Titanium Dioxide Coatings Doubly-Doped with Ca and Ag Ions as Corrosion Resistant, Biocompatible, and Bioactive Materials for Medical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples’ Preparation
2.2. Surface Characterization
2.3. Corrosion Tests
2.4. Immersion Tests
2.5. Wettability
2.6. Biological Evaluation (Cell Viability Assays)
3. Results and Discussion
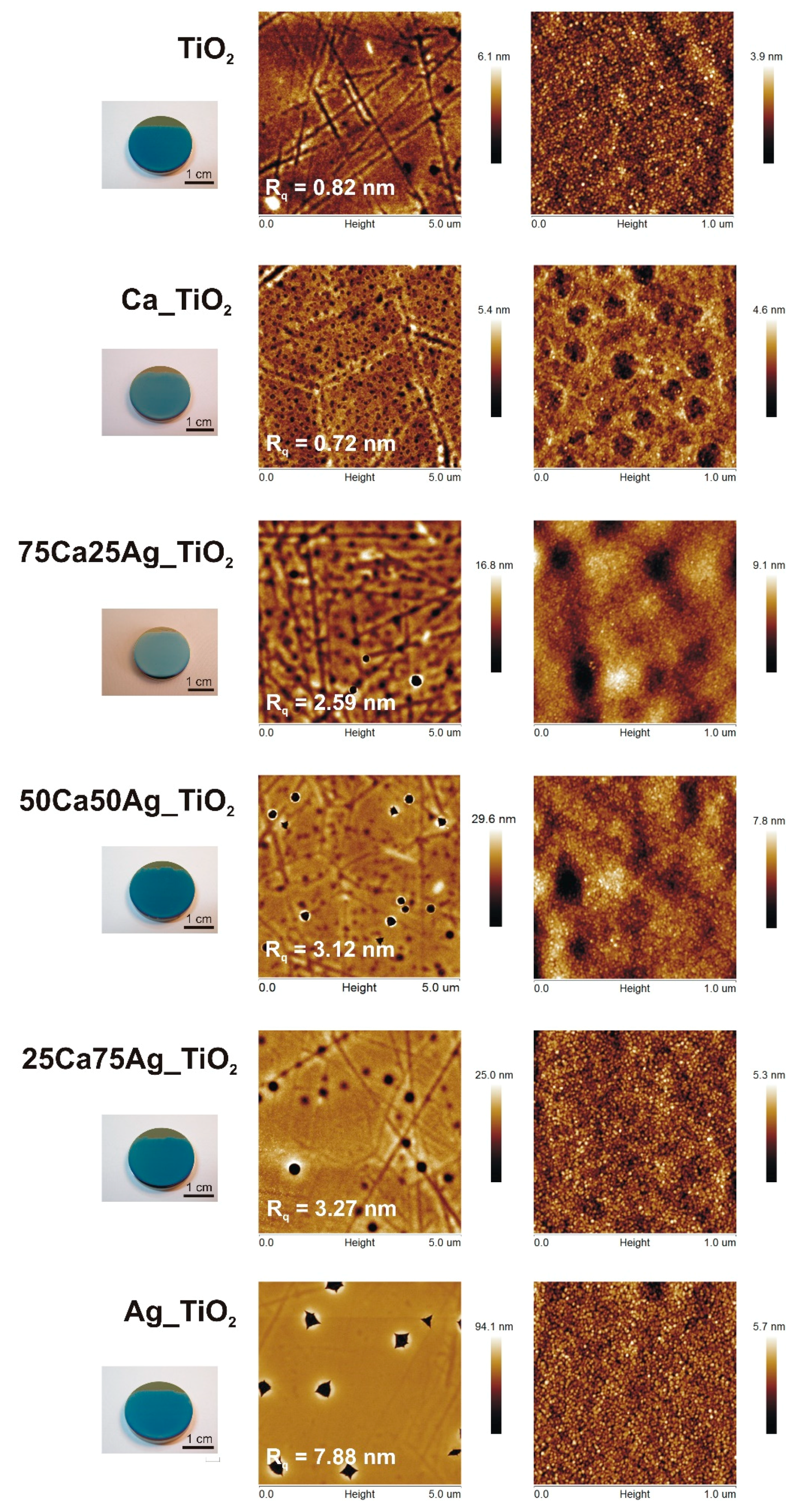
3.1. Surface Characterization
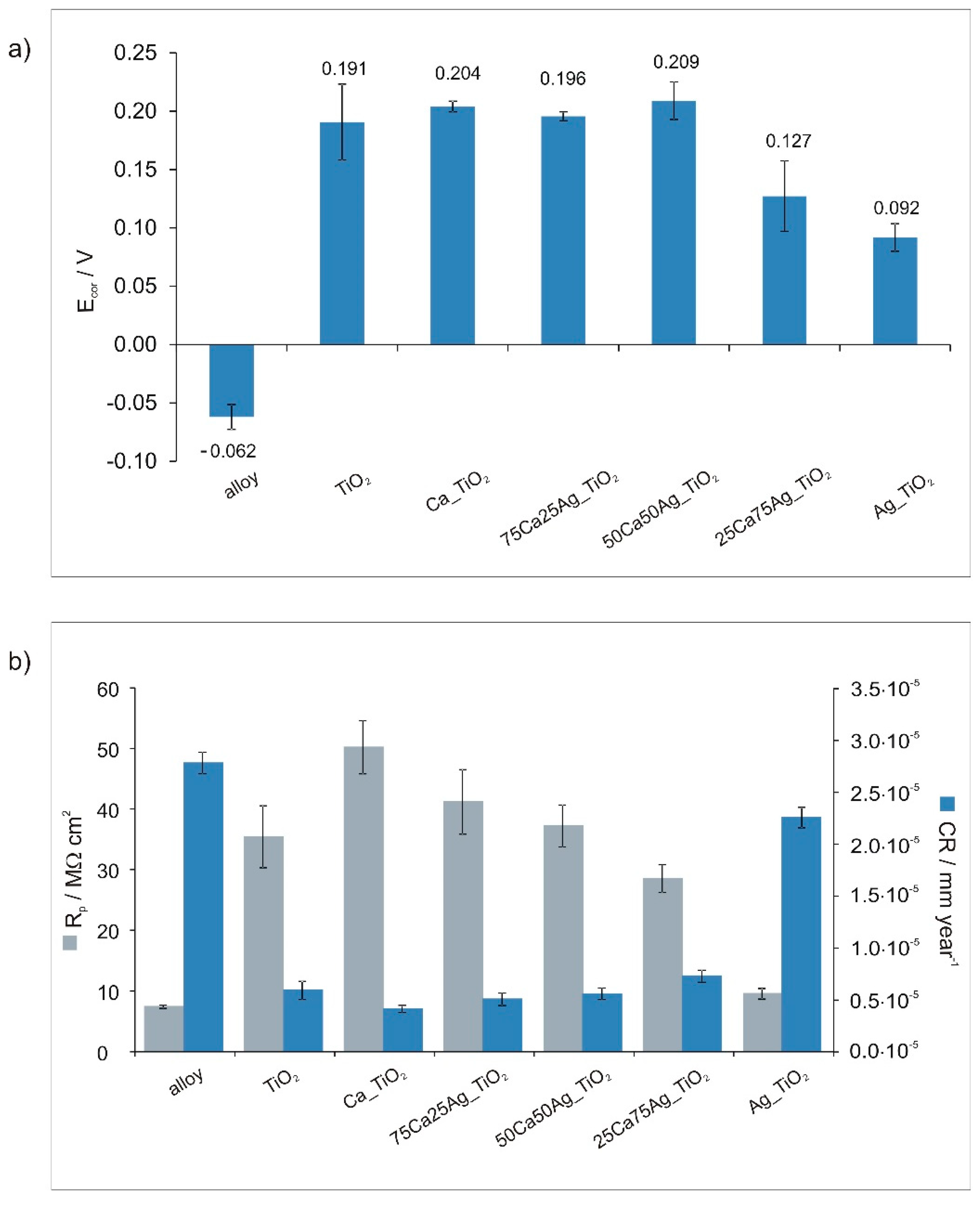
3.2. Corrosion Tests
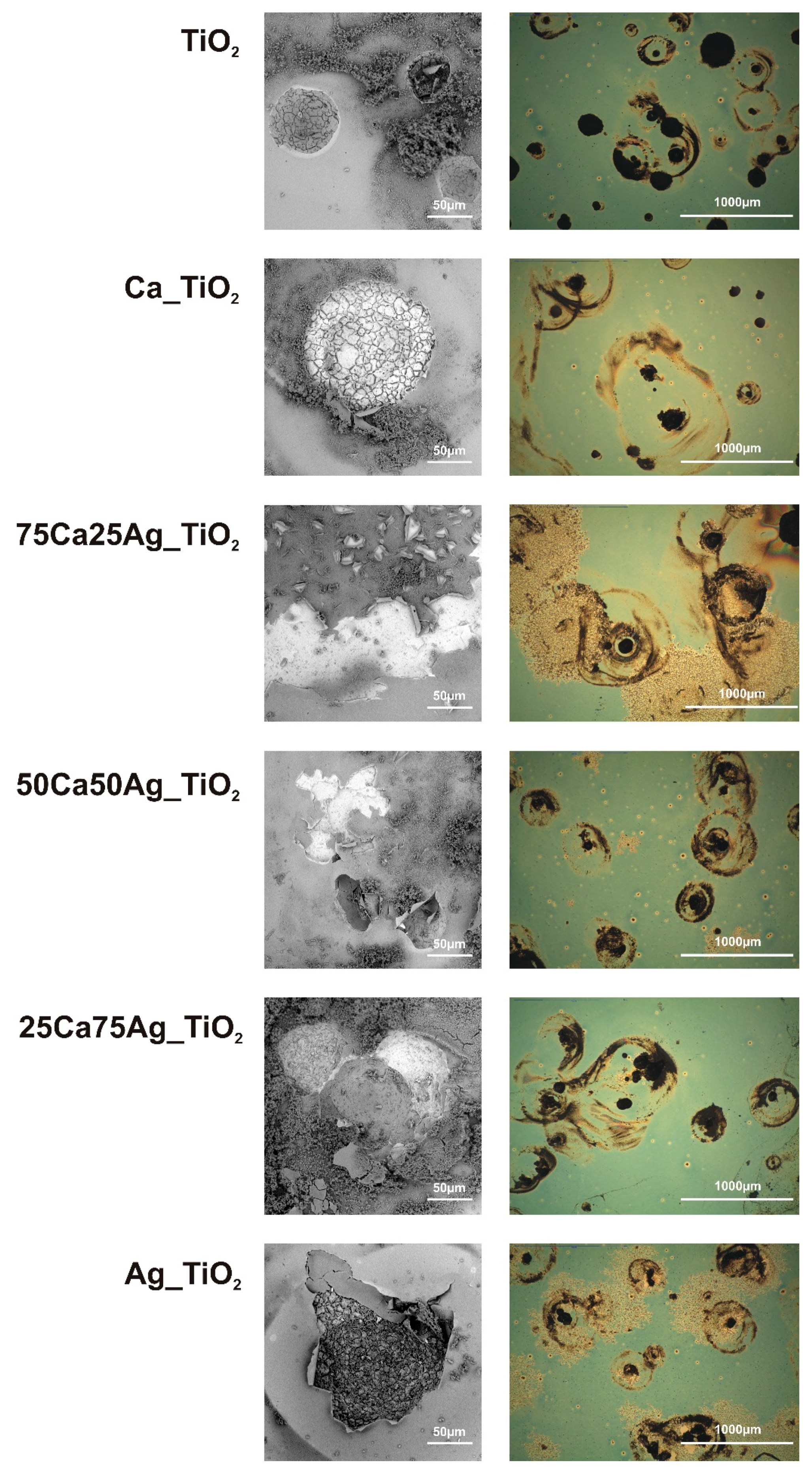
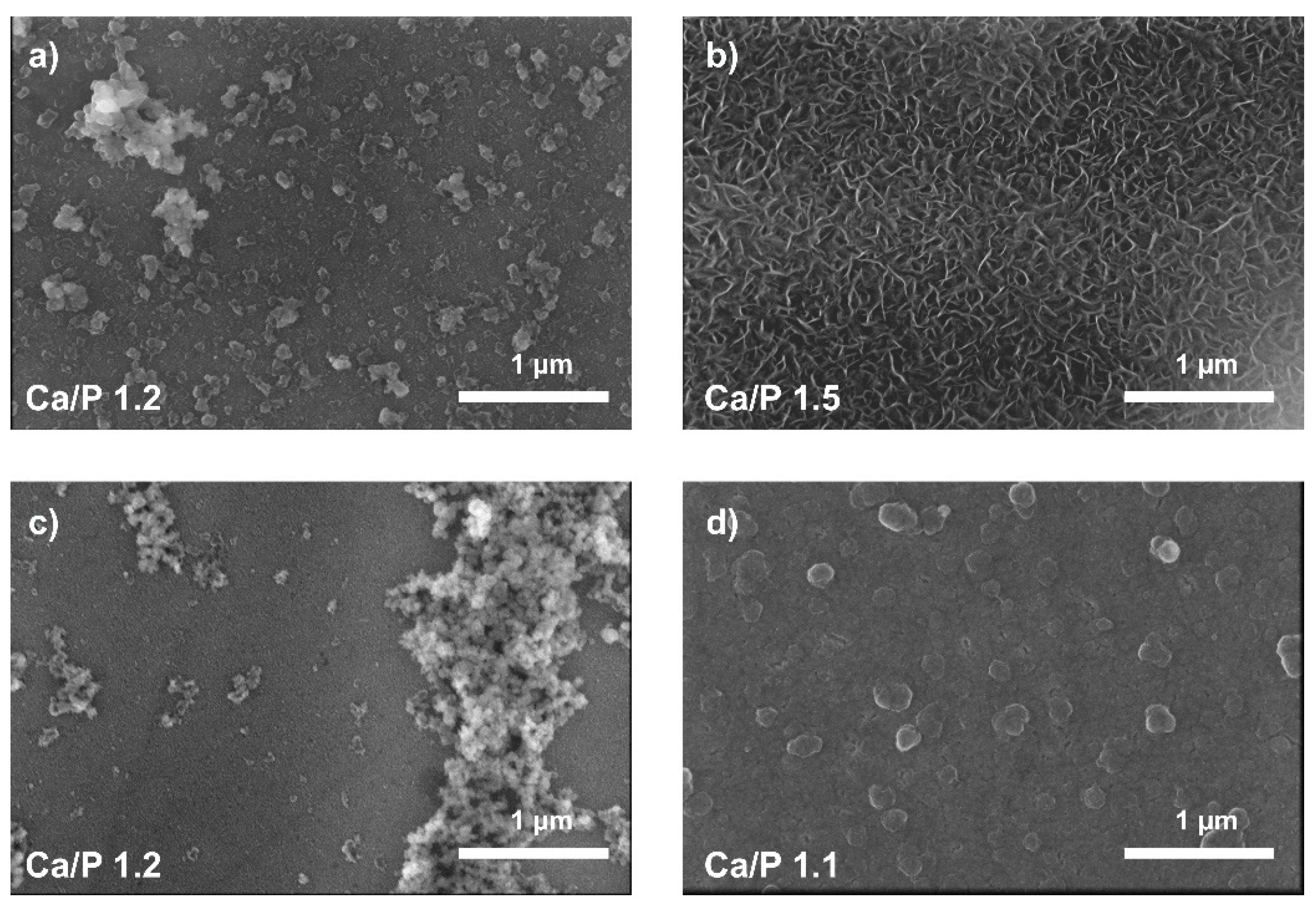
3.3. Immersion Test
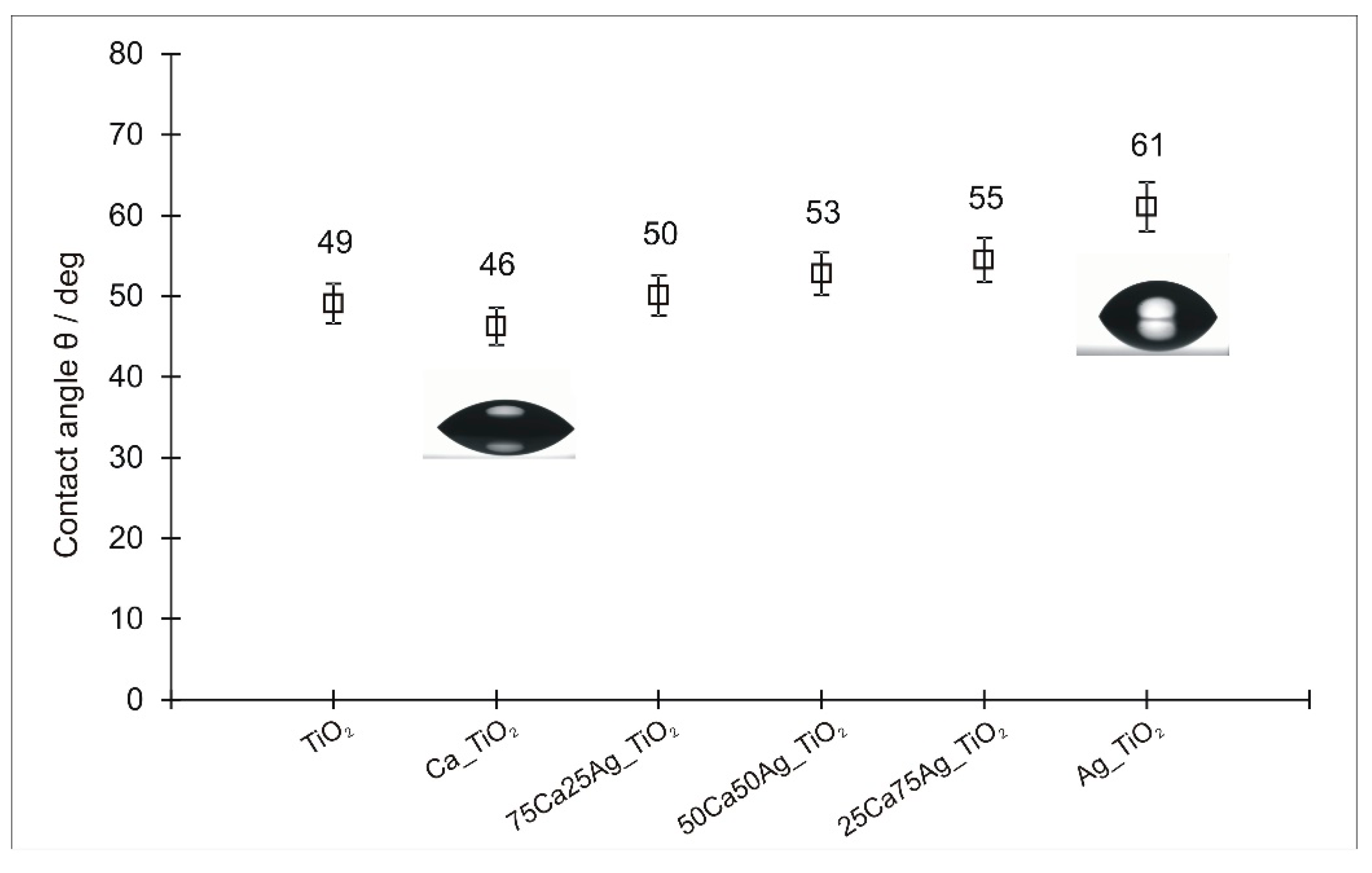
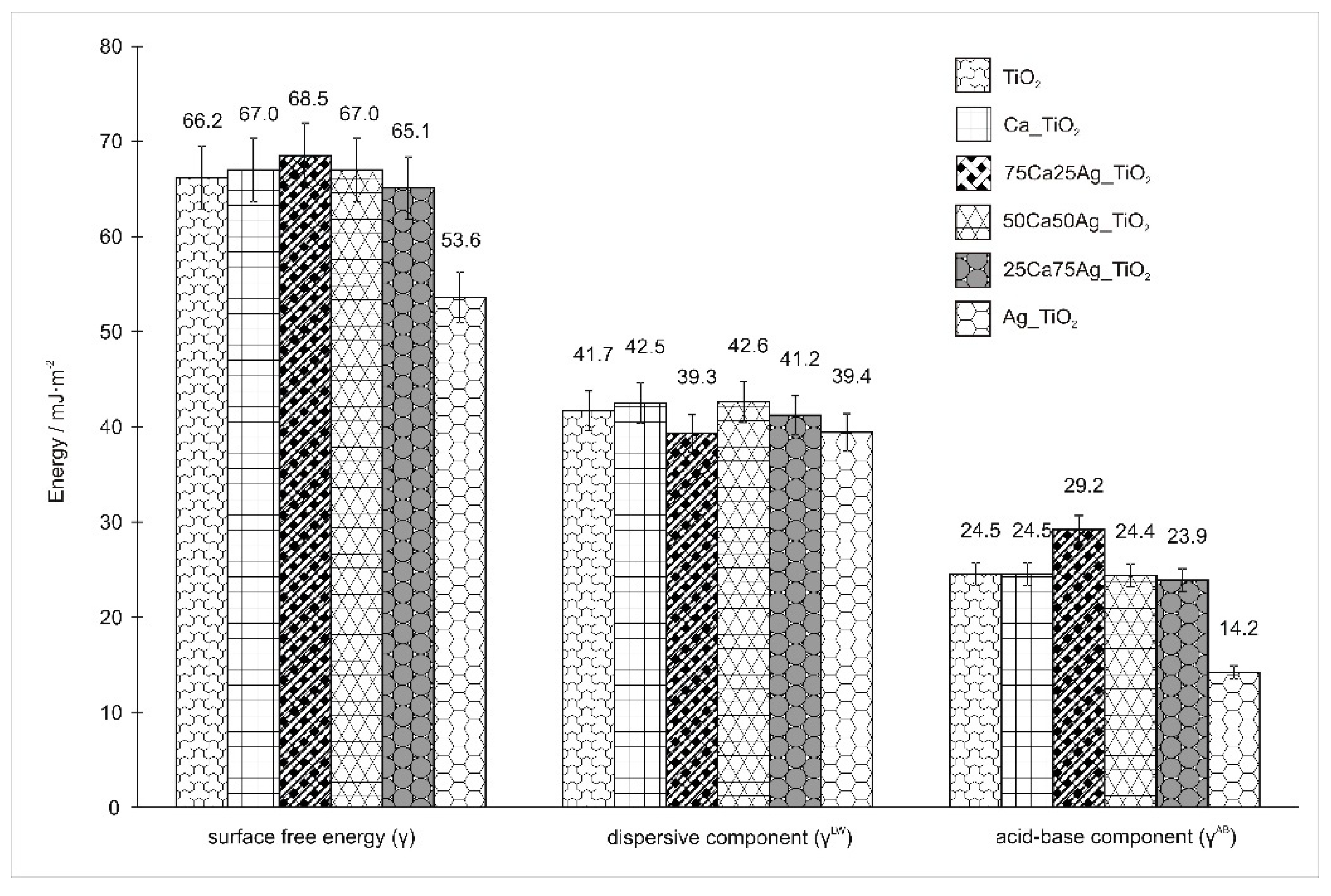
3.4. Wettability
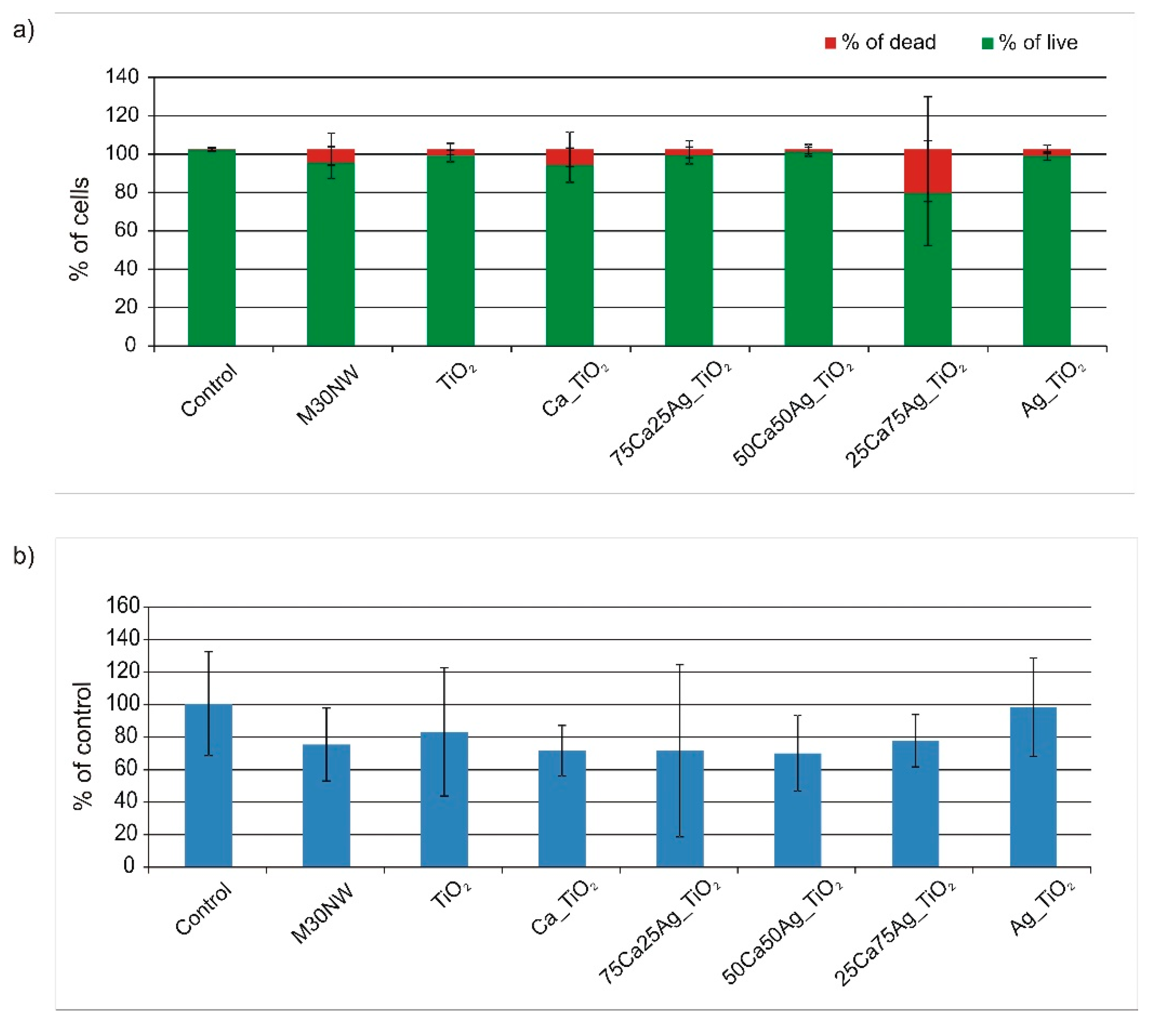
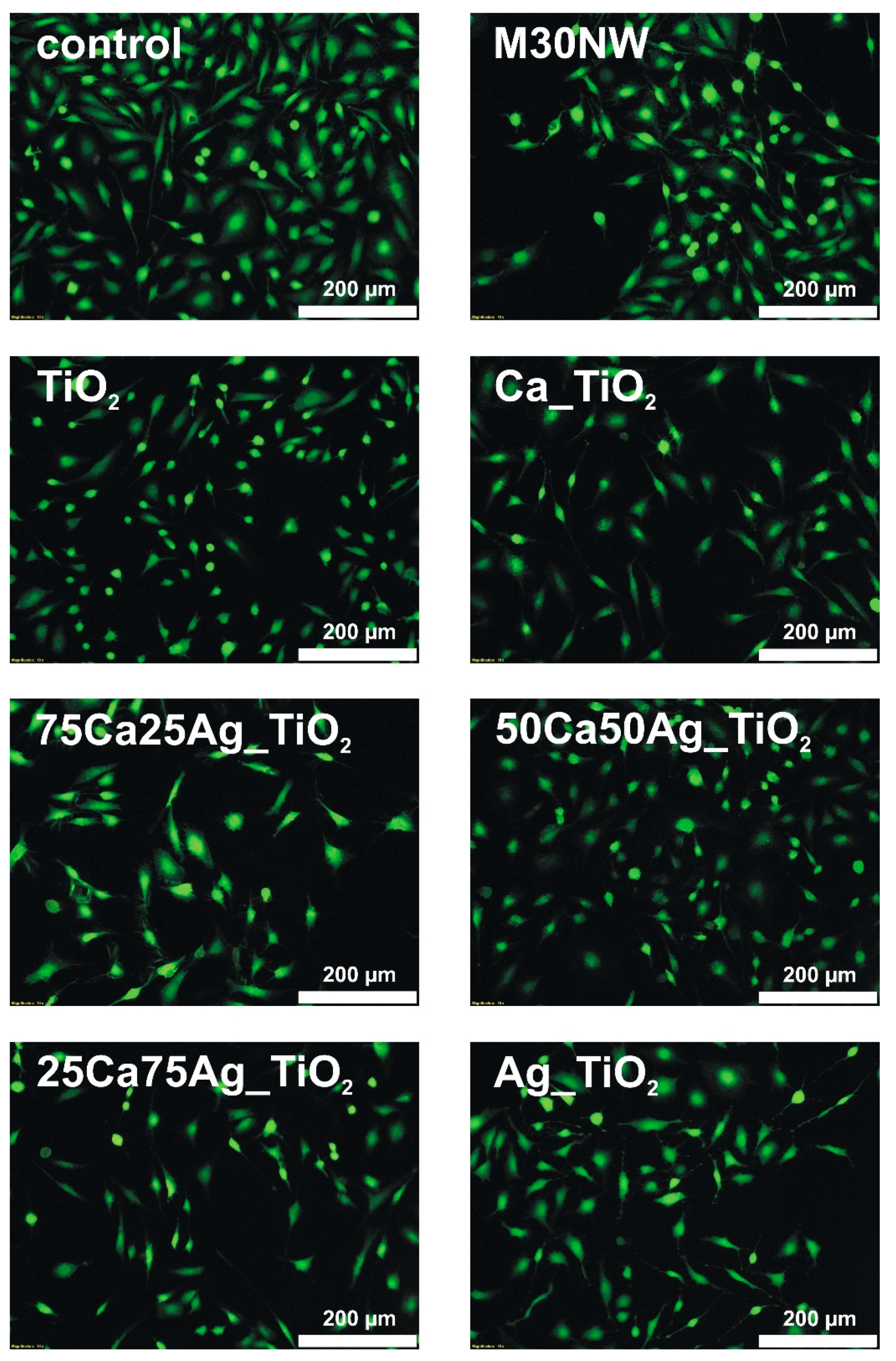
3.5. Biological Evaluation: Cell Viability and Proliferation Ability Assays
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fu, T.; Wen, C.S.; Lu, J.; Zhou, Y.M.; Ma, S.G.; Dong, B.H.; Liu, B.G. Sol-gel derived TiO2 coating on plasma nitrided 316L stainless steel. Vacuum 2012, 86, 1402–1407. [Google Scholar] [CrossRef]
- Velten, D.; Biehl, V.; Aubertin, F.; Valeske, B.; Possart, W.; Breme, J. Preparation of TiO2 layers on cp-Ti and Ti6Al4V by thermal and anodic oxidation and by sol gel coating techniques and their characterization. J. Biomed. Mater. Res. 2002, 59, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, J.; Lv, K.; Zhang, W.; Ding, X.; Yang, G.; Liu, X.; Jiang, X. Surface thermal oxidation on titanium implants to enhance osteogenic activity and in vivo osseointegration. Sci. Rep. 2016, 6, 31769. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, D.; Mazur, M.; Indyka, J.; Jurkowska, A.; Kalisz, M.; Domanowski, P.; Kaczmarek, D.; Domaradzki, J. Mechanical and structural properties of titanium dioxide deposited by innovative magnetron sputtering process. Mater. Sci. Pol. 2015, 33, 660–668. [Google Scholar] [CrossRef]
- Dreesen, L.; Cecchet, F.; Lucas, S. DC magnetron sputtering deposition of titanium oxide nanoparticles: Influence of temperature, pressure and deposition time on the deposited layer morphology, the wetting and optical surface properties. Plasma Process. Polym. 2009, 6, S849–S854. [Google Scholar] [CrossRef]
- Altmayer, J.; Barth, S.; Mathur, S. Influence of precursor chemistry on CVD grown TiO2 coatings: Differential cell growth and biocompatibility. RSC Adv. 2013, 3, 11234–11239. [Google Scholar] [CrossRef]
- Sobczyk-Guzenda, A.; Pietrzyk, B.; Szymanowski, H.; Gazicki-Lipman, M.; Jakubowski, W. Photocatalytic activity of thin TiO2 films deposited using sol–gel and plasma enhanced chemical vapor deposition methods. Ceram. Int. 2013, 39, 2787–2794. [Google Scholar] [CrossRef]
- Sobczyk-Guzenda, A.; Owczarek, S.; Fijalkowski, M.; Batory, D.; Gazicki-Lipman, M. Morphology, structure and photowettability of TiO2 coatings doped with copper and fluorine. Ceram. Int. 2018, 44, 5076–5085. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. Pure anatase phase titanium dioxide films prepared by mist chemical vapor deposition. Nanomaterials 2018, 8, 827. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and characterization of TiO2 thin films by sol-gel method. J. Sol-Gel Sci. Technol. 2002, 25, 137–145. [Google Scholar] [CrossRef]
- Piwoński, I. Preparation method and some tribological properties of porous titanium dioxide layers. Thin Solid Film. 2007, 515, 3499–3506. [Google Scholar] [CrossRef]
- Burnat, B.; Dercz, G.; Błaszczyk, T. Structural analysis and corrosion studies on an ISO 5832-9 biomedical alloy with TiO2 sol-gel layers. J. Mater. Sci.: Mater. Med. 2014, 25, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.M.; Mahapatra, C.; Kim, H.W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Carrera-Figueiras, C.; Pérez-Padilla, Y.; Estrella-Gutiérrez, M.A.; Uc-Cayetano, E.G.; Juárez-Moreno, J.A.; Avila-Ortega, A. Surface science engineering through sol-gel process. Appl. Surf. Sci. 2019. [Google Scholar] [CrossRef]
- Viitala, R.; Jokinen, M.; Peltola, T.; Gunnelius, K.; Rosenholm, J.B. Surface properties of in vitro bioactive and non-bioactive sol–gel derived materials. Biomaterials 2002, 23, 3073–3086. [Google Scholar] [CrossRef]
- Park, J.W.; Park, K.B.; Suh, J.Y. Effects of calcium ion incorporation on bone healing of Ti6Al4V alloy implants in rabbit tibiae. Biomaterials 2007, 28, 3306–3313. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Jang, J.H. Surface characteristics and in vitro biocompatibility of a manganese-containing titanium oxide surface. Appl. Surf. Sci. 2015, 258, 977–985. [Google Scholar] [CrossRef]
- Park, J.W. Increased bone apposition on a titanium oxide surface incorporating phosphate and strontium. Clin. Oral Implant. Res. 2011, 22, 230–234. [Google Scholar] [CrossRef]
- Anne Pauline, S.; Kamachi Mudali, U.; Rajendran, N. Fabrication of nanoporous Sr incorporated TiO2 coating on 316L SS: Evaluation of bioactivity and corrosion protection. Mater. Chem. Phys. 2013, 142, 27–36. [Google Scholar] [CrossRef]
- Burnat, B.; Robak, J.; Batory, D.; Leniart, A.; Piwoński, I.; Skrzypek, S.; Brycht, M. Surface characterization, corrosion properties and bioactivity of Ca-doped TiO2 coatings for biomedical applications. Surf. Coat. Technol. 2015, 280, 291–300. [Google Scholar] [CrossRef]
- Burnat, B.; Robak, J.; Leniart, A.; Piwoński, I.; Batory, D. The effect of concentration and source of calcium ions on anticorrosion properties of Ca-doped TiO2 bioactive sol-gel coatings. Ceram. Int. 2017, 43, 13735–13742. [Google Scholar] [CrossRef]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.; Albouy, P.A.; Ayral, A.; Basa, P.; Csik, G.; Nagy, N.; Roualdes, S.; Rouessac, V.; Safran, G.; Suhajda, A.; et al. Antibacterial properties of Ag–TiO2 composite sol–gel coatings. RSC Adv. 2015, 5, 59070–59081. [Google Scholar] [CrossRef]
- Cotolan, N.; Rak, M.; Bele, M.; Cör, A.; Muresan, L.M.; Milošev, I. Sol-gel synthesis, characterization and properties of TiO2 and Ag-TiO2 coatings on titanium substrate. Surf. Coat. Technol. 2016, 307, 790–799. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Haenle, M.; Fritsche, A.; Zietz, C.; Bader, R.; Heidenau, F.; Mittelmeier, W.; Gollwitzer, H. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: In vitro effectiveness against MRSA and mechanical properties. J. Mater. Sci. Mater. Med. 2011, 22, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, X.; Yang, H. Modification of the antibacterial activity of Zn/TiO2 nano-materials through different anions doped. Vacuum 2014, 101, 193–199. [Google Scholar] [CrossRef]
- Pérez-Jorge Peremarch, C.; Perez Tanoira, R.; Arenas, M.A.; Matykina, E.; Conde, A.; De Damborenea, J.J.; Barrena, E.G.; Esteban, J. Bacterial adherence to anodized titanium alloy. J. Phys. Conf. Ser. 2010, 252, 012011. [Google Scholar] [CrossRef]
- Pérez-Jorge, C.; Conde, A.; Arenas, M.A.; Pérez-Tanoira, R.; Matykina, E.; de Damborenea, J.J.; Gómez-Barrena, E.; Esteban, J. In vitro assessment of Staphylococcus epidermidis and Staphylococcus aureus adhesion on TiO2 nanotubes on Ti–6Al–4V alloy. J. Biomed. Mater. Res. A 2012, 100, 1696–1705. [Google Scholar] [CrossRef]
- Arenas, M.A.; Pérez-Jorge, C.; Conde, A.; Matykina, E.; Hernández-López, J.M.; Pérez-Tanoira, R.; de Damborenea, J.J.; Gómez-Barrena, E.; Esteba, J. Doped TiO2 anodic layers of enhanced antibacterial properties. Colloids Surf. 2013, 105, 106–112. [Google Scholar] [CrossRef]
- Choi, J.; Park, H.; Hoffmann, M.R. Combinatorial doping of TiO2 with platinum (Pt), chromium (Cr), vanadium (V), and nickel (Ni) to achieve enhanced photocatalytic activity with visible light irradiation. J. Mater. Res. 2010, 25, 149–158. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, L. Multifunction Sr, Co and F co-doped microporous coating on titanium of antibacterial, angiogenic and osteogenic activities. Sci. Rep. 2016, 6, 29069. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; Zhang, H.; Qiao, H.; Zhang, X.; Jia, T.; Han, S.; Gao, Y.; Xiao, H.; Yang, H. Fabrication of silver-and strontium-doped hydroxyapatite/TiO2 nanotube bilayer coatings for enhancing bactericidal effect and osteoinductivity. Ceram. Int. 2017, 43, 992–1007. [Google Scholar] [CrossRef]
- Rajendran, A.; Vinoth, G.; Nivedhitha, J.; Iyer, K.M.; Pattanayak, D.K. Ca-Ag coexisting nano-structured titania layer on Ti metal surface with enhanced bioactivity, antibacterial and cell compatibility. Mater. Sci. Eng. 2019, 99, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Yang, M.; Lu, J.; Zhao, X.; Wang, G. Effect of Ca and Ag doping on structure, in-vitro mineralization, and anti-bacterial properties of TiO2 coatings. J. Chin. Ceram. Soc. 2019, 47, 692–700. [Google Scholar]
- Yetim, A.F.; Yildiz, F.; Alsaran, A.; Celik, A. Surface modification of 316L stainless steel with plasma nitriding. Kov. Mater. 2008, 46, 105–115. [Google Scholar]
- Junping, Y.; Wei, L.; Keya, S. Study of Ce-modified antibacterial 316L stainless steel. China Foundry 2012, 9, 307–312. [Google Scholar]
- Burnat, B.; Błaszczyk, T.; Leniart, A. Effects of serum proteins on corrosion behavior of ISO 5832-9 alloy modified by titania coatings. J. Solid State Electrochem. 2014, 18, 3111–3119. [Google Scholar] [CrossRef][Green Version]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 75th ed.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- García-Serrano, J.; Gómez-Hernández, E.; Ocampo-Fernández, M.; Pal, U. Effect of Ag doping on the crystallization and phase transition of TiO2 nanoparticles. Curr. Appl. Phys. 2009, 9, 1097–1105. [Google Scholar] [CrossRef]
- ASTM G102-89(2015)e1, Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM International: West Conshohocken, PA, USA, 2015.
- Zhang, X.; Li, M.; He, X.; Huang, X.; Hang, R.; Tang, B. Effects of silver concentrations on microstructure and properties of nanostructured titania films. Mater. Des. 2015, 65, 600–605. [Google Scholar] [CrossRef]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Zhang, W.; Du, K.; Yan, C.; Wang, F. Preparation and characterization of a novel Si-incorporated ceramic film on pure titanium by plasma electrolytic oxidation. Appl. Surf. Sci. 2008, 254, 5216–5223. [Google Scholar] [CrossRef]
- de Rooij, A. The Oxidation of Silver by Atomic Oxygen. ESA J. 1989, 13, 363–392. [Google Scholar]
- Bociaga, D.; Sobczyk-Guzenda, A.; Komorowski, P.; Balcerzak, J.; Jastrzebski, K.; Przybyszewska, K.; Kaczmarek, A. Surface characteristics and biological evaluation of Si-DLC coatings fabricated using magnetron sputtering method on Ti6Al7Nb substrate. Nanomaterials 2019, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.; Levi, D.S.; Mohanchandra, K.P.; Carman, G.P. Superhydrophilic surface treatment for thin film NiTi vascular applications. Mater. Sci. Eng. 2009, 29, 2436–2441. [Google Scholar] [CrossRef]
- Williams, D.F. Advances in Biomaterials; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Junkar, I. Chapter Two—Interaction of cells and platelets with biomaterial surfaces treated with gaseous plasma. In Advances in Biomembranes and Lipid Self-Assembly; Iglic, A., Kulkarni, C.H., Rappolt, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 23, pp. 25–59. [Google Scholar]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. Part A 2003, 66, 247–259. [Google Scholar] [CrossRef]
- Heynes, C.A.; Norde, W. Globular proteins at solid/liquid interfaces. Colloids Surf. B: Biointerfaces 1994, 2, 517–566. [Google Scholar] [CrossRef]
- Israelachvili, J.; Wennerstrom, H. Role of hydration and water structure in biological and colloidal interactions. Nature 1996, 379, 219–225. [Google Scholar] [CrossRef]
- Sit, P.S.; Marchant, R.E. Surface-dependent differences in fibrin assembly visualized by atomic force microscopy. Surf. Sci. 2001, 491, 421–432. [Google Scholar] [CrossRef]
- Kidoaki, S.; Matsuda, T. Mechanistic aspects of protein/material interactions probed by atomic force microscopy. Colloids Surf. B Biointerfaces 2002, 23, 153–163. [Google Scholar] [CrossRef]
- Xu, L.C.; Logan, B.E. Interaction forces between colloids and protein-coated surfaces measured using an atomic force microscope. Environ. Sci. Technol. 2005, 39, 3592–3600. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.C.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [PubMed]
- Ogwu, A.A.; Okpalugo, T.I.T.; Ali, N.; Maguire, P.D.; McLaughlin, J.A.D. Endothelial cell growth on silicon modified hydrogenated amorphous carbon thin films. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 105–113. [Google Scholar] [CrossRef]
- Kongdee, A.; Bechtold, T.; Teufel, L. Modification of cellulose fiber with silk sericin. J. Appl. Polym. Sci. 2005, 96, 1421–1428. [Google Scholar] [CrossRef]
- Liao, T.T.; Zhang, T.F.; Li, S.S.; Deng, Q.Y.; Wu, B.J.; Zhang, Y.Z.; Zhou, Y.J.; Guo, Y.B.; Leng, Y.X.; Huang, N. Biological responses of diamond-like carbon (DLC) films with different structures in biomedical application. Mater. Sci. Eng. 2016, 69, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Riehle, M.O.; Johnstone, H.; Affrossman, S.; Curtis, A.S.G. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials 2002, 23, 2945–2954. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, C. New depths in cell behaviour: Reactions of cells to nanotopography. Biochem. Soc. Symp. 1999, 65, 15–26. [Google Scholar]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 1996, 12, 463–518. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, J.S.; Park, K.S.; Khang, G.; Lee, Y.M.; Lee, H.B. Response of MG63 osteoblast-like cells onto polycarbonate membrane surfaces with different micropore sizes. Biomaterials 2004, 25, 4699–4707. [Google Scholar] [CrossRef]


| Coating | TiBut [mL] | EtOH [mL] | HNO3 [mL] | H2O [mL] | Ca(NO3)2 [mL] | AgNO3 [mL] | Ca/Ag Ratio |
|---|---|---|---|---|---|---|---|
| TiO2 | 7 | 20 | 0.64 | 0.5 | – | – | – |
| Ca_TiO2 | 7 | 20 | 0.64 | – | 0.500 | – | – |
| 75Ca25Ag_TiO2 | 7 | 20 | 0.64 | – | 0.375 | 0.125 | 3:1 |
| 50Ca50Ag_TiO2 | 7 | 20 | 0.64 | – | 0.250 | 0.250 | 1:1 |
| 25Ca75Ag_TiO2 | 7 | 20 | 0.64 | – | 0.125 | 0.375 | 1:3 |
| Ag_TiO2 | 7 | 20 | 0.64 | – | – | 0.500 | – |
| Coating | Thickness/nm |
|---|---|
| TiO2 | 77 ± 4 |
| Ca_TiO2 | 74 ± 4 |
| 75Ca25Ag_TiO2 | 80 ± 4 |
| 50Ca50Ag_TiO2 | 75 ± 4 |
| 25Ca75Ag_TiO2 | 77 ± 4 |
| Ag_TiO2 | 78 ± 4 |
| Coating | i0.2/nA·cm−2 | Eb/V |
|---|---|---|
| TiO2 | 3.3 ± 1.1 | 1.600 ± 0.013 |
| Ca_TiO2 | 3.0 ± 2.2 | 1.524 ± 0.029 |
| 75Ca25Ag_TiO2 | 3.8 ± 1.8 | 1.579 ± 0.024 |
| 50Ca50Ag_TiO2 | 3.2 ± 3.3 | 1.613 ± 0.003 |
| 25Ca75Ag_TiO2 | 6.2 ± 1.7 | 1.569 ± 0.054 |
| Ag_TiO2 | 22.9 ± 2.7 | 1.593 ± 0.021 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnat, B.; Olejarz, P.; Batory, D.; Cichomski, M.; Kaminska, M.; Bociaga, D. Titanium Dioxide Coatings Doubly-Doped with Ca and Ag Ions as Corrosion Resistant, Biocompatible, and Bioactive Materials for Medical Applications. Coatings 2020, 10, 169. https://doi.org/10.3390/coatings10020169
Burnat B, Olejarz P, Batory D, Cichomski M, Kaminska M, Bociaga D. Titanium Dioxide Coatings Doubly-Doped with Ca and Ag Ions as Corrosion Resistant, Biocompatible, and Bioactive Materials for Medical Applications. Coatings. 2020; 10(2):169. https://doi.org/10.3390/coatings10020169
Chicago/Turabian StyleBurnat, Barbara, Patrycja Olejarz, Damian Batory, Michal Cichomski, Marta Kaminska, and Dorota Bociaga. 2020. "Titanium Dioxide Coatings Doubly-Doped with Ca and Ag Ions as Corrosion Resistant, Biocompatible, and Bioactive Materials for Medical Applications" Coatings 10, no. 2: 169. https://doi.org/10.3390/coatings10020169
APA StyleBurnat, B., Olejarz, P., Batory, D., Cichomski, M., Kaminska, M., & Bociaga, D. (2020). Titanium Dioxide Coatings Doubly-Doped with Ca and Ag Ions as Corrosion Resistant, Biocompatible, and Bioactive Materials for Medical Applications. Coatings, 10(2), 169. https://doi.org/10.3390/coatings10020169







