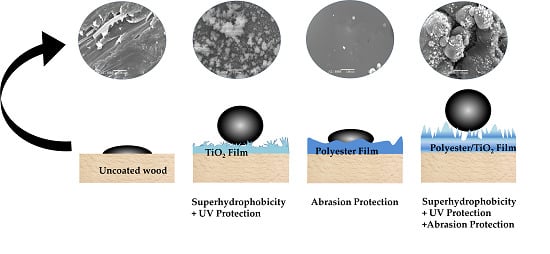
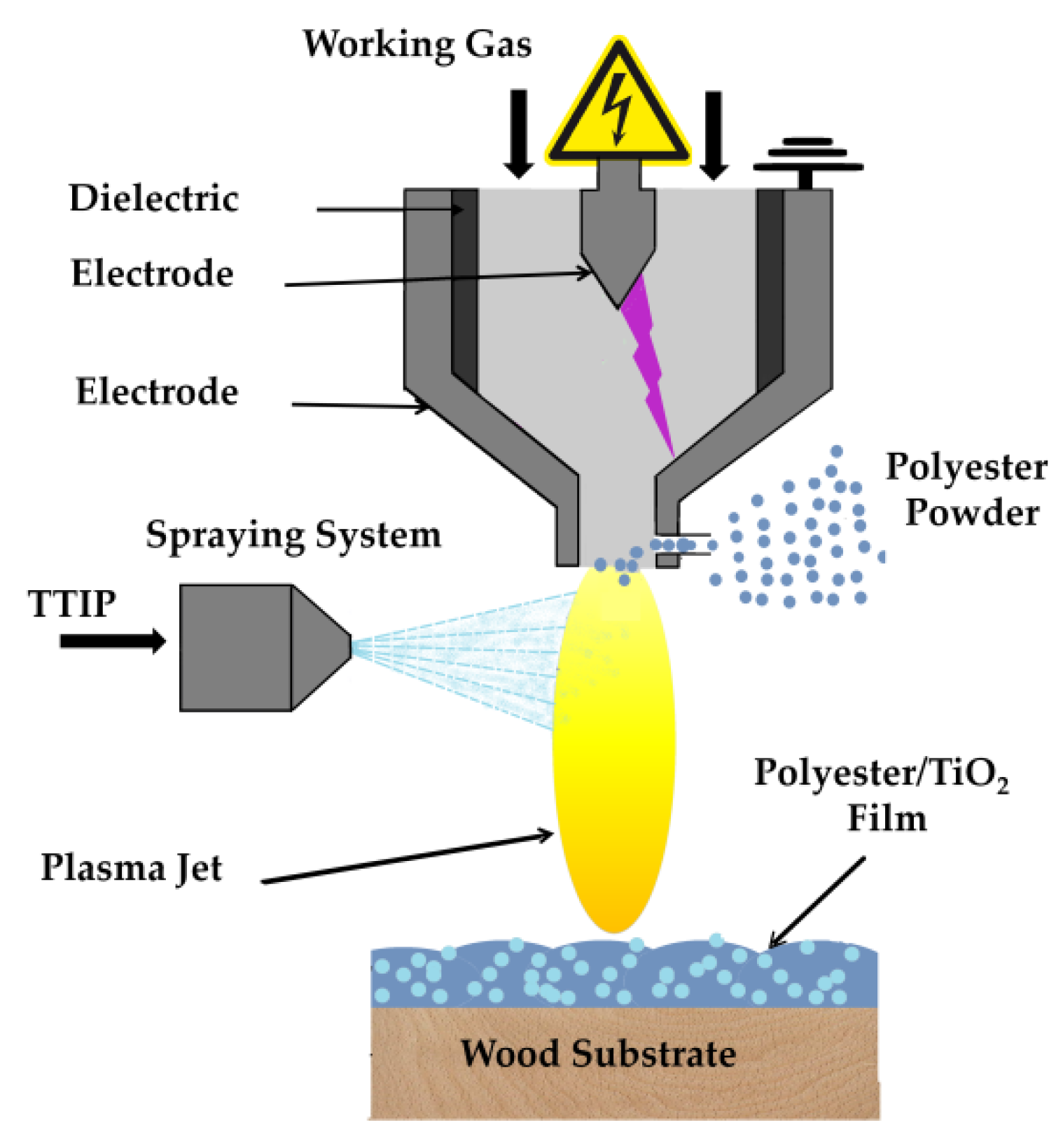
One-Step Deposition of Polyester/TiO2 Coatings by Atmospheric Pressure Plasma Jet on Wood Surfaces for UV and Moisture Protection
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
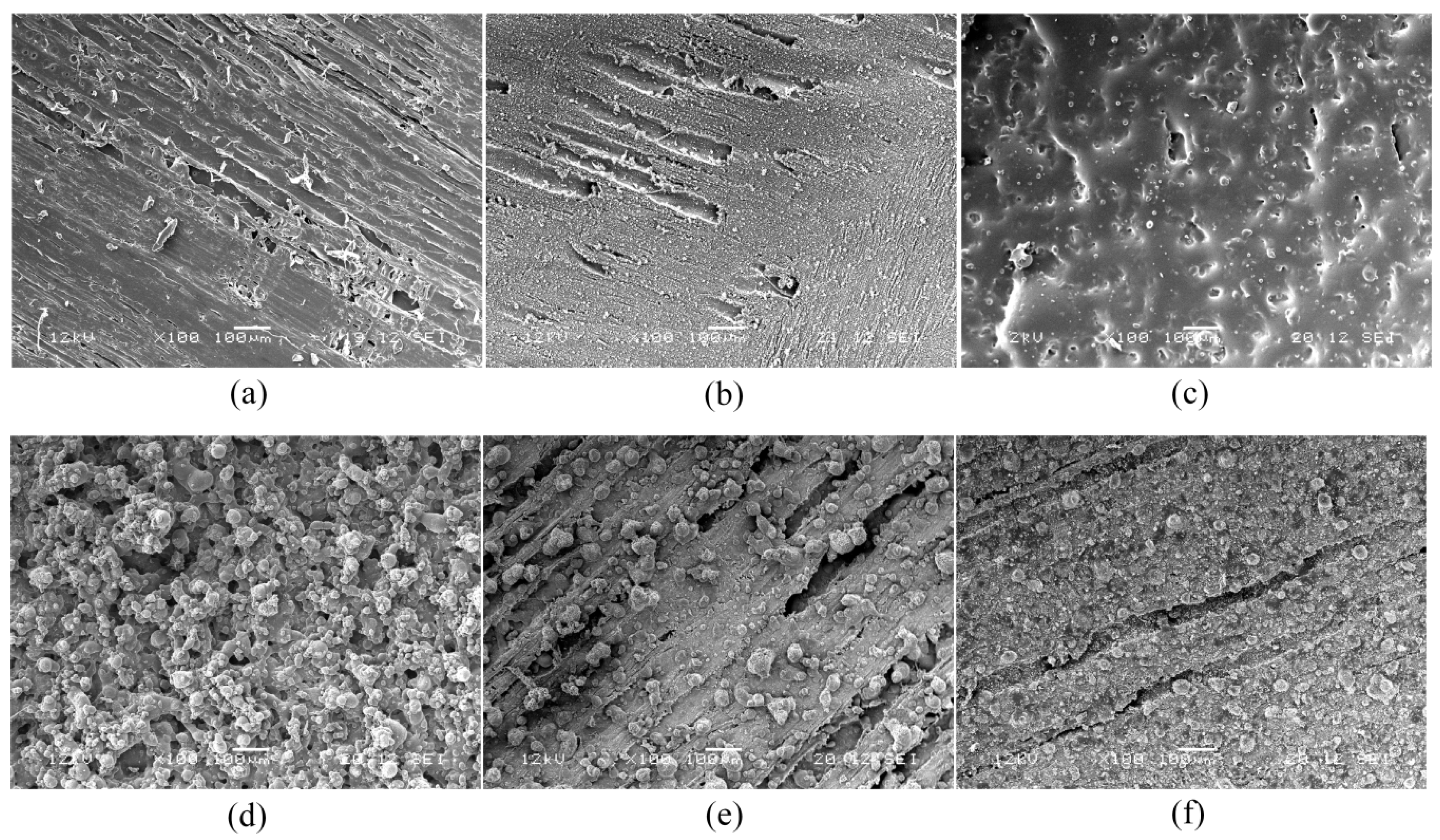
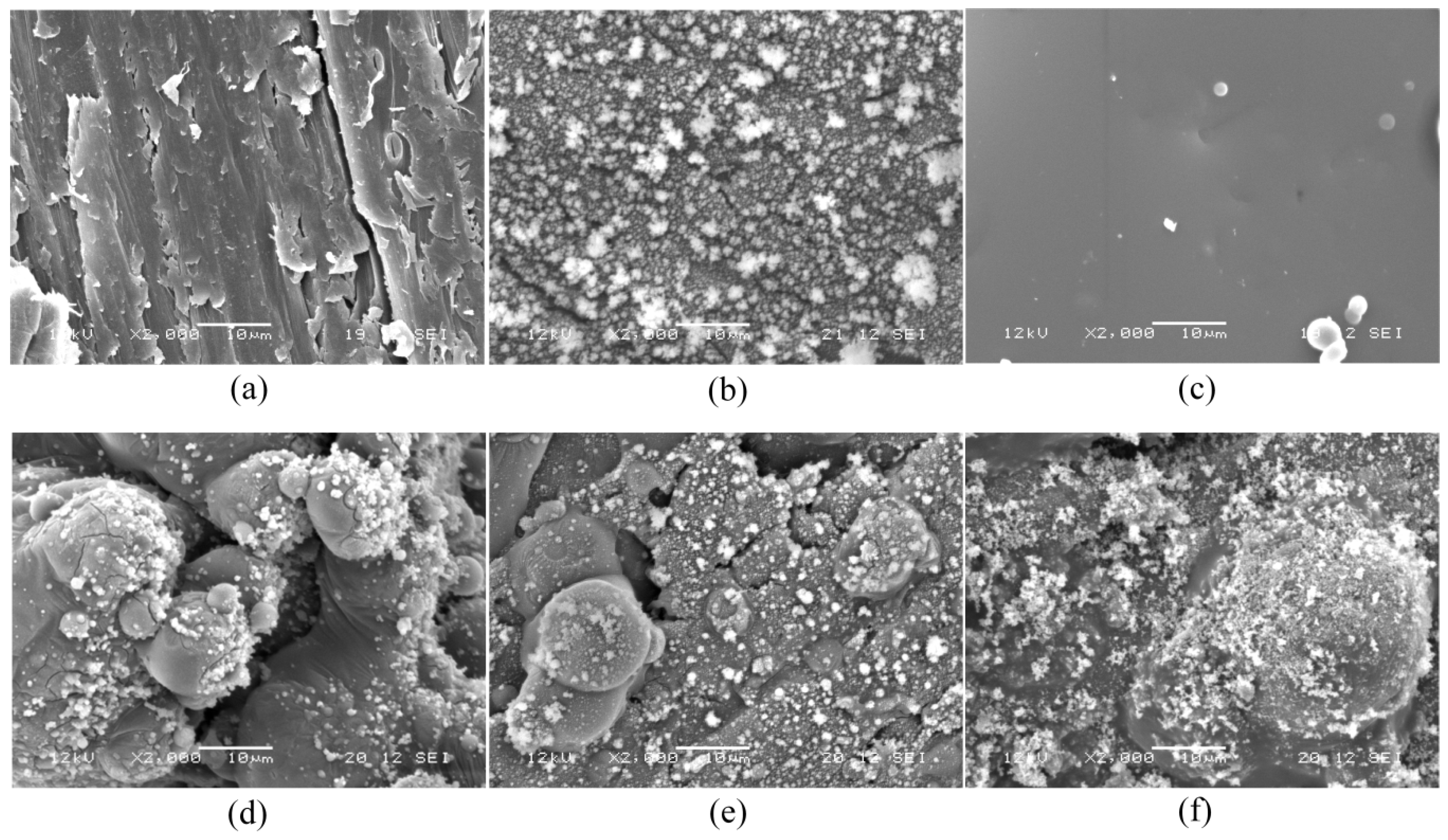
3.1. Characterization of Coatings on Wood by Scanning Electron Microscopy (SEM)
3.2. Characterization of Coatings by Fourier Transform Infrared (FTIR) Spectroscopy
3.3. Characterization of Coatings by X-ray Photoelectron Spectroscopy (XPS)
3.4. Ultraviolet (UV) Protection
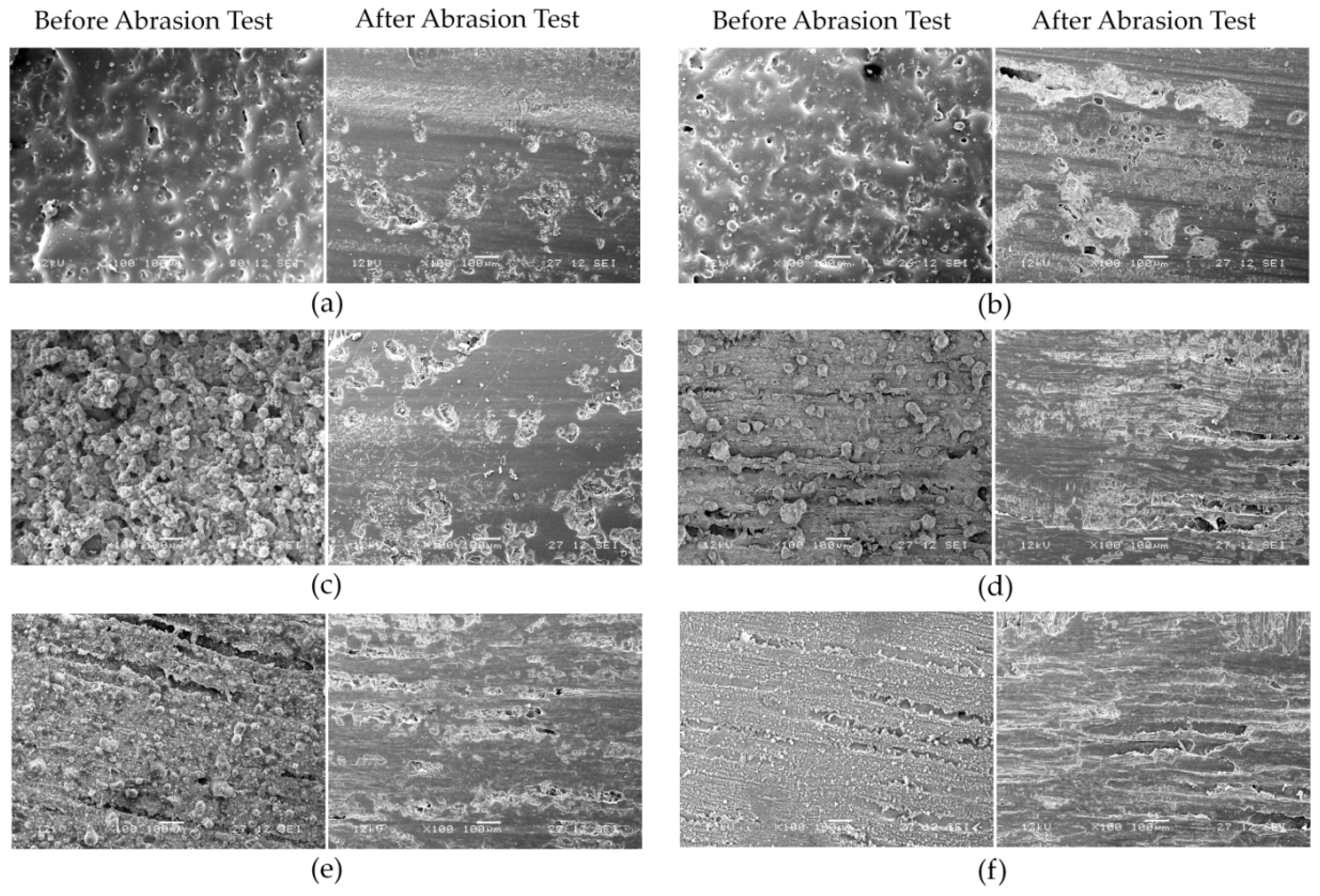
3.5. Abrasion Test
3.6. Contact Angle Analysis (Water)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fengel, D.; Wegener, G. Wood Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1984. [Google Scholar]
- Feist, W.C.; Hon, D.S. Chemistry of weathering and protection. In The Chemistry of Solid Wood; Rowell, R.M., Ed.; American Chemical Society: Washington, DC, USA, 1984; Volume 207, pp. 401–451. [Google Scholar]
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Yano, S.; Iwata, K.; Kurita, K. Physical properties and structure of organic–inorganic hybrid materials produced by sol–gel process. Mater. Sci. Eng. C 1998, 6, 75–90. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/Inorganic Hybrid Network Materials by the Sol-Gel Approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- Mir, S.H.; Nagahara, L.A.; Thundat, T.; Mokarian-Tabari, P.; Furukawa, H. Organic-inorganic hybrid functional materials: An integrated platform for applied technologies. J. Electrochem. Soc. 2018, 165, 3137–3156. [Google Scholar] [CrossRef]
- Flores Tandy, L.M.; Perez Bueno, J.J.; Meas Vong, Y. Multifunctional Polymer/Nano-TiO2 Photochromic Hybrid Coatings as a Barrier for Protection against Corrosion. In Handbook of Research on Diverse Applications of Nanotechnology in Biomedicine, Chemistry, and Engineering; United States of America by Engineering Science Reference (an imprint of IGI Global): Hershey, PA, USA, 2014. [Google Scholar]
- Altan, M.; Yildirim, H. Mechanical and Antibacterial Properties of Injection Molded PP/TiO2 Nano-Composites: Effects of Surface Modification. J. Mater. Sci. Technol. 2012, 28, 686–692. [Google Scholar] [CrossRef]
- Segura González, E.A.; Olmos, D.; Lorente, M.Á.; Vélaz, I.; González-Benito, J. Preparation and Characterization of Polymer Composite Materials Based on PLA/TiO2 for Antibacterial Packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Cerrada, M.L.; Fernández-García, M. Titanium Dioxide–Polymer Nanocomposites with Advanced Properties. In Nano-Antimicrobials: Progress and Prospects; Springer: Berlin, Germany, 2012; pp. 119–149. [Google Scholar]
- Ghaebi Mehmandoust, S.; Alizadeh, R.; Babaluo, A.A. Kinetic study of the poly(vinyl chloride)/titanium dioxide nanocomposites photodegradation under accelerated ultraviolet and visible light exposure. Polym. Adv. Technol. 2014, 25, 799–808. [Google Scholar] [CrossRef]
- Ghanem, A.; Badawy, A.; Ismail, N.; Tian, Z.; Rehim, M.; Rabia, A. Photocatalytic activity of hyperbranched polyester/TiO2 nanocomposites. Appl. Catal. Gen. 2014, 472, 191–197. [Google Scholar] [CrossRef]
- Koltsakidou, A.; Terzopoulou, Z.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Biobased Poly(ethylene furanoate) Polyester/TiO2 Supported Nanocomposites as Effective Photocatalysts for Anti-inflammatory/Analgesic Drugs. Molecules 2019, 24, 564. [Google Scholar] [CrossRef]
- Peña, J.; Vallet-Regí, M.; San Román, J. TiO2-polymer composites for biomedical applications. J. Biomed. Mater. Res. 1997, 35, 129–134. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Liu, X.H.; Fu, Y.B. Studies on the structures and optical properties of TiO2 doped with transition metals. In Proceedings of the 8th Pacific Rim International Congress on Advanced Materials and Processing, Waikoloa, HI, USA, 4–9 August 2013; pp. 295–305. [Google Scholar]
- Fanelli, F.; Mastrangelo, A.; Fracassi, F. Aerosol-Assisted Atmospheric Cold Plasma Deposition and Characterization of Superhydrophobic Organic–Inorganic Nanocomposite Thin Films. Langmuir 2014, 30, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Dembele, A.; Rahman, M.; Reid, I.; Twomey, B.; MacElroy, D.; Dowling, D.P. Deposition of Hybrid Organic–Inorganic Composite Coatings Using an Atmospheric Plasma Jet System. J. Nanosci. Nanotechnol. 2011, 11, 8730–8737. [Google Scholar] [CrossRef] [PubMed]
- Buske, C. Environmentally friendly and cost-saving Atmospheric-pressure plasma technology. Innov. Surf. Technol. 2009, 2, 18–22. [Google Scholar]
- Dong, S.; Zhao, Z.; Dauskardt, R.H. Highly transparent bilayer organosilicate multifunctional coatings on plastics deposited by atmospheric plasma deposition with dual organic and inorganic precursors in air. ACS Appl. Mater. Interf. 2015, 7, 17929–17934. [Google Scholar] [CrossRef]
- Pappas, D. Status and potential of atmospheric plasma processing of materials. J. Vac. Sci. Technol. A Vac. Surf. Films 2011, 29, 020801. [Google Scholar] [CrossRef]
- Joshi, S.V.; Sivakumar, G.; Raghuveer, T.; Dusane, R.O. Hybrid plasma-sprayed thermal barrier coatings using powder and solution precursor feedstock. J. Ther. Spray Technol. 2014, 23, 616–624. [Google Scholar] [CrossRef]
- Lohia, A.; Sivakumar, G.; Ramakrishna, M.; Joshi, S.V. Deposition of Nanocomposite Coatings employing a Hybrid APS + SPPS Technique. J. Therm. Spray Technol. 2014, 23, 1054–1064. [Google Scholar] [CrossRef]
- Zhang, W.; Li, G.; Zhang, Q.; Yang, G.; Zhang, G.; Mu, H. Bimodal TBCs with low thermal conductivity deposited by a powder-suspension co-spray process. J. Mater. Sci. Technol. 2018, 34, 1293–1304. [Google Scholar] [CrossRef]
- Jnido, G.; Ohms, G.; Viöl, W. Deposition of TiO2 Thin Films on Wood Substrate by an Air Atmospheric Pressure Plasma Jet. Coatings 2019, 9, 441. [Google Scholar] [CrossRef]
- Köhler, R.; Sauerbier, P.; Militz, H.; Viöl, W. Atmospheric Pressure Plasma Coating of Wood and MDF with Polyester Powder. Coatings 2017, 7, 171. [Google Scholar] [CrossRef]
- Köhler, R.; Sauerbier, P.; Ohms, G.; Viöl, W.; Militz, H. Wood Protection through Plasma Powder Deposition—An Alternative Coating Process. Forests 2019, 10, 898. [Google Scholar] [CrossRef]
- Wallenhorst, L.; Gurău, L.; Gellerich, A.; Militz, H.; Ohms, G.; Viöl, W. UV-blocking properties of Zn/ZnO coatings on wood deposited by cold plasma spraying at atmospheric pressure. Appl. Surf. Sci. 2018, 434, 1183–1192. [Google Scholar] [CrossRef]
- Fakhouri, H.; Ben Salem, D.; Carton, O.; Pulpytel, J.; Arefi-Khonsari, F. Highly effcient photocatalytic TiO2 coatings deposited by open air atmospheric pressure plasma jet with aerosolized TTIP precursor. J. Phys. D Appl. Phys. 2014, 47, 265301. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference ∆E—A survey. J. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Ozen, E.; Yeniocak, M.; Colak, M.; Koca, I. Colourability of wood material with punica granatum and morus nigra extracts. Bioresources 2014, 9, 2797–2807. [Google Scholar] [CrossRef][Green Version]
- Sun, Q.; Lu, Y.; Zhang, H.; Zhao, H.; Yu, H.; Xu, J.; Fu, Y.; Yang, D.; Liu, Y. Hydrothermal fabrication of rutile TiO2 submicrospheres on wood surface: An efficient method to prepare UV-protective wood. Mater. Chem. Phys. 2012, 133, 253–258. [Google Scholar] [CrossRef]
- Pavel, P.; Aljaž, V.; Marko, P.; Andrijana, S.; Mohor, M.; Angela, S.; Urban, N.; Boris, O. Structural studies of TiO2/wood coatings prepared by hydrothermal deposition of rutile particles from TiCl4 aqueous solutions on spruce (Picea Abies) wood. Appl. Surf. Sci. 2016, 372, 125–138. [Google Scholar]
- Santos, L.; Carone, L.P.; Einloft, O.; Ligabue, R.A. Preparation and properties of aromatic polyester/TiO2 nanocomposites from polyethylene terephthalate. Mat. Res. 2016, 19, 158–166. [Google Scholar] [CrossRef][Green Version]
- Sirohi, S.; Singh, R.; Jain, N.; Pani, B.; Dutt, K.; Nain, R. Synthesis and characterization of multifunctional ZnO/polyester green composite films. J. Polym. Res. 2017, 24, 193. [Google Scholar] [CrossRef]
- George, G.A. High resolution XPS of organic polymers—The scienta ESCA 300 data base. Polym. Int. 1994, 33, 439–440. [Google Scholar] [CrossRef]
- Optics, Photonics-Optical Coatings, Part 4: Specific Test Methods; ISO 9211-4; International Organization for Standardization; ISO: Geneva, Switzerland, 2012.
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]






| Sample | Deposition Technique | Working Distance (mm) | Powder Feed Speed (mm/h) | TTIP Carrier Gas N2 (L/min) |
|---|---|---|---|---|
| Polyester/TiO2 thick film | PSP + LPPS | 24 | 150 | 14 |
| Polyester/TiO2 thin film | PSP + LPPS | 24 | 50 | 17 |
| Polyester/TiO2 film | PSP | 20 | 50 | - |
| Polyester thick film | PSP | 24 | 150 | - |
| Polyester thin film | PSP | 24 | 50 | - |
| TiO2 film | LPPS | 24 | - | 14 |
| Sample | Deposition Technique | Powder Feed Rates (mm/h) | Thickness (µm) |
|---|---|---|---|
| Polyester thick film | PSP | 150 | 114 |
| Polyester/TiO2 thick film | PSP + LPPS | 150 | 112 |
| Polyester thin film | PSP | 50 | 36 |
| Polyester/TiO2 thin film | PSP + LPPS | 50 | 22 |
| Polyester/TiO2 film | PSP | 50 | 14 |
| TiO2 film | LPPS | - | 0.35 |
| Sample | Surface Composition (at.%) | |||
|---|---|---|---|---|
| C | O | Ti | N | |
| Uncoated wood | 69.2 | 30.8 | - | - |
| Polyester thick film | 62.60 | 33.98 | - | 3.42 |
| Polyester/TiO2 thick film | 49.85 | 39.96 | 7.26 | 2.93 |
| Polyester/TiO2 thin film | 48.45 | 41.78 | 9.53 | 0.24 |
| Polyester/TiO2 film via (PSP) | 48.69 | 40.53 | 7.57 | 3.21 |
| Species | Sample | ∆L* | ∆a* | ∆b* | ∆E* |
|---|---|---|---|---|---|
| Beech | Control | −3.36 | 2.13 | 8.15 | 9.49 |
| Polyester/TiO2 thick film | −2.49 | 0.71 | 1.02 | 2.82 | |
| Polyester/TiO2 thin film | −2.57 | 1.96 | 1.81 | 3.76 | |
| Polyester/TiO2 film via (PSP) | −2.01 | 0.75 | 0.7 | 2.62 | |
| Pine | Control | −3.66 | 1.76 | 12.83 | 13.36 |
| Polyester/TiO2 thick film | −0.48 | −0.24 | 1.76 | 1.85 | |
| Polyester/TiO2 thin film | −2.72 | 1.22 | 4.66 | 7.76 | |
| Polyester/TiO2 film via (PSP) | −1.45 | 1.10 | 3.90 | 3.86 |
| Sample | Roughness before Abrasion Sa (µm) | Roughness after Abrasion Sa (µm) |
|---|---|---|
| Polyester thick film | 18.83 | 10.34 |
| Polyester thin film | 21.39 | 5.08 |
| Polyester/ TiO2 thick film | 40.09 | 8.50 |
| Polyester/ TiO2 thin film | 8.98 | 5.26 |
| Polyester/ TiO2 film via (PSP) | 8.06 | 3.25 |
| TiO2 film | 5.29 | 3.14 |
| Samples | Contact Angle (°) before Abrasion | Contact Angle (°) after Abrasion |
|---|---|---|
| Uncoated wood | 45 ± 3 | - |
| Polyester thick film | 55 ± 2 | 73 ± 3 |
| TiO2 film | >160 | 45 ± 4 |
| Polyester/TiO2 thick film | >170 | 102 ± 2 |
| Polyester/TiO2 thin film | >170 | 81 ± 1 |
| Polyester/TiO2 film via (PSP) | 65 ± 4 | 80 ± 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jnido, G.; Ohms, G.; Viöl, W. One-Step Deposition of Polyester/TiO2 Coatings by Atmospheric Pressure Plasma Jet on Wood Surfaces for UV and Moisture Protection. Coatings 2020, 10, 184. https://doi.org/10.3390/coatings10020184
Jnido G, Ohms G, Viöl W. One-Step Deposition of Polyester/TiO2 Coatings by Atmospheric Pressure Plasma Jet on Wood Surfaces for UV and Moisture Protection. Coatings. 2020; 10(2):184. https://doi.org/10.3390/coatings10020184
Chicago/Turabian StyleJnido, Ghiath, Gisela Ohms, and Wolfgang Viöl. 2020. "One-Step Deposition of Polyester/TiO2 Coatings by Atmospheric Pressure Plasma Jet on Wood Surfaces for UV and Moisture Protection" Coatings 10, no. 2: 184. https://doi.org/10.3390/coatings10020184
APA StyleJnido, G., Ohms, G., & Viöl, W. (2020). One-Step Deposition of Polyester/TiO2 Coatings by Atmospheric Pressure Plasma Jet on Wood Surfaces for UV and Moisture Protection. Coatings, 10(2), 184. https://doi.org/10.3390/coatings10020184