medium, where a circular form was observed (diameter = 20.5 µm).
It is important to note that the corrosion rate of NiCoB coating in both of the studied media was lower than the corrosion rate of IrO2-Ta2O5/Ti coatings, indicating, in consequence, better resistance against corrosion.
Taking into consideration the observed corrosion rate of the NiCoB/CS coating in the MNC (0.04 mm/year) and MAL media (0.01 mm/year) and the measured coating thickness (0.057 mm), an estimate of the coating’s service life was calculated. Assuming uniform corrosion and a constant corrosion rate, the service life for NiCoB/CS was estimated to be 1.14 years in MNC medium and 2.85 years in MAL medium. In the case of the IrO2-Ta2O5/Ti coating, a 0.1mm thickness was considered, yielding an expected service life of 1.04 in the MNC medium and 2.56 years in the MAL medium. Despite the difference in materials, thickness, and corrosion rate, the estimated service lives of the two coatings are very similar. From these results the advantage of NiCoB/CS is clear, due to a better protection against corrosion being observed for lower thickness than for lower raw materials quantities used in their preparation and lower preparation costs.
3.5. Electrochemical Response of the Coating
The anodic behavior of the coating was evaluated from the polarization curves obtained in each one of the studied media. The shape of the curves indicates the oxidation behavior of the material, assumed as the corresponding corrosion behavior. A continuous increase in the current with the applied potential is associated with the oxidation of the material in soluble products. In contrast, if the current remains constant when the potential is increased it is assumed that the formation of insoluble products passivates the material. A passive material is not appropriate for ICCP applications. In Figure 4, the polarization curves of the NiCoB/CS coating (Figure 4a) and IrO2-Ta2O5/Ti (Figure 4b) in the MNC and MAL solutions are shown. From these curves, the corrosion behavior and the associated parameters were evaluated. Differences in corrosion potential for the NiCoB/CS coating in both media (Table 3) were observed and no passivation was evidenced. The corrosion potential of the IrO2-Ta2O5/Ti coating was very similar in both media (Table 3). However, the curve from the MAL medium exhibited a region with passive tendencies in the anodic part and an active region in the MNC medium.
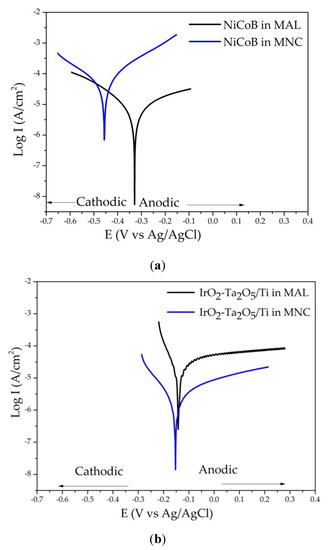
Figure 4.
Polarization curves in Ca(OH)2 and NaCl 3.5 wt% media for the (a) NiCoB/CS and (b) IrO2-Ta2O5/Ti coatings.

Table 3.
Corrosion potential values for the NiCoB/CS and IrO2-Ta2O5/Ti coatings.
3.6. Electrocatalytic Activity for the Oxygen (ȠO2) and Chlorine (ȠCl2) Evolution
Cyclic voltammetry curves were obtained at a scan rate of 50 mV/s from the open circuit potential in the cathodic direction for both media, as shown in Figure 5. For the NiCoB anode, oxygen and hydrogen evolution were observed as the anodic and cathodic reactions, indicating that the electrode and then its surface did not undergo any significant changes during the potential scan. Similar results were reported by Campillo [19]. For the IrO2-Ta2O5/Ti coating, electrochemical signals between 1.0 V and 1.2 V were only observed in the MNC medium (dot line). These signals were attributed to the formation of iridium oxides producing a passivation of the surface. The passive behavior was assigned to the oxidation of Ir4+/Ir6+ [28,29,30] and the oxide could be responsible for the passivation. From Figure 4b it is observed that on IrO2-Ta2O5/Ti coating, higher overpotentials were required for the oxygen and hydrogen evolution than on NiCoB/CS coating, indicating that the working potential interval of NiCoB was lower than for the IrO2-Ta2O5/Ti coating.
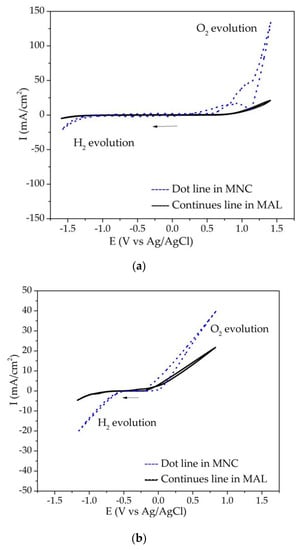
Figure 5.
Cyclic voltammetry at 50 mV/s from the Eoc of the (a) IrO2-Ta2O5/Ti y (b) NiCoB/CS coatings.
Concerning the applications of the studied samples in ICCP systems, it was necessary to evaluate their behavior for oxygen and chlorine evolution. Oxygen and chlorine evolution in electrocatalytic materials such as ruthenium oxide have been reported at 12.5 mA/cm2 and 0–2 mA/cm2, respectively (Trassati [31]). However, in the application of ICCP systems in concrete structures, the impressed current density is typically between 8–12 mA/m2 to control corrosion and 1–2 mA/m2 to prevent it all together [32]. A maximum current density of 800 mA/m2 is recommended for impressed current protection systems according to ISO/DIS 12696 standards [33] for concrete structures with anodes of mixed metal oxide (MMO) on a titanium substrate.
Based on the maximum value recommended by the ISO/DIS 12696 standards, the electrocatalytic activity of the coating was analyzed to evaluate the oxygen and chlorine evolution conditions. These results were compared to the reported values for IrO2-Ta2O5/Ti coatings.
From the linear scanning voltammetry curves (relative to Eoc) for both coatings (Figure 6), the electrocatalytic activity for chlorine (Figure 6a) and oxygen (Figure 6b) evolution was determined.
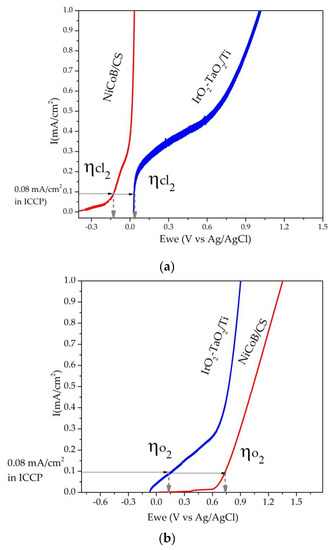
Figure 6.
Linear scanning voltammetry curves of the NiCoB/CS and IrO2-Ta2O5/Ti coatings for (a) chlorine and (b) oxygen evolution.
At the typical maximum current applied in ICCP systems 800 mA/m2 (0.08 mA/cm2) to reach the oxygen and chlorine evolution, the observed overpotentials were evaluated. The potential for chlorine evolution was -0.11 V versus Ag/AgCl for the NiCoB coating (Figure 6a) and 0.05 V versus Ag/AgCl for the IrO2-Ta2O5/Ti coating. This indicates a good electrocatalytic activity in the NiCoB/CS coating by achieving oxygen evolution at lower values of potential than that observed on the IrO2-Ta2O5/Ti coating. Oxygen evolution was carried out at 0.7 V versus Ag/AgCl in the NiCoB coating and 0.12 V versus Ag/AgCl in the IrO2-Ta2O5/Ti coating, and oxygen evolution was achieved at a higher potential than in IrO2-Ta2O5/Ti, as shown in Figure 6b. However, the electrocatalytic activity indicates that the NiCoB coating is a promising electrocatalytic material for application in ICCP systems for concrete structures. The IrO2-Ta2O5/Ti curves exhibited an increase in current at positive potentials, which could show a different mechanism involved during chlorine evolution. Song [30] found that this mechanism was related to the resistivity of iridium and tantalum oxides. Oxygen and chlorine evolution at further cathodic overpotential values indicated promising functionality as an electrocatalytic material and viability for application in cathodic protection systems.
3.7. Exchange Current Density (J0) and Tafel’s Slope (ba)
The exchange current density is indicative of the electrochemical kinetic behavior of the studied system. The exchange current density was calculated based the relationship J0 = RT/nFRct, where n = 2 (Ni/Ni2+) (Table 4). The exchange current density of NiCoB/CS coating was similar, as IrO2-Ta2O5/Ti, indicating that the electrochemical reactions occur at similar rates. This similarity to values reported in the literature supports the claim that NiCoB/CS is a good electrocatalytic material candidate for ICCP systems because of its similar electrocatalytic activity to IrO2-Ta2O5/Ti [34].

Table 4.
Exchange current density (J0) and Tafel’s slope of NiCoB/CS and IrO2-Ta2O5/Ti coatings.
The Tafel slope is often used to identify the mechanism involved in the electrochemical reaction observed as well as with the number of steps in this mechanism [35]. In this way, the values evaluated from this Tafel slope can provide further insight into the evolution reaction mechanism [36].
The Tafel slope values for the NiCoB/CS indicated that the coating was a good electrocatalytic material in an alkaline medium (Table 4). However, the electrocatalytic properties were lower in a medium with chloride ions than the IrO2-Ta2O5/Ti coating, which had a smaller Tafel slope, larger electrocatalytic activity, and faster kinetics. The Tafel slopes of the coating were related to the M + OH− → MOH + e− reaction, where a slope of 2RT/F was attributed to Tafel slopes of 120 mV/dec (Suen 2017). The differences in Tafel slope values were associated with variations in the evolution reaction mechanism in the different materials (IrO2-Ta2O5/Ti and NiCoB coatings).
4. Conclusions
The NiCoB/CS coating had similar properties to the IrO2-Ta2O5/Ti coating. Good electrocatalytic activity was observed, with overpotential values for oxygen and chlorine evolution of 0.7 V and −0.11 V, respectively. Furthermore, the coating had a high resistance to corrosion in alkaline conditions (0.015 ± 0.005 mm/year), an exchange current density in the range of 10−5–10−7 A/cm2, and Tafel slopes 117 and 147 mV/dec. The exchange current density values of the NiCoB/CS coating were within the same order of magnitude as the IrO2-Ta2O5/Ti coating, indicating that the oxygen and chlorine evolution kinetics of both materials were similar. However, the Tafel slope values suggested that the evolution mechanisms of the two materials were different. The NiCoB/CS coating’s evolution reaction mechanism exhibited good electrocatalytic activity with fast kinetics.
The high resistance to corrosion, low overpotential values, exchange current density, and Tafel slopes of NiCoB/CS coating indicate that the material is a promising electrocatalytic material for oxygen and chlorine evolution in ICCP systems. Furthermore, its application in steel structures within concrete would involve lower costs than an IrO2-Ta2O5 coating. It is recommended that the resistance to corrosion of the NiCoB/CS coating be further optimized to increase service life. For this purpose, preliminary treatments could be used during the deposition of the coating materials. The use of this coating and any further improvements offer the industry a substantial cost benefit.
Author Contributions
Writing and experimental analysis, A.L.M.; Validation of the corrosion analysis and chemical reactants contribution, J.T.P.Q. and M.R.B.; Electrochemical tests and analysis and interpretation of electrochemical results, R.O.-B., G.T. and Y.M.-V.; Validation analysis of the XRD information, J.M.S.L.; direction and administration of the project, M.M.M. and R.O.-B. All authors have read and agreed to the published version of the manuscript.
Funding
No specific funding was received was received nor granted for this project.
Acknowledgments
The first author is grateful to Consejo Nacional de Ciencia y Tecnología, (CONACyT) for the scholarship provided during the development of this project, as well as the Instituto Mexicano del Transporte (IMT) and the Centro de Investigación y Desarrollo en Electroquímica (CIDETEQ) for the help facilities provided in the use of their installations and equipment. Finally, the author also thanks Ing. Maura Arroyo Olvera for the support provided in the characterization of SEM and EDS samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. NACE Report International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; Gretchen Jacobson: Houston, TX, USA, 2016; p. 240. [Google Scholar]
- Rincon, I.T.; Carruyo, A.R.; Andrade, C.; Helene, P.R.; Dias, J. Manual de Inspeccion, Evaluacion y Diagnostico de Corrosion en Estructuras de Hormigon Armado; Editorial Venezolana: Venezuela, 1997; ISBN 980-296-541-3. [Google Scholar]
- Ávila, J.; Genescá, J. Más allá de la Herrumbre II. La Lucha Contra la Corrosión; FCE-Fondo de Cultura Económica: Mexico City, Mexico, 1995; ISBN 968-16-3153-6. [Google Scholar]
- Wilson, K.; Jawed, M.; Ngala, V. The selection and use of cathodic protection systems for the repair of reinforced concrete structures. Constr. Build. Mater. 2013, 39, 19–25. [Google Scholar] [CrossRef]
- Chess, P.M.; Broomfield, J.P. (Eds.) Cathodic Protection of Steel in Concrete; Routledge, T&F: London, UK, 2005; ISBN 0203223039. [Google Scholar]
- Vercesi, G.P.; Rolewicz, J.; Hinden, J.; Branch, G. Characterization of DSA-type oxygen evolving electrodes. Choice of base metal. Thermochim. Acta 1991, 176, 31–47. [Google Scholar] [CrossRef]
- Devilliers, D.; Groult, H.; Pouilleau, J.; Mahe, E. Electrochemical behaviour of platinum-coated Ta/Ta2O5 electrodes. Electrochim. Acta 1999, 44, 2307–2315. [Google Scholar]
- Yan, Z.; Li, G.; Wang, J.; Zhang, Z.; Feng, Z.; Tang, M.; Zhang, R. Electrocatalytic study of IrO2-Ta2O5 coated anodes with pretreated titanium substrates. J. Alloys Compd. 2016, 680, 60–66. [Google Scholar] [CrossRef]
- Xu, L.; Xin, Y.; Wang, J. A comparative study on IrO2-Ta2O5 coated titanium electrodes prepared with different methods. Electrochim. Acta 2009, 54, 1820–1825. [Google Scholar] [CrossRef]
- Huang, C.A.; Yang, S.W.; Chen, C.Z.; Hsu, F.Y. Electrochemical behavior of IrO2-Ta2O5/Ti anodes prepared with different surface pretreatments of Ti substrate. Surf. Coat. Technol. 2017, 320, 270–278. [Google Scholar] [CrossRef]
- Krýsa, J.; Kule, L.; Mráz, R.; Rousar, I. Effect of coating thickness and surface treatment of titanium on the properties of IrO2-Ta2O5 anodes. J. Appl. Electrochem. 1996, 26, 999–1005. [Google Scholar] [CrossRef]
- Morimitsu, M.; Otogawa, R.; Matsunaga, M. Effects of cathodizing on the morphology and composition. Electrochim. Acta 2000, 46, 401–406. [Google Scholar] [CrossRef]
- Yi, Z.; Kangning, C.; Wei, W.; Wang, J.; Lee, S. Effect of IrO2 loading on RuO2-IrO2-TiO2 anodes: A study of microstructure and working life for the chlorine evolution reaction. Ceram. Int. 2007, 33, 1087–1091. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, Y.; Zhang, Z.; Li, G.; Li, H.; Wang, J.; Feng, Z.; Tang, M.; Yuan, X.; Zhang, R.; et al. A study on the performance of IrO2-Ta2O5 coated anodes with surface treated Ti substrates. Electrochim. Acta 2015, 157, 345–350. [Google Scholar] [CrossRef]
- Trasatti, S.; Buzzanca, G. Ruthenium dioxide: A new interesting electrode material. Solid state structure and electrochemical behaviour. J. Electroanal. Chem. 1971, 29, 4–8. [Google Scholar] [CrossRef]
- Bocca, C.; Barbucci, A.; Delucchi, M.; Cerisola, G. Nickel-Cobalt oxide-coated electrodes: Influence of the preparation technique on oxygen evolution reaction (OER) in an alkaline solution. Int. J. Hydrogen Energy 1999, 24, 21–26. [Google Scholar] [CrossRef]
- Panić, V.V.; Nikolić, B.Ž. Sol-gel prepared active ternary oxide coating on titanium in cathodic protection. J. Serb. Chem. Soc. 2007, 72, 1393–1402. [Google Scholar] [CrossRef]
- Li, X.; Walsh, F.C.; Pletcher, D. Nickel based electrocatalysts for oxygen evolution in high current density, alkaline water electrolysers. Phys. Chem. Chem. Phys. 2011, 13, 1162–1167. [Google Scholar] [CrossRef]
- Campillo, B.; Sebastian, P.J.; Gamboa, S.A.; Albarran, J.L.; Caballero, L.X. Electrodeposited Ni-Co-B alloy: Application in water electrolysis. Mater. Sci. Eng. C 2002, 19, 115–118. [Google Scholar] [CrossRef]
- Rodriguez, C.; Campillo, B.; Albarran, J.L.; Genesca, J.; Caballero, L.X.; Perez, R. Corrosion behavior of electrolytic NiCoB coatings. Corros. Rev. 1999, 17, 137–149. [Google Scholar] [CrossRef]
- Gamboa, S.A.; Gonzalez-Rodriguez, J.G.; Valenzuela, E.; Campillo, B.; Sebastian, P.J.; Reyes-Rojas, A. Evaluation of the corrosion resistance of Ni-Co-B coatings in simulated PEMFC environment. Electrochim. Acta 2006, 51, 4045–4051. [Google Scholar] [CrossRef]
- Astm, G. Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Mixed Metal Oxide Anodes for Cathodic Protection Applications. Available online: www.telprocompanies.com › uploads › telpro_data_sheets_2014 (accessed on 25 February 2020).
- Jacob, W.R. Impressed current Anodes. Electrochem. Prot. 2010, 4.20, 2781–2800. [Google Scholar]
- Lee, J.-Y.; Kang, D.-K.; Lee, K.; Chang, D. An Investigation on the Electrochemical Characteristics of Ta2O5-IrO2 Anodes for the Application of Electrolysis Process. Mater. Sci. Appl. 2011, 2, 237–243. [Google Scholar]
- Comninellis, C.; Vercesi, G.P. Characterization of DSA-Type electrodes: Choice of a coating. J. Appl. Electrochem. 1991, 21, 335–345. [Google Scholar] [CrossRef]
- Bekish, Y.N.; Poznyak, S.K.; Tsybulskaya, L.S.; Gaevskaya, T.V.; Kukareko, V.A.; Mazanik, A.V. Electrodeposited Ni-Co-B Alloy Coatings: Preparation and Properties. J. Electrochem. Soc. 2014, 161, D620–D627. [Google Scholar] [CrossRef]
- Ren, Z.; Quan, S.; Gao, J.; Li, W.; Zhu, Y.; Liu, Y.; Chai, B.; Wang, Y. The electrocatalytic activity of IrO2-Ta2O5 anode materials and electrolyzed oxidizing water preparation and sterilization effect. RSC Adv. 2015, 5, 8778–8786. [Google Scholar] [CrossRef]
- Mattos-Costa, F.I.; de Lima-Neto, P.; Machado, S.A.S.; Avaca, L.A. Characterisation of surfaces modified by sol-gel derived RuxIr1−xO2 coatings for oxygen evolution in acid medium.pdf. Electrochim. Acta 1998, 44, 1515–1523. [Google Scholar] [CrossRef]
- Li, B.S.; Lin, A.; Gan, F.X. Preparation and electrocatalytic properties of Ti/IrO2-Ta2O5 anodes for oxygen evolution. Trans. Nonferr. Met. Soc. China (Engl. Ed.) 2006, 16, 1193–1199. [Google Scholar] [CrossRef]
- Trasatti, S. Electrocatalysis in the Anodic Evolution Oxygen and Chlorine. Electrochim. Acta 1984, 29, 1503–1512. [Google Scholar] [CrossRef]
- Carmona, J.; Garcés, P.; Climent, M.A. Efficiency of a conductive cement-based anodic system for the application of cathodic protection, cathodic prevention and electrochemical chloride extraction to control corrosion in reinforced concrete structures. Corros. Sci. 2015, 96, 102–111. [Google Scholar] [CrossRef]
- Draft International Standard ISO/DIS 12696 Cathodic protection of steel in concrete, CEN Parallel Processing. In CEN Parallel Processing; International Organization for Standardization: Geneva, Switzerland, 2009.
- Borja-Arco, E.; Sandoval, O.J.; Escalante-García, J.; Sandoval-González, A.; Sebastian, P.J. Microwave assisted synthesis of ruthenium electrocatalysts for oxygen reduction reaction in the presence and absence of aqueous methanol. Int. J. Hydrogen Energy 2011, 36, 103–110. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, N.; Fernandes, R.; Kadrekar, R.; Dashora, A.; Yadav, A.K.; Bhattacharyya, D.; Jha, S.N.; Miotello, A.; Kothari, D.C. Co-Ni-B nanocatalyst for efficient hydrogen evolution reaction in wide pH range. Appl. Catal. B Environ. 2016, 192, 126–133. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).