A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics
Abstract
1. Introduction
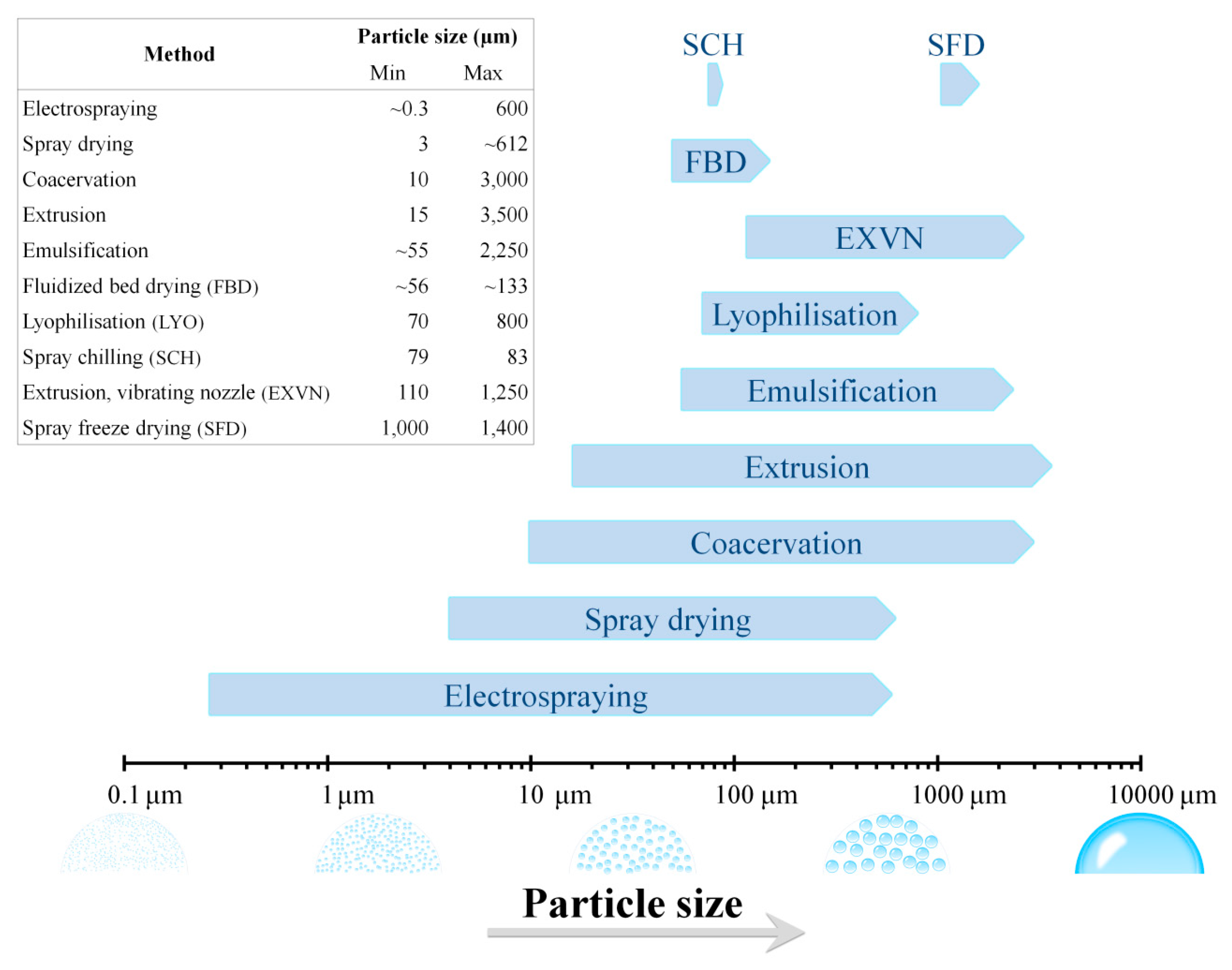
2. Methods for Microencapsulation of Probiotics
3. Edible Coating Materials
4. Proteins
5. Polysaccharides
5.1. Anionic Polysaccharides
5.2. Cationic Polysaccharides
5.3. Non-ionic Polysaccharides
5.4. Amphoteric Polysaccharides
6. Lipids
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO/WHO. Probiotics in Food. In Health and Nutritional Properties and Guidelines for Evaluation; FAO/WHO: Rome, Italy, 2006; p. 56. [Google Scholar]
- Dunne, C. Adaptation of bacteria to the intestinal niche: Probiotics and gut disorder. Inflamm. Bowel Dis. 2001, 7, 136–145. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Liu, X.; Wang, B.; Cao, H. Saccharomyces Boulardii, a Yeast Probiotic, Regulates Serotonin Transporter in the Intestine. Gastroenterology 2019, 156, 26. [Google Scholar] [CrossRef]
- Gu, Y.; Zhou, G.; Qin, X.; Huang, S.; Wang, B.; Cao, H. The Potential Role of Gut Mycobiome in Irritable Bowel Syndrome. Front. Microbiol. 2019, 10, 1894. [Google Scholar] [CrossRef]
- Cayzeele-Decherf, A.; Pélerin, F.; Leuillet, S.; Douillard, B.; Housez, B.; Cazaubiel, M.; Jacobson, G.K.; Jüsten, P.; Desreumaux, P. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: An individual subject meta-analysis. World J. Gastroenterol. 2017, 23, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, M.L.; Das, N. Optimization of exopolysaccharide production by probiotic yeast Lipomyces starkeyi VIT-MN03 using response surface methodology and its applications. Ann. Microbiol. 2019, 69, 515–530. [Google Scholar] [CrossRef]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Jacouton, E.; Michel, M.-L.; Torres-Maravilla, E.; Chain, F.; Langella, P.; Humaran, L.G.B. Elucidating the Immune-Related Mechanisms by Which Probiotic Strain Lactobacillus casei BL23 Displays Anti-tumoral Properties. Front. Microbiol. 2019, 9, 9. [Google Scholar] [CrossRef]
- Lebeer, S.; A Bron, P.; Marco, M.L.; Van Pijkeren, J.-P.; Motherway, M.O.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of Probiotic Lactobacilli in Acidic Environments Is Enhanced in the Presence of Metabolizable Sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef]
- Tripathi, M.; Giri, S. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- McClements, D.J. Requirements for food ingredient and nutraceutical delivery systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Garti, N., McClements, D.J., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 3–18. [Google Scholar] [CrossRef]
- Monnard, P.-A.; Oberholzer, T.; Luisi, P. Entrapment of nucleic acids in liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 1997, 1329, 39–50. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival during Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Pavli, F.; Tassou, C.; Nychas, G.-J.E.; Chorianopoulos, N. Probiotic Incorporation in Edible Films and Coatings: Bioactive Solution for Functional Foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Yang, W.; Wang, L.; Ban, Z.; Yan, J.; Lu, H.; Zhang, X.; Wu, Q.; Aghdam, M.S.; Luo, Z.; Li, L. Efficient microencapsulation of Syringa essential oil; the valuable potential on quality maintenance and storage behavior of peach. Food Hydrocoll. 2019, 95, 177–185. [Google Scholar] [CrossRef]
- Ayoub, A.; Sood, M.; Singh, J.; Bandral, J.; Gupta, N.; Bhat, A. Microencapsulation and its applications in food industry. J. Pharmacogn. Phytochem. 2019, 8, 32–37. [Google Scholar]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.; Olivas, G.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Krochta, J. Edible Packaging Materials. Annu. Rev. Food Sci. Technol. 2010, 1, 415–448. [Google Scholar] [CrossRef] [PubMed]
- Fazilah, N.F.; Hamidon, N.H.; Bin Ariff, A.; Khayat, M.E.; Wasoh, H.; Halim, M. Microencapsulation of Lactococcus lactis Gh1 with Gum Arabic and Synsepalum dulcificum via Spray Drying for Potential Inclusion in Functional Yogurt. Molecules 2019, 24, 1422. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.A.A.; De Almeida, C.A.M.; Mattarugo, N.M.D.S.; Ferri, E.A.V.; Bittencourt, P.R.S.; Colla, E.; Drunkler, D.A. Soy extract and maltodextrin as microencapsulating agents for Lactobacillus acidophilus: A model approach. J. Microencapsul. 2018, 35, 705–719. [Google Scholar] [CrossRef]
- Silva, M.; Tulini, F.L.; Matos, F.E.; Oliveira, M.G.; Thomazini, M.; Favaro-Trindade, C.S. Application of spray chilling and electrostatic interaction to produce lipid microparticles loaded with probiotics as an alternative to improve resistance under stress conditions. Food Hydrocoll. 2018, 83, 109–117. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Semyonov, D.; Ramon, O.; Kaplun, Z.; Levin-Brener, L.; Gurevich, N.; Shimoni, E. Microencapsulation of Lactobacillus paracasei by spray freeze drying. Food Res. Int. 2010, 43, 193–202. [Google Scholar] [CrossRef]
- Dolly, P.; Anishaparvin, A.; Joseph, G.S.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (mtcc 5422) by spray-freeze-drying method and evaluation of survival in simulated gastrointestinal conditions. J. Microencapsul. 2011, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Boonanuntanasarn, S.; Ditthab, K.; Jangprai, A.; Nakharuthai, C. Effects of Microencapsulated Saccharomyces cerevisiae on Growth, Hematological Indices, Blood Chemical, and Immune Parameters and Intestinal Morphology in Striped Catfish, Pangasianodon hypophthalmus. Probiotics Antimicrob. Proteins 2018, 11, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Kumar, S.B.; Prabhasankar, P.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum MTCC 5422 in fructooligosaccharide and whey protein wall systems and its impact on noodle quality. J. Food Sci. Technol. 2014, 52, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- Coghetto, C.C.; Brinques, G.B.; Siqueira, N.; Pletsch, J.; Soares, R.M.; Ayub, M.A.Z. Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. J. Funct. Foods 2016, 24, 316–326. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Morfin, R.C.; Pérez-Masiá, R.; Sánchez, G.; López-Rubio, A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT 2016, 69, 438–446. [Google Scholar] [CrossRef]
- Anselmo, A.C.; McHugh, K.J.; Webster, J.; Langer, R.; Jaklenec, A. Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv. Mater. 2016, 28, 9486–9490. [Google Scholar] [CrossRef]
- Priya, A.J.; Vijayalakshmi, S.P.; Raichur, A. Enhanced Survival of Probiotic Lactobacillus acidophilusby Encapsulation with Nanostructured Polyelectrolyte Layers through Layer-by-Layer Approach. J. Agric. Food Chem. 2011, 59, 11838–11845. [Google Scholar] [CrossRef]
- Penhasi, A. Microencapsulation of probiotic bacteria using thermo-sensitive sol-gel polymers for powdered infant formula. J. Microencapsul. 2015, 32, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus acidophilus in moist-heat-resistant multilayered microcapsules. J. Food Eng. 2017, 192, 11–18. [Google Scholar] [CrossRef]
- Deshpande, H.; Kharat, V.; Katke, S. Studies on Process Standardization and Sensory Evaluation of Probiotic Chocolate. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1527–1534. [Google Scholar] [CrossRef]
- Lee, S.; Kirkland, R.; Grunewald, Z.I.; Sun, Q.; Wicker, L.; De La Serre, C.B. Beneficial Effects of Non-Encapsulated or Encapsulated Probiotic Supplementation on Microbiota Composition, Intestinal Barrier Functions, Inflammatory Profiles, and Glucose Tolerance in High Fat Fed Rats. Nutrients 2019, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- De Prisco, A.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by vibrating technology of the probiotic strain Lactobacillus reuteri DSM 17938 to enhance its survival in foods and in gastrointestinal environment. LWT 2015, 61, 452–462. [Google Scholar] [CrossRef]
- Pop, O.; Brandau, T.; Schwinn, J.; Vodnar, D.C.; Socaciu, C. The influence of different polymers on viability of Bifidobacterium lactis 300b during encapsulation, freeze-drying and storage. J. Food Sci. Technol. 2014, 52, 4146–4155. [Google Scholar] [CrossRef]
- Setijawati, D.; Nursyam, H.; Salis, H. Carrageenan: The difference between PNG and KCL gel precipitation method as Lactobacillus acidophilus encapsulation material. IOP Conf. Ser. Earth Environ. Sci. 2018, 137, 12073. [Google Scholar] [CrossRef]
- Ding, W.; Shah, N. Effect of Various Encapsulating Materials on the Stability of Probiotic Bacteria. J. Food Sci. 2009, 74, M100–M107. [Google Scholar] [CrossRef]
- Bosnea, L.; Moschakis, T.; Nigam, P.; Biliaderis, C. Growth adaptation of probiotics in biopolymer-based coacervate structures to enhance cell viability. LWT 2017, 77, 282–289. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Barin, J.S.; Lopes, E.J.; Cichoski, A.J.; Flores, E.M.D.M.; Silva, C.D.B.D.; De Menezes, C.R. Development, characterization and viability study of probiotic microcapsules produced by complex coacervation followed by freeze-drying. Ciência Rural 2019, 49, 49. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Spray drying, freeze drying and related processes for food ingredient and nutraceutical encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 73–109. [Google Scholar]
- Oxley, J. Spray cooling and spray chilling for food ingredient and nutraceutical encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 110–130. [Google Scholar]
- Ali, M.E.; Lamprecht, A. Spray freeze drying as an alternative technique for lyophilization of polymeric and lipid-based nanoparticles. Int. J. Pharm. 2017, 516, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and methods of microencapsulation of probiotic microorganisms. Iran. J. Biotechnol. 2007, 5, 1–18. [Google Scholar]
- Whelehan, M.; Marison, I.W. Microencapsulation using vibrating technology. J. Microencapsul. 2011, 28, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L. On the capillary phenomena of jets. Proc. R. Soc. Lond. 1879, 29, 71–97. [Google Scholar]
- Olivares, A.; Silva, P.; Altamirano, C. Microencapsulation of probiotics by efficient vibration technology. J. Microencapsul. 2017, 34, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, S.; Tosi, A.; Balestra, C.; Nastruzzi, C.; Luca, G.; Mancuso, F.; Calafiore, R.; Calvitti, M. Production and Characterization of Alginate Microcapsules Produced by a Vibrational Encapsulation Device. J. Biomater. Appl. 2008, 23, 123–145. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Li, D.-T.; Xu, M.; Chen, H.-Y.; Zhang, Z.; Tang, Z.-X. Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yoghurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Garti, N.; Aserin, A. Micelles and microemulsions as food ingredient and nutraceutical delivery systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 211–251. [Google Scholar]
- Singh, M.; Hemant, K.; Ram, M.; Shivakumar, H. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar]
- Jaworek, A. Electrohydrodynamic microencapsulation technology. In Encapsulations; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 1–45. [Google Scholar]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Aghdam, M.S.; Li, L. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Boil. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Zhong, W.; Li, B.; Mequanint, K.; Luo, G.; Xing, M. Biomedical Applications of Layer-by-Layer Self-Assembly for Cell Encapsulation: Current Status and Future Perspectives. Adv. Heal. Mater. 2018, 8, 1800939. [Google Scholar] [CrossRef] [PubMed]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Complex Coacervation as a Novel Microencapsulation Technique to Improve Viability of Probiotics under Different Stresses. Food Bioprocess Technol. 2014, 7, 2767–2781. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M.; Maghsoudlou, Y.; Ghorbani, M. The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Res. Int. 2017, 96, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Holkem, A.; Raddatz, G.C.; Barin, J.S.; Flores, E.M.M.; Müller, E.; Codevilla, C.F.; Jacob-Lopes, E.; Grosso, C.R.F.; De Menezes, C.R. Production of microcapsules containing Bifidobacterium BB-12 by emulsification/internal gelation. LWT 2017, 76, 216–221. [Google Scholar] [CrossRef]
- Mandal, S.; Hati, S.; Puniya, A.K.; Khamrui, K.; Singh, K. Enhancement of survival of alginate-encapsulated Lactobacillus casei NCDC 298. J. Sci. Food Agric. 2014, 94, 1994–2001. [Google Scholar] [CrossRef]
- Mandal, S.; Puniya, A.; Singh, K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int. Dairy J. 2006, 16, 1190–1195. [Google Scholar] [CrossRef]
- Gaudreau, H.; Champagne, C.P.; Remondetto, G.E.; Gomaa, A.; Subirade, M. Co-encapsulation of Lactobacillus helveticus cells and green tea extract: Influence on cell survival in simulated gastrointestinal conditions. J. Funct. Foods 2016, 26, 451–459. [Google Scholar] [CrossRef]
- Nag, A.; Han, K.-S.; Singh, H. Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int. Dairy J. 2011, 21, 247–253. [Google Scholar] [CrossRef]
- Heidebach, T.; Foerst, P.; Kulozik, U. Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic cells. Int. Dairy J. 2009, 19, 77–84. [Google Scholar] [CrossRef]
- Heidebach, T.; Foerst, P.; Kulozik, U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J. Food Eng. 2010, 98, 309–316. [Google Scholar] [CrossRef]
- Paula, D.D.A.; Martins, E.M.F.; Costa, N.D.A.; De Oliveira, P.M.; De Oliveira, E.B.; Ramos, A.M. Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. Int. J. Boil. Macromol. 2019, 133, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.C.E.; Chaves, K.S.; Gebara, C.; Infante, F.N.; Grosso, C.R.; Gigante, M.L. Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res. Int. 2014, 66, 424–431. [Google Scholar] [CrossRef]
- Zer, B.; Uzun, Y.S.; Kirmaci, H.A.; Ozer, B. Effect of Microencapsulation on Viability of Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-12 During Kasar Cheese Ripening. Int. J. Dairy Technol. 2008, 61, 237–244. [Google Scholar] [CrossRef]
- Ozer, B.; Kirmaci, H.A.; Şenel, E.; Atamer, M.; Hayaloglu, A. Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white-brined cheese by microencapsulation. Int. Dairy J. 2009, 19, 22–29. [Google Scholar] [CrossRef]
- Valero-Cases, E.; Frutos, M.J.; Fernandez, M.J.F. Effect of different types of encapsulation on the survival of Lactobacillus plantarum during storage with inulin and in vitro digestion. LWT 2015, 64, 824–828. [Google Scholar] [CrossRef]
- Samedi, L.; Charles, A.L. Viability of 4 Probiotic Bacteria Microencapsulated with Arrowroot Starch in the Simulated Gastrointestinal Tract (GIT) and Yoghurt. Foods 2019, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, X.; Sun, Z.W.; Park, H.J.; Cha, D.-S. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr. Polym. 2011, 83, 1479–1485. [Google Scholar] [CrossRef]
- Albertini, B.; Vitali, B.; Passerini, N.; Cruciani, F.; Di Sabatino, M.; Rodriguez, L.; Brigidi, P. Development of microparticulate systems for intestinal delivery of Lactobacillus acidophilus and Bifidobacterium lactis. Eur. J. Pharm. Sci. 2010, 40, 359–366. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Qaziyani, S.D.; Pourfarzad, A.; Gheibi, S.; Nasiraie, L.R. Effect of encapsulation and wall material on the probiotic survival and physicochemical properties of synbiotic chewing gum: Study with univariate and multivariate analyses. Heliyon 2019, 5, e02144. [Google Scholar] [CrossRef]
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Ben Salah, R. Encapsulation in alginate and alginate coated-chitosan improved the survival of newly probiotic in oxgall and gastric juice. Int. J. Boil. Macromol. 2013, 61, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nualkaekul, S.; Lenton, D.; Cook, M.; Khutoryanskiy, V.V.; Charalampopoulos, D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012, 90, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Ying-Zi, J.; Huan-Xin, W.; Peng, H.; Xiao-Yan, C. The Application and Immobilization of Bifidobacterium with Carboxymethyl Chitin and Calcium Alginate (Original article in Chinese). Sci. Technol. Eng. 2018, 18, 37–43. [Google Scholar]
- Iyer, C.; Phillips, M.; Kailasapathy, K. Release studies of Lactobacillus casei strain Shirota from chitosan-coated alginate-starch microcapsules in ex vivo porcine gastrointestinal contents. Lett. Appl. Microbiol. 2005, 41, 493–497. [Google Scholar] [CrossRef] [PubMed]
- And, C.I.; Kailasapathy, K. Effect of Co-encapsulation of Probiotics with Prebiotics on Increasing the Viability of Encapsulated Bacteria under In Vitro Acidic and Bile Salt Conditions and in Yogurt. J. Food Sci. 2005, 70, M18–M23. [Google Scholar] [CrossRef]
- Mirzaei, H.; Pourjafar, H.; Homayouni, A. Effect of calcium alginate and resistant starch microencapsulation on the survival rate of Lactobacillus acidophilus La5 and sensory properties in Iranian white brined cheese. Food Chem. 2012, 132, 1966–1970. [Google Scholar] [CrossRef]
- Praepanitchai, O.-A.; Noomhorm, A.; Anal, A.K. Survival and Behavior of Encapsulated Probiotics (Lactobacillus plantarum) in Calcium-Alginate-Soy Protein Isolate-Based Hydrogel Beads in Different Processing Conditions (pH and Temperature) and in Pasteurized Mango Juice. BioMed Res. Int. 2019, 2019, 9768152–9768158. [Google Scholar] [CrossRef]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Boil. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef]
- Shu, G.; He, Y.; Chen, L.; Song, Y.; Cao, J.; Chen, H. Effect of Xanthan⁻Chitosan Microencapsulation on the Survival of Lactobacillus acidophilus in Simulated Gastrointestinal Fluid and Dairy Beverage. Polymers 2018, 10, 588. [Google Scholar] [CrossRef]
- McMaster, L.D.; Kokott, S.A. Micro-encapsulation of Bifidobacterium lactis for incorporation into soft foods. World J. Microbiol. Biotechnol. 2005, 21, 723–728. [Google Scholar] [CrossRef]
- Ameeta, S.; Thompkinson, D.K.; Kumar, M.H.; Latha, S. Prebiotics in the microencapsulating matrix enhance the viability of probiotic Lactobacillus acidophilus LA1. Int. J. Fermented Foods 2013, 2, 12. [Google Scholar]
- Gbassi, G.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins☆. Int. J. Food Microbiol. 2009, 129, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Chitosan coated alginate–xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Boil. Macromol. 2015, 72, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Chavarri, M.; Maranon, I.; Ares, R.; Ibanez, F.C.; Marzo, F.; Villarán, M.D.C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Etchepare, M.D.A.; Raddatz, G.C.; Flores, E.M.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Grosso, C.R.F.; De Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT 2016, 65, 511–517. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Das, N. Process optimization for microencapsulation of probiotic yeasts. Front. Boil. 2018, 13, 197–207. [Google Scholar] [CrossRef]
- Falco, C.Y.; Amadei, F.; Dhayal, S.K.; Cárdenas, M.; Tanaka, M.; Risbo, J. Hybrid coating of alginate microbeads based on protein-biopolymer multilayers for encapsulation of probiotics. Biotechnol. Prog. 2019, 35, e2806. [Google Scholar] [CrossRef]
- Yeung, T.W.; Üçok, E.F.; Tiani, K.A.; McClements, D.J.; Sela, D.A. Microencapsulation in Alginate and Chitosan Microgels to Enhance Viability of Bifidobacterium longum for Oral Delivery. Front. Microbiol. 2016, 7, 1946. [Google Scholar] [CrossRef]
- Hébrard, G.; Hoffart, V.; Beyssac, E.; Cardot, J.-M.; Alric, M.; Subirade, M. Coated whey protein/alginate microparticles as oral controlled delivery systems for probiotic yeast. J. Microencapsul. 2010, 27, 292–302. [Google Scholar] [CrossRef]
- Doherty, S.; Gee, V.; Ross, R.P.; Stanton, C.; Fitzgerald, G.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.; Anandharamakrishnan, C. Effect of whey protein – alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Malmo, C.; La Storia, A.; Mauriello, G. Microencapsulation of Lactobacillus reuteri DSM 17938 Cells Coated in Alginate Beads with Chitosan by Spray Drying to Use as a Probiotic Cell in a Chocolate Soufflé. Food Bioprocess Technol. 2011, 6, 795–805. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. J. Microencapsul. 2002, 19, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Flores-Belmont, I.A.; Palou, E.; López-Malo, A.; Jiménez-Munguía, M.-T. Simple and double microencapsulation of Lactobacillus acidophilus with chitosan using spray drying. Int. J. Food Stud. 2015, 4, 188–200. [Google Scholar] [CrossRef]
- O’Riordan, K.; Andrews, D.; Buckle, K.; Conway, P. Evaluation of microencapsulation of a Bifidobacterium strain with starch as an approach to prolonging viability during storage. J. Appl. Microbiol. 2001, 91, 1059–1066. [Google Scholar] [CrossRef]
- Pinto, S.; Verruck, S.; Vieira, C.R.; Prudencio, E.S.; Amante, E.R.; Amboni, R.D.D.M.C. Influence of microencapsulation with sweet whey and prebiotics on the survival of Bifidobacterium-BB-12 under simulated gastrointestinal conditions and heat treatments. LWT 2015, 64, 1004–1009. [Google Scholar] [CrossRef]
- Ying, D.; Sanguansri, L.; Weerakkody, R.; Bull, M.; Singh, T.; Augustin, M.A. Effect of encapsulant matrix on stability of microencapsulated probiotics. J. Funct. Foods 2016, 25, 447–458. [Google Scholar] [CrossRef]
- Zou, Q.; Liu, X.; Zhao, J.; Tian, F.; Zhang, H.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium bifidum F-35 in Whey Protein-Based Microcapsules by Transglutaminase-Induced Gelation. J. Food Sci. 2012, 77, 270. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Spray freeze drying method for microencapsulation of Lactobacillus plantarum. J. Food Eng. 2015, 166, 95–103. [Google Scholar] [CrossRef]
- Ömür, Ş.; Ocak, B. Development and Characterization of Collagen Hydrolysate Films Incorporated with Melaleuca Alternifolia (Tea Tree) Essential Oil. In Proceedings of the International Agriculture, Environment and Health Congress, Aydin, Turkey, 26–28 October 2018. [Google Scholar]
- Park, H.J.; Chinnan, M.S. Gas and water vapor barrier properties of edible films from protein and cellulosic materials. J. Food Eng. 1995, 25, 497–507. [Google Scholar] [CrossRef]
- Ryu, S.; Rhim, J.; Roh, H.; Kim, S. Preparation and Physical Properties of Zein-Coated High-Amylose Corn Starch Film. LWT 2002, 35, 680–686. [Google Scholar] [CrossRef]
- Handa, A.; Gennadios, A.; Hanna, M.; Weller, C.; Kuroda, N. Physical and Molecular Properties of Egg-white Lipid Films. J. Food Sci. 1999, 64, 860–864. [Google Scholar] [CrossRef]
- Ket-On, A.; Pongmongkol, N.; Somwangthanaroj, A.; Janjarasskul, T.; Tananuwong, K. Properties and storage stability of whey protein edible film with spice powders. J. Food Sci. Technol. 2016, 53, 2933–2942. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R.; Krochta, J. Water Vapor Permeability and Solubility of Films from Hydrolyzed Whey Protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.; Olsen, C.; Olson, D.; Chiou, B.; Yee, E.; Bechtel, P.; McHugh, T. Water Vapor Permeability of Mammalian and Fish Gelatin Films. J. Food Sci. 2006, 71, 202. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.; Krochta, J. Water Vapor Permeability of Caseinate-Based Edible Films as Affected by pH, Calcium Crosslinking and Lipid Content. J. Food Sci. 1993, 58, 904–907. [Google Scholar] [CrossRef]
- Khwaldia, K. Water Vapor Barrier and Mechanical Properties of Paper-Sodium Caseinate and Paper-Sodium Caseinate-Paraffin Wax Films. J. Food Biochem. 2010, 34, 998–1013. [Google Scholar] [CrossRef]
- Banerjee, R.; Chen, H. Functional Properties of Edible Films Using Whey Protein Concentrate. J. Dairy Sci. 1995, 78, 1673–1683. [Google Scholar] [CrossRef]
- Gennadios, A.; Brandenburg, A.H.; Park, J.W.; Weller, C.L.; Testin, R.F. Water vapor permeability of wheat gluten and soy protein isolate films. Ind. Crop. Prod. 1994, 2, 189–195. [Google Scholar] [CrossRef]
- Cho, S.Y.; Rhee, C. Mechanical properties and water vapor permeability of edible films made from fractionated soy proteins with ultrafiltration. LWT 2004, 37, 833–839. [Google Scholar] [CrossRef]
- Cho, S.Y.; Park, J.-W.; Batt, H.P.; Thomas, R.L. Edible films made from membrane processed soy protein concentrates. LWT 2007, 40, 418–423. [Google Scholar] [CrossRef]
- Anker, M.; Berntsen, J.; Hermansson, A.-M.; Stading, M. Improved water vapor barrier of whey protein films by addition of an acetylated monoglyceride. Innov. Food Sci. Emerg. Technol. 2002, 3, 81–92. [Google Scholar] [CrossRef]
- Mauer, L.; Smith, D.; Labuza, T. Water vapor permeability, mechanical, and structural properties of edible β-casein films. Int. Dairy J. 2000, 10, 353–358. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent Advances in Edible Coatings for Fresh and Minimally Processed Fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Rosa, M.D.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Mater. 2019, 12, 2454. [Google Scholar] [CrossRef]
- Rankin, J.; Wolff, I.; Davis, H.; Rist, C. Permeability of Amylose Film to Moisture Vapor, Selected Organic Vapors, and the Common Gases. Ind. Eng. Chem. Chem. Eng. Data Ser. 1958, 3, 120–123. [Google Scholar] [CrossRef]
- Nogueira, G.; Fakhouri, F.; Velasco, J.I.; De Oliveira, R.A. Active Edible Films Based on Arrowroot Starch with Microparticles of Blackberry Pulp Obtained by Freeze-Drying for Food Packaging. Polymers 2019, 11, 1382. [Google Scholar] [CrossRef]
- Razzaq, H.A.; Pezzuto, M.; Santagata, G.; Silvestre, C.; Cimmino, S.; Larsen, N.; Duraccio, D. Barley β-glucan-protein based bioplastic film with enhanced physicochemical properties for packaging. Food Hydrocoll. 2016, 58, 276–283. [Google Scholar] [CrossRef]
- Dashipour, A.; Razavilar, V.; Hosseini, H.; Alibadi, S.S.; German, J.B.; Ghanati, K.; Khakpour, M.; Khaksar, R. Antioxidant and antimicrobial carboxymethyl cellulose films containing Zataria multiflora essential oil. Int. J. Boil. Macromol. 2015, 72, 606–613. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.; Xia, W. Preparation and characterization of pullulan–chitosan and pullulan–carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Fakhouri, F.; Tanada-Palmu, P.S.; Grosso, C.R. Characterization of composite biofilms of wheat gluten and cellulose acetate phthalate. Braz. J. Chem. Eng. 2004, 21, 261–264. [Google Scholar] [CrossRef]
- Plotto, A. Coatings for Fresh Fruits and Vegetables; Informa UK Limited: London, UK, 2011; pp. 185–242. [Google Scholar]
- Yang, L.; Paulson, A. Mechanical and water vapour barrier properties of edible gellan films. Food Res. Int. 2000, 33, 563–570. [Google Scholar] [CrossRef]
- Bifani, V.; Ramírez, C.; Ihl, M.; Rubilar, M.; García, A.; Zaritzky, N. Effects of murta (Ugni molinae Turcz) extract on gas and water vapor permeability of carboxymethylcellulose-based edible films. LWT 2007, 40, 1473–1481. [Google Scholar] [CrossRef]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Film-forming properties of guar gum, tara gum and locust bean gum. Food Hydrocoll. 2020, 98, 105007. [Google Scholar] [CrossRef]
- Bertuzzi, M.; Vidaurre, E.C.; Armada, M.; Gottifredi, J. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Park, H.; Weller, C.; Vergano, P.; Testin, R. Permeability and Mechanical Properties of Cellulose-Based Edible Films. J. Food Sci. 1993, 58, 1361–1364. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Shaw, P.E. Moisture permeability of edible films made with fatty acid and hydroxypropyl methyl cellulose. J. Agric. Food Chem. 1990, 38, 1799–1803. [Google Scholar] [CrossRef]
- Grandtner, G.; Cavrot, J.-P.; Bandur, G.; Rusnac, L.; Krausz, P.; Granet, R.; Joly, N.; Martin, P. Synthesis of fructooligosaccharide-based plastic films starting from inulin. e-Polymers 2005, 5, 1–7. [Google Scholar] [CrossRef]
- Hanlon, J.F. Institute of Packaging Professionals. Handbook of Package Engineering, 2nd ed.; Technomic Pub. Co.: Lancaster, PA, USA, 1992. [Google Scholar]
- Harnkarnsujarit, N.; Li, Y. Structure-property modification of microcrystalline cellulose film using agar and propylene glycol alginate. J. Appl. Polym. Sci. 2017, 134, 45533. [Google Scholar] [CrossRef]
- Zarabadi, M.H.; Kadivar, M.; Keramat, J. Production and evaluation the properties of laminated oat protein film and electrospun nylon. J. Food Process. Preserv. 2017, 42, e13513. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Boil. Macromol. 2019, 143, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Hettiarachchy, N.; Coleman, M. Mechanical and Barrier Properties of Rice Bran Films. J. Food Sci. 1997, 62, 395–398. [Google Scholar] [CrossRef]
- Biduski, B.; Da Silva, F.T.; Silva, W.M.F.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Zavareze, E. Impact of acid and oxidative modifications, single or dual, of sorghum starch on biodegradable films. Food Chem. 2017, 214, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; De La Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioprocess Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Coimbra, M.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2017, 58, 1864–1877. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Zynudheen, A.A.; Parvathy, U. Microencapsulation of Bioactive Food Ingredients and Controlled Release A Review. MOJ Food Process. Technol. 2016, 2. [Google Scholar] [CrossRef]
- Achinna, P.; Kuna, A. Microencapsulation technology: A review. J. Res. ANGRAU 2010, 38, 86–102. [Google Scholar]
- Tzia, C.; Tasios, L.; Spiliotaki, T.; Chranioti, C.; Giannou, V.; Varzakas, T. Edible Coatings and Films to Preserve Quality of Fresh Fruits and Vegetables. In Engineering Aspects of Membrane Separation and Application in Food Processing; Informa UK Limited: London, UK, 2015; Volume 20152939, pp. 531–570. [Google Scholar]
- Vieira, M.G.A.; Da Silva, M.A.; Santos, L.; Beppu, M.M. Natural-based plasticizers and biopolymer films: A review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef]
- Lacroix, M. Mechanical and Permeability Properties of Edible Films and Coatings for Food and Pharmaceutical Applications. In Edible Films and Coatings for Food Applications; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 347–366. [Google Scholar]
- Desai, K.G.; Park, H.J. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Morrison, N.A.; Clark, R.C.; Chen, Y.L.; Talashek, T.; Sworn, G. Gelatin alternatives for the food industry. In Progress in Colloid and Polymer Science; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2007; Volume 114, pp. 127–131. [Google Scholar]
- Gómez-Guillen, M.C.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Nualkaekul, S.; Cook, M.; Khutoryanskiy, V.V.; Charalampopoulos, D. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res. Int. 2013, 53, 304–311. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, W. Coacervation Processes. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 125–137. [Google Scholar]
- Thies, C. Microencapsulation of Flavors by Complex Coacervation. In Encapsulation and Controlled Release Technologies in Food Systems; Wiley: Hoboken, NJ, USA, 2007; pp. 149–170. [Google Scholar]
- Meng, Y.; Cloutier, S. Gelatin and Other Proteins for Microencapsulation. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 227–239. [Google Scholar]
- Weinbreck, F.; De Vries, R.; Schrooyen, P.; De Kruif, C.G. Complex Coacervation of Whey Proteins and Gum Arabic. Biomacromolecules 2003, 4, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.-X.; Huang, G.-Q.; Wang, S.; Sun, Y.-T. Microencapsulation of capsanthin by soybean protein isolate-chitosan coacervation and microcapsule stability evaluation. J. Appl. Polym. Sci. 2013, 131, 131. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y.-P.; Guo, S.; Phillips, G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocoll. 2014, 39, 301–318. [Google Scholar] [CrossRef]
- Wang, X.; Luo, K.; Liu, S.; Adhikari, B.; Chen, J. Improvement of gelation properties of soy protein isolate emulsion induced by calcium cooperated with magnesium. J. Food Eng. 2019, 244, 32–39. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, M.; Qin, F.; Adhikari, B.; He, Z.; Chen, J. Enhanced CaSO4-induced gelation properties of soy protein isolate emulsion by pre-aggregation. Food Chem. 2018, 242, 459–465. [Google Scholar] [CrossRef]
- Mulvihill, D.M.; Donovan, M. Whey proteins and their thermal denaturation—A review. Irish J. Food Sci. Technol. 1987, 11, 43–75. [Google Scholar]
- Augustin, M.A.; Oliver, C.M. Use of Milk Proteins for Encapsulation of Food Ingredients. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 211–226. [Google Scholar]
- Visker, M.H.P.W.; Heck, J.; Van Valenberg, H.; Van Arendonk, J.; Bovenhuis, H. Short communication: A new bovine milk-protein variant: α-Lactalbumin variant D. J. Dairy Sci. 2012, 95, 2165–2169. [Google Scholar] [CrossRef]
- Sarode, A.; Sawale, P.; Khedkar, C.; Kalyankar, S.; Pawshe, R. Casein and Caseinate: Methods of Manufacture. In Encyclopedia of Food and Health; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 676–682. [Google Scholar]
- DeJong, G.; Koppelman, S.J. Transglutaminase Catalyzed Reactions: Impact on Food Applications. J. Food Sci. 2002, 67, 2798–2806. [Google Scholar] [CrossRef]
- Gangurde, H.; Patil, P.; Chordiya, M.; Baste, N. Whey protein. Sch. Res. J. 2011, 1, 69. [Google Scholar] [CrossRef]
- O’Mahony, J.; Fox, P. Milk: An Overview. In Milk Proteins; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 19–73. [Google Scholar]
- Morr, C.V.; Ha, E.Y.W. Whey protein concentrates and isolates: Processing and functional properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.A.; Kung, B.; Senaratne, V.; Dalgleish, D.G.; Flores, A. Gelation of commercial fractions of β-lactoglobulin and α-lactalbumin. Int. Dairy J. 1997, 7, 79–85. [Google Scholar] [CrossRef]
- Santos, M.B.; Da Costa, N.R.; Garcia-Rojas, E.E. Interpolymeric Complexes Formed Between Whey Proteins and Biopolymers: Delivery Systems of Bioactive Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 792–805. [Google Scholar] [CrossRef]
- Layman, D.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.B.; Wedholm-Pallas, A.; Lindmark-Månsson, H.; Andrén, A. Different proteomic profiles of sweet whey and rennet casein obtained after preparation from raw versus heat-treated skimmed milk. Dairy Sci. Technol. 2010, 90, 641–656. [Google Scholar] [CrossRef][Green Version]
- Gruber, J. Polysaccharide-Based Polymers in Cosmetics. In Principles of Polymer Science and Technology in Cosmetics and Personal Care; Desmond Goddard, J.V.G.E., Ed.; CRC Press: London, UK, 1999; pp. 325–389. [Google Scholar] [CrossRef]
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef]
- Kwiecień, I.; Kwiecień, M. Application of Polysaccharide-Based Hydrogels as Probiotic Delivery Systems. Gels 2018, 4, 47. [Google Scholar] [CrossRef]
- Burnside, E. Hydrocolloids and Gums as Encapsulating Agents. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 241–252. [Google Scholar]
- Chakraborty, S. Carrageenan for encapsulation and immobilization of flavor, fragrance, probiotics, and enzymes: A review. J. Carbohydr. Chem. 2017, 36, 1–19. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Thuy, T.T.T.; Urakawa, H.; Kajiwara, K. Structural characteristics of carrageenan gels: Temperature and concentration dependence. Food Hydrocoll. 2002, 16, 515–522. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Wandrey, C.; Bartkowiak, A.; Harding, S. Materials for encapsulation. In Encapsulation Technologies for Active Food Ingredients and Food Processing, 1st ed.; Zuidam, N.J., Nedovic, V., Eds.; Springer-Verlag: New York, NY, USA, 2009; pp. 31–100. [Google Scholar] [CrossRef]
- Jansson, P.-E.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from xanthomonas campestris. Carbohydr. Res. 1975, 45, 275–282. [Google Scholar] [CrossRef]
- Petri, D.F. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 132. [Google Scholar] [CrossRef]
- Kool, M.M.; Gruppen, H.; Sworn, G.; Schols, H.A. The influence of the six constituent xanthan repeating units on the order–disorder transition of xanthan. Carbohydr. Polym. 2014, 104, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.M.; Schols, H.A.; Delahaije, R.; Sworn, G.; Wierenga, P.A.; Gruppen, H. The influence of the primary and secondary xanthan structure on the enzymatic hydrolysis of the xanthan backbone. Carbohydr. Polym. 2013, 97, 368–375. [Google Scholar] [CrossRef]
- Wu, M.; Qu, J.; Shen, Y.; Dai, X.; Wei, W.; Shi, Z.; Li, G.; Ma, T. Gel properties of xanthan containing a single repeating unit with saturated pyruvate produced by an engineered Xanthomonas campestris CGMCC 15155. Food Hydrocoll. 2019, 87, 747–757. [Google Scholar] [CrossRef]
- Bergmann, D.; Furth, G.; Mayer, C. Binding of bivalent cations by xanthan in aqueous solution. Int. J. Boil. Macromol. 2008, 43, 245–251. [Google Scholar] [CrossRef]
- Cook, M.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 2012, 162, 56–67. [Google Scholar] [CrossRef]
- Fareez, I.M.; Lim, S.M.; Mishra, R.K.; Ramasamy, K. Microencapsulation of Lactobacillus SP. Using Chitosan-Alginate-Xanthan Gum-?-Cyclodextrin and Characterization of its Cholesterol Reducing Potential and Resistance Against pH, Temperature and Storage. J. Food Process. Eng. 2016, 40, e12458. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Boil. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Nussinovitch, A. Hydrocolloids in Flavor Encapsulation. In Water-Soluble Polymer Applications in Foods; Nussinovitch, A., Ed.; Blackwell Science: Oxford, UK, 2003; pp. 31–113. [Google Scholar] [CrossRef]
- Karlton-Senaye, B.; Ibrahim, S. Impact of gums on the growth of probiotics. Agro Food Ind. Hi-Tech 2013, 24, 10–14. [Google Scholar]
- Izydorczyk, M.; Cui, S.; Wang, Q. Polysaccharide Gums: Structures, Functional Properties, and Applications. Food Carbohydr. Chem. Phys. Prop. Appl. 2005. [Google Scholar] [CrossRef]
- Wallick, D. Cellulose Polymers in Microencapsulation of Food Additives. In Microencapsulation in the Food Industry; Elsevier BV: Amsterdam, The Netherlands, 2014; pp. 181–193. [Google Scholar]
- Narayanan, D.; Kumar, A.S.; Chennazhi, K.P. Versatile carboxymethyl chitin and chitosan nanomaterials: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 574–598. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Heinze, T.; El Seoud, O.A.; Koschella, A. Etherification of Cellulose. In Springer Series on Polymer and Composite Materials; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 429–477. [Google Scholar]
- Liu, H.; Yang, Q.; Zhang, L.; Zhuo, R.; Jiang, X. Synthesis of carboxymethyl chitin in aqueous solution and its thermo- and pH-sensitive behaviors. Carbohydr. Polym. 2016, 137, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Ștefănescu, B.E.; Pop, I.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- And, Z.L.; Han, J. Film-forming Characteristics of Starches. J. Food Sci. 2005, 70, 31. [Google Scholar] [CrossRef]
- BeMiller, J.N. Starches: Conversions, modifications, and uses. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; BeMiller, J.N., Ed.; AACC International Press: Amsterdam, The Netherlands, 2019; pp. 191–221. [Google Scholar] [CrossRef]
- Van Der Veen, B.A.; Van Alebeek, G.-J.W.M.; Uitdehaag, J.C.M.; Dijkstra, B.W.; Dijkhuizen, L. The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. JBIC J. Boil. Inorg. Chem. 2000, 267, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, M.P. The Potential of Cyclodextrins as Novel Active Pharmaceutical Ingredients: A Short Overview. Molecules 2016, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Pariot, N.; Edwards-Lévy, F.; Andry, M.-C.; Levy, M.-C. Cross-linked β-cyclodextrin microcapsules: Preparation and properties. Int. J. Pharm. 2000, 211, 19–27. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Muzzafar, A.; Sharma, V. Microencapsulation of probiotics for incorporation in cream biscuits. J. Food Meas. Charact. 2018, 12, 2193–2201. [Google Scholar] [CrossRef]
- Synthesis of Carboxymethyl Chitosan and its Rheological Behaviour in Pharmaceutical and Cosmetic Emulsions. J. Appl. Pharm. Sci. 2017, 7, 70–78.
- Zhao, L.; Zhu, B.; Jia, Y.; Hou, W.; Su, C. Preparation of Biocompatible Carboxymethyl Chitosan Nanoparticles for Delivery of Antibiotic Drug. BioMed Res. Int. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shit, S.C.; Shah, P. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Aydin, F.; Kahve, H.; Ardic, M. Lipid-based edible films. J. Sci. Eng. Res. 2017, 4, 86–92. [Google Scholar]
- Wolfmeier, U.; Schmidt, H.; Michalczyk, G.; Payer, W.; Dietsche, W.; Boehlke, K.; Hohner, G.; Wildgruber, J.; Heinrichs, F.-L. Waxes. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Hall, D.J. Edible coatings from lipids, waxes, and resins. In Edible Coatings and Films to Improve Food Quality, 2nd ed.; Baldwin, E.A., Hagenmaier, R., Bai, J., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 79–101. [Google Scholar] [CrossRef]
- Rao, A.; Shiwnarain, N.; Maharaj, I. Survival of Microencapsulated Bifidobacterium pseudolongum in Simulated Gastric and Intestinal Juices. Can. Inst. Food Sci. Technol. J. 1989, 22, 345–349. [Google Scholar] [CrossRef]
- Xie, M. Phospholipids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 214–217. [Google Scholar] [CrossRef]
- Jackson, L. Microencapsulation and the food Industry. Lebensm. Wiss. Technol. 1991, 24, 289–297. [Google Scholar]
- Singh, H.; Thompson, A.; Liu, W.; Corredig, M. Liposomes as food ingredients and nutraceutical delivery systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 287–318. [Google Scholar]
- Sarao, L.K.; Arora, M.; M, A. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
| Probiotic | Method * | Coating Material ** | Particle Size (µm) | Reference | |
|---|---|---|---|---|---|
| Inner | External | ||||
| Lactobacillus paracasei subsp. paracasei E6, Lactobacillus paraplantarum B1 | CC | WPI + GA | - | 10–15 | [58] |
| Lactobacillus paracasei subsp. paracasei E6, Lactobacillus paraplantarum B1 | CC + EX | WPI + GA | A | 2000–3000 | [41] |
| Lactobacillus acidophilus La-5 | CC + LYO | G + GA | - | 227.05 | [42] |
| Lactobacillus plantarum BL011 | ES | A, A + P | - | 7–2000 | [29] |
| Bifidobacterium longum BIOMA 5920 | ES | A + CLG | - | 300–600 | [55] |
| Lactobacillus acidophilus TISTR 1338 | ES | A + Z | - | 500–600 | [55] |
| Bifidobacterium animalis subsp. lactis Bb12 | ES | WPC + Pul | - | 1.1–4.7 | [55] |
| Lactobacillus plantarum CECT 748 T | ES | WPC | - | ND ** | [30] |
| Lactobacillus acidophilus PTCC 1643, Bifidobacterium bifidum PTCC 1644 | EM | A + CaO | YCW | 90–117 | [59] |
| Bifidobacterium BB-12 | EM | A + CaO | - | 54–55 | [60] |
| Lactobacillus casei NCDC-298 | EM | A + RS + SO | SA, BW, PLL | ND | [61] |
| Lactobacillus casei NCDC-298 | EM | A + SO | - | ND | [62] |
| Lactobacillus helveticus R0052 | EM | P + CaO | WPI | 90–130 | [63] |
| Lactobacillus casei 431 | EM | SC + CaO, SC + GG + CaO | - | 287–399 | [64] |
| Lactobacillus paracasei ssp. paracasei F19, Bifidobacterium lactis Bb12 | EM | SC + Tgase + SFO | - | 165 ± 23 | [65] |
| Lactobacillus acidophilus | EM | CGNs + VF | - | ND | [39] |
| Lactobacillus rhamnosus Lr-32, Bifidobacterium longum Bl-05, Lactobacillus salivarius Ls-33, Lactobacillus plantarum Lpc-37, Lactobacillus acidophilus NCFM, Lactobacillus paracasei Lp-115, Bifidobacterium lactis type Bl-04, Bifidobacterium lactis Bi-07, Lactobacillus rhamnosus HOWARU Bifidobacterium bifidum HOWARU | EM | A + VF, GUG + VF, XG + VF, LBG + VF, CGN + VF | - | ND | [40] |
| Lactobacillus F19, Bifidobacterium lactis Bb12 | EM + LYO | SC + Tgase + SFO, SC + RS + Tgase + SFO | - | ND | [66] |
| Lactobacillus plantarum (Digestive Care, USA) | EM + CC | G + CoO, G + GA | - | 66.07 ± 3.24 105.66 ± 3.24 | [67] |
| Lactobacillus acidophilus La5 | EM + CC | P + B | WPI | 230–270 | [68] |
| Lactobacillus acidophilus LA-5, Bifidobacterium bifidum BB-12 | EM, EX | k-CGN + CoO, A | - | 500–1000 | [69] |
| Bifidobacterium bifidum BB-12, Lactobacillus acidophilus LA-5 | EM, EX | k-CGN + CoO, A | - | 300–400 200–300 | [70] |
| Lactobacillus plantarum CECT 220 (ATCC 8014) | EM, EX | A + XG + I + OO, A + XG + I | - | 600–900 1860–2250 | [71] |
| Lactobacillus plantarum MF369875.1, Weissela paramesenteroides CP023501.1, Enterococcus faecalis HQ802261.1, Lactobacillus paraplantarum AB362736.1 | EX + LYO | AS + WP, M + WP | - | 382.8 349.92–458.91 | [72] |
| Lactobacillus acidophilus TISTR 1338 | EM + ES + FBD | A + EA + SA | - | 450 ± 50 | [34] |
| Lactobacillus casei ATCC 393 | EX | A | Ch, CMCS | 2200 ± 100 | [73] |
| Lactobacillus acidophilus, Lactobacillus bulgaricus | EX | A + GUG | - | 2000–5000 | [35] |
| Lactobacillus bulgaricus (Hangzhou Wahaha Group. Co. Ltd.) | EX | A + Milk | - | 750 ± 12 to 890 ± 25 | [51] |
| Bifidobacterium lactis BI07 Lactobacillus acidophilus LA14 | EX | A + XG, A + CAP | - | 1000–2000 | [74] |
| Lactobacillus casei W8® | EX | P | - | ND | [36] |
| Lactobacillus acidophilus 547, Bifidobacterium bifidum ATCC 1994, Lactobacillus casei 01 | EX | A | A + PLL, Ch, A | 1890 | [75] |
| Lactobacillus reuteri (PTCC 1655) | EX | A | - | 1000–2250 | [76] |
| Lactobacillus plantarum TN8 | EX | A | Ch | 20 | [77] |
| Lactobacillus plantarum NCIMB 8826 | EX | A, P | Ch | 2500–3500 | [78] |
| Bifidobacterium sp. | EX | A + CMCH | - | ND | [79] |
| Lactobacillus acidophilus, Lactobacillus bulgaricus | EX | A + GUG | - | ND | [35] |
| Lactobacillus casei Shirota (Yakult®) | EX | A + RS | Ch | 500 | [80] |
| Lactobacillus acidophilus CSCC 2400 and 2409 | EX | A + RS | A, Ch, PLL | 450–500 | [81] |
| Lactobacillus acidophilus La5 | EX | A + RS | - | 50–80 | [82] |
| Lactobacillus plantarum TISTR 050 | EX | A + SPI | - | 3030 ± 30 to 3440 ± 60 | [83] |
| Lactobacillus plantarum ATCC:13643 | EX | CMC, CMC + k-CGN | - | 86–95 | [84] |
| Lactobacillus acidophilus (Synbiotech Biotechnology Co., Ltd.) | EX | XG + Ch | XG | ND | [85] |
| Bifidobacterium lactis | EX | XG + GG | - | 20–2200 | [86] |
| Lactobacillus acidophilus LA1 | EX | A + CS + GUG, A + CS + FOS | - | 15–180 | [87] |
| Lactobacillus plantarum 299v, 800, and CIP A159 | EX + LYO | A | WP | ND | [88] |
| Lactobacillus plantarum LAB13 | EX + LYO | A, A + XG | Ch | 1343.2 ± 4.8 | [89] |
| Lactobacillus gasseri, Bifidobacterium bifidum (CECT) | EX + LYO | A + Ch | - | 340–360 | [90] |
| Lactobacillus plantarum LAB12 | EX + LYO | A + XG, A + XG + β-CD | Ch | 1302–1335 | [89] |
| Lactobacillus acidophilus La-14 (Danisco) | EX + LYO | A, A + RS + Ch | - | 112.5; 114.5 | [91] |
| Yarrowia lipolytica VIT-MN01, Kluyveromyces lactis VIT-MN02, Lipomyces starkeyi VIT-MN03, Saccharomycopsis fibuligera VIT-MN04, Brettanomyces custersianus VIT-MN07 | EX + LYO | A + BBG A + FMBG A + GA A + OBG A + PMBG A + RBG | WPI,Ch | 700–750 750–800 ND 600–650 750–800 850–899 | [92] |
| Lactobacillus rhamnosus GG, LMG 18243 | EX, LBL | A | Ch + LF + SC, Ch + DS | 130 ± 47 | [93] |
| Lactobacillus reuteri DSM 17938 | EXVN | A | Ch | 110 ± 5 | [37] |
| Bifidobacterium longum subsp. infantis (UMA 298, UMA 299, MA 300, UMA 305), Bifidobacterium longum subsp. longum (UMA 306, UMA 318, UMA 401, UMA 402) | EXVN | A | Ch | 310–340 | [94] |
| Saccharomyces boulardii (Ultralevure, n° 325988.5) | EXVN | A + WPI, WPI A, A + WPI | WPI, A A, WPI | 900–1250 800–1200 | [95] |
| Bifidobacterium lactis 300b | EXVN | A + HPMC, A + CMC, A + MCC, A + MS, A + D, A + Pul | - | 1054–1066 | [38] |
| Lactobacillus rhamnosus GG ATCC 53103 | EXVN | WPI | - | 200 | [96] |
| Bifidobacterium animalis spp lactis NCC 2818 (BL818) | FBD | HPMC | HPCP | 55.6–132.7 | [33] |
| Bacillus coagulans ATCC 7050 | LBL | A | Ch | ND | [31] |
| Lactobacillus acidophilus | LBL | Ch | CMC | ND | [32] |
| Lactobacillus plantarum MTCC 5422 | LYO | FOS + WPI, FOS + DWPI | - | 70–90 | [28] |
| Lactobacillus plantarum MTCC 5421 | LYO, SDY | A + WPI, A + DWPI | - | ND | [97] |
| Lactobacillus casei DSM 20011, Lactobacillus reuteri DSM 20016, Lactobacillus bulgaricus DSM 20081 | LYO + EXVN | A | - | 600–800 | [49] |
| Saccharomyces cerevisiae DSY-5 | LYO | GUG | - | ND | [27] |
| Lactobacillus acidophilus LA3, Bifidobacterium animalis subsp. 103 lactis BLC1 | SCH, CC + LYO | VF | G + GA | 79–83 | [23] |
| Lactobacillus reuteri DSM 17938 | SDY | A | Ch | 3 | [98] |
| Lactobacillus acidophilus La-05, Bifidobacterium lactis Bb-12 | SDY | CAP | - | 5–50 | [99] |
| Lactobacillus acidophilus NRRL (B-4495) | SDY | Ch + I, Ch + M, G + M | Ch + I, Ch + M | 11.39 13.94 21.37 | [100] |
| Lactococcus lactis Gh1 | SDY | GA + seed, leaf, or pulp extract of miracle fruit (Synsepalum dulcificum) | - | ND | [21] |
| Bifidobacterium PL1 | SDY | MS | - | 5 | [101] |
| Lactobacillus acidophilus La-05 | SDY | SE + M | - | 4.97 ± 2.33 to 8.82 ± 4.07 | [22] |
| Bifidobacterium lactis Bb12 | SDY | SW, SWI, SWP | - | ND | [102] |
| Lactobacillus rhamnosus GG | SDY | WPI, HWP, DGS, RS, SFO | - | ND | [103] |
| Bifidobacterium bifidum F-35 | SDY, EM | WPI, WPI + SO | - | 20 200 | [104] |
| Saccharomyces boulardii, Lactobacillus acidophilus LA-5, Bifidobacterium bifidum BB-12 | SDY + SCH, SCH + SDY | GA + β-CD, HPO | HPO, GA + β-CD, | 4.88–24.06 244.55–612.54 | [24] |
| Lactobacillus paracasei LMG P-21380 | SFD | MT | - | 1000–1400 | [25] |
| Lactobacillus plantarum MTCC 5422 | SFD | A + WPI, WPI + FOS, A + DWPI, DWPI + FOS, | - | 53.99–105.07 | [105] |
| Lactobacillus plantarum MTCC 5422 | SFD, LYO, SDY | WPI | - | ND | [26] |
| Coating Material | Water Vapour Permeability | O2 and CO2 Permeability | ||||
|---|---|---|---|---|---|---|
| WVP | PO2 | PCO2 | ||||
| Test Conditions | (10−12 g·m−1·s−1·Pa−1) | Test Conditions | (10−10 L·m−1·d−1·Pa−1) | References | ||
| Proteins | ||||||
| Collagen (CLG) | 25 °C, 50% RH | 211 ± 44 | - * | - | - | [106] |
| Zein (Z) | 21–30 °C, 0/85% RH | 116 | 20 °C, 60% RH | 0.31 | 2.31 | [107] |
| 25 °C, 50% RH | 3900 | - | - | - | [108] | |
| Egg albumen (EA)g | 25 °C, 100/50% RH | 2310 | - | - | - | [109] |
| Heat denatured whey protein isolate (DWPI) | 25 °C, 50% RH | 922 | - | - | - | [110] |
| Hydrolysed whey protein (HWP) | 25 °C, 35% RH | 944 | - | - | - | [111] |
| Mammalian gelatin films | 25 °C, 0/81% RH | 523 | - | - | - | [112] |
| Sodium caseinate (SC) | 25 °C, 0/81% RH | 425 | 25 °C, 77% RH | 8.8 | 52.78 | [113,114] |
| 23 °C, 55% RH | 3580 | - | - | - | [115] | |
| Soy protein isolate (SPI) | 5-35 °C, 100/50% and 100/70%RH | 1600–4400 | - | - | - | [116] |
| 25 °C, 50% RH | 830 | - | - | - | [117] | |
| 25 °C, 50% RH | 3540 | 25 C, 50% RH | - | - | [118] | |
| Whey protein (WP) | 25 °C, 100/55% RH | 616–4170 | 25 °C, 50% RH | 0.012 | 2.13 | - |
| Whey protein concentrate (WPC) | 23 °C, 55% RH | 2960 | - | - | - | [115] |
| Whey protein isolate (WPI) | 23 °C, 50% RH | 3830 | - | - | - | [119] |
| 23 °C, 55% RH | 3370 | - | - | - | [115] | |
| β-casein | 22.5 °C,53/11% and 53/76% RH | 179–523 | - | - | - | [120] |
| Polysaccharides | ||||||
| Alginate (A) | 20 °C, 100/50% RH | 3900 | - | - | - | [121] |
| 25 °C, 50% RH | 102 | 58.3 | 139 | [122] | ||
| Amylose | 25 °C, 100/0% RH | 370 | - | - | - | [123] |
| Arrowroot starch (AS) | 25 °C, 75% RH | 41.9 | - | - | - | [124] |
| Barley β-glucan-protein alkaline extracts | 25 °C, 50% RH | 400 | - | - | - | [125] |
| Barley β-glucan-protein non alkaline extracts | 25 °C, 50% RH | 1400.0 | - | - | - | [125] |
| Carboxymethyl cellulose (CMC) | 25 °C, 75% RH | 298 | - | - | - | [126] |
| Carboxymethyl chitosan (CMCS) | 25 °C, 53% RH | 236 | - | - | - | [127] |
| Carrageenan (CGN) | 25 °C, 100/50% RH | 1900 | 3.62 | - | - | [121] |
| Cellulose acetate phthalate (CAP) | 25 °C, 52% RH | 69.8–116 | - | - | - | [128] |
| Chitosan (Ch) | 25 °C, 100/50% RH | 490 | 25 °C, 93% RH | 0.0014 | - | [129] |
| 25 °C, 53% RH | 181 | - | - | - | [127] | |
| Gellan gum (GG) | 21 °C, 0/54% RH | 158 | - | - | - | [130,131] |
| Guar gum (GUG), | 25 °C, 53% RH | 128 | - | - | - | [132] |
| Resistant starch (RS) | 25 °C, 50% RH | 1.17 × 104 | - | - | - | [108,133] |
| Hydroxypropyl cellulose - poloxamer (HPCP), | 30 °C, 11% RH | 52–66 | 30 °C, 0% RH | 2.59–3.2 | - | [134] |
| Hydroxypropyl methylcellulose (HPMC) | 27 °C, 0/85% RH | 105 | 25 °C, 50% RH | 0.12–1.16 | - | [129,135] |
| Inulin (I) | - | - | - | - | - | [136] |
| Locust bean gum (LBG) | 25 °C, 53% RH | 114 | - | - | - | [132] |
| Methyl cellulose (MC) | 35 °C, 0/90% RH | 55.6 | - | - | - | [137] |
| 25 °C, 0/75% RH | 76–92 | 30 °C, 0% RH | 2.17–12.96 | 69-743 | [129] | |
| Microcrystalline cellulose (MCC) | 25 °C, 50% RH | 277 | - | - | - | [138] |
| Oats protein | 25 °C, 100% RH | 760–1570 | - | - | - | [139] |
| Pearl millet starch | 30 °C, 75% RH | 206 | - | - | - | [140] |
| Pectin (P) | - | - | 6.6–29.5 | 472 | [129] | |
| Pullulan (Pul) | 25 °C, 53% RH | 106 | 25 °C, 96% RH | - | - | [127] |
| Rice bran gum (RBG) | 25 °C, 55% RH | 8 × 105 – 9.2 × 105 | 35°C, 55% RH, | 1.18 × 10−5 – 5.46 × 10−6 | - | [141] |
| Starch | 23 °C, 74/50% RH | 2170 | 20 °C, 63.8% RH | 1591 | 29209 | [129] |
| Starch from red sorghum (St-RedS) | 25 °C, 0/75% RH | 45.6–61.5 | - | - | - | |
| St-RedS Oxidize-Acid-modified | 25 °C, 0/75% RH | 34–41.7 | - | - | - | [142] |
| St-RedS Acid-modified | 25 °C, 0/75% RH | 27.9–58.7 | - | - | - | [142] |
| St-RedS Acid and oxidize-modified | 25 °C, 0/75% RH | 63.4–69.1 | - | - | - | [142] |
| Xanthan gum (XG) low-density | 38 °C, 0/90% RH | 0.7–0.97 | 25 °C, 90% RH | 7.43 | 21.7 | [129] |
| XG high-density | 38 °C, 0/90% RH | 0.24 | 25 °C, 90% RH | 2.1 | 120 | [129] |
| Yeast cell wall (YCW) | 24 °C, 10% RH | 280 | - | - | - | [143] |
| Lipids | ||||||
| Bee wax (BW) | 25 °C, 0/100% RH | 0.58 | 25 °C, 0% RH | 1.06 | - | [129] |
| Vegetable or animal fat | 23 °C, 12/56% RH | 2.2-34.7 | - | - | - | [121] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pech-Canul, A.d.l.C.; Ortega, D.; García-Triana, A.; González-Silva, N.; Solis-Oviedo, R.L. A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings 2020, 10, 197. https://doi.org/10.3390/coatings10030197
Pech-Canul AdlC, Ortega D, García-Triana A, González-Silva N, Solis-Oviedo RL. A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings. 2020; 10(3):197. https://doi.org/10.3390/coatings10030197
Chicago/Turabian StylePech-Canul, Angel de la Cruz, David Ortega, Antonio García-Triana, Napoleón González-Silva, and Rosa Lidia Solis-Oviedo. 2020. "A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics" Coatings 10, no. 3: 197. https://doi.org/10.3390/coatings10030197
APA StylePech-Canul, A. d. l. C., Ortega, D., García-Triana, A., González-Silva, N., & Solis-Oviedo, R. L. (2020). A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings, 10(3), 197. https://doi.org/10.3390/coatings10030197





