Characterization of a C-Based Coating Applied on an AA6063 Alloy and Developed by a Novel Electrochemical Synthesis Route
Abstract
1. Introduction
2. Experimental Details
2.1. Electrochemical Synthesis of C-based Coatings
2.2. Structural and Microstructural Characterization Procedure
2.3. Fatigue Testing Procedure
- DT = 6.35 mm;
- DG = 2DT = 12.70 mm;
- RC = 8DT = 50.80 mm;
- LS = 16DT = 101.6 mm.
2.4. Polarization Resistance Testing Procedure
3. Results and Discussion
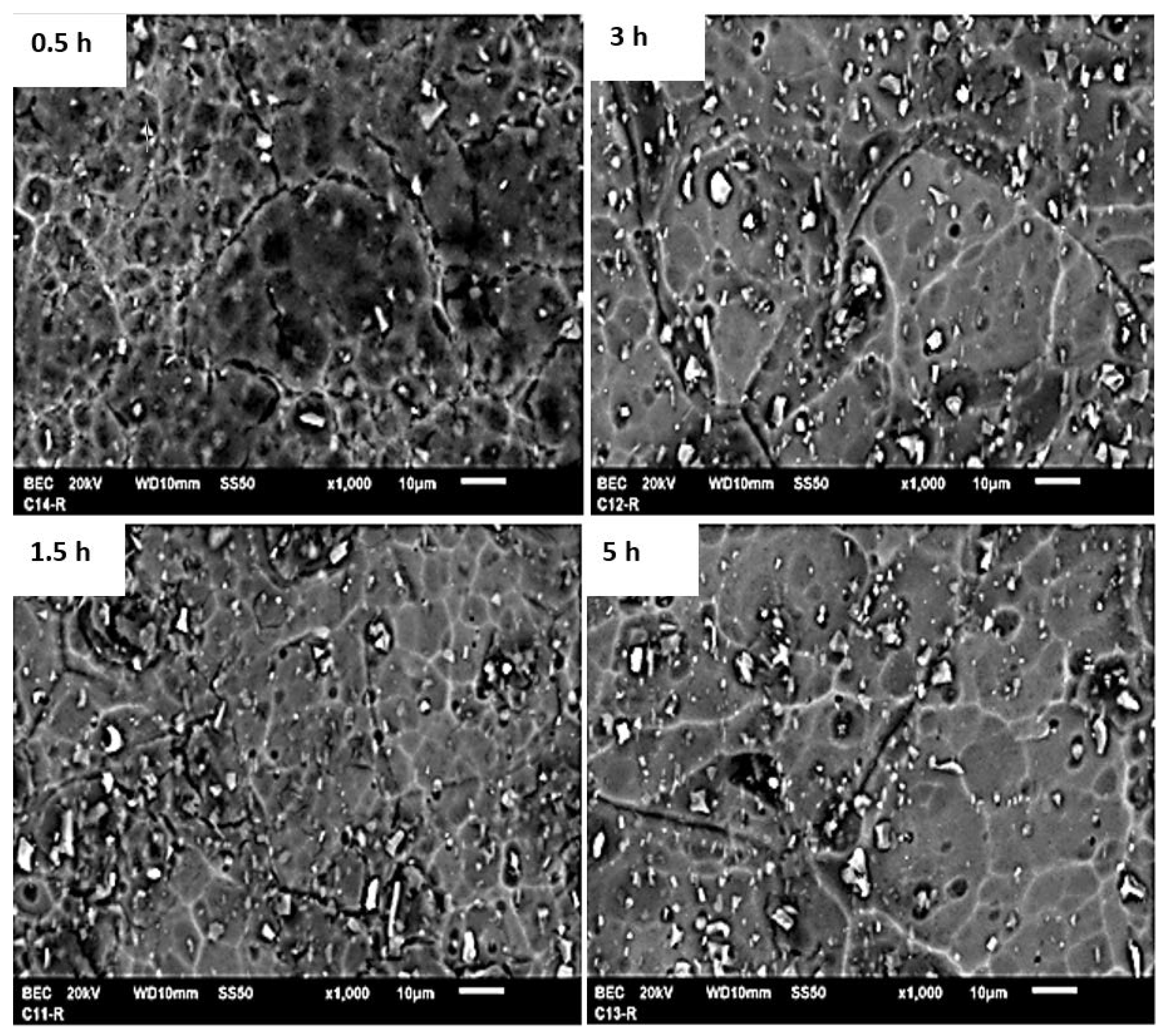
3.1. Structural and Microstructural Characterization Results
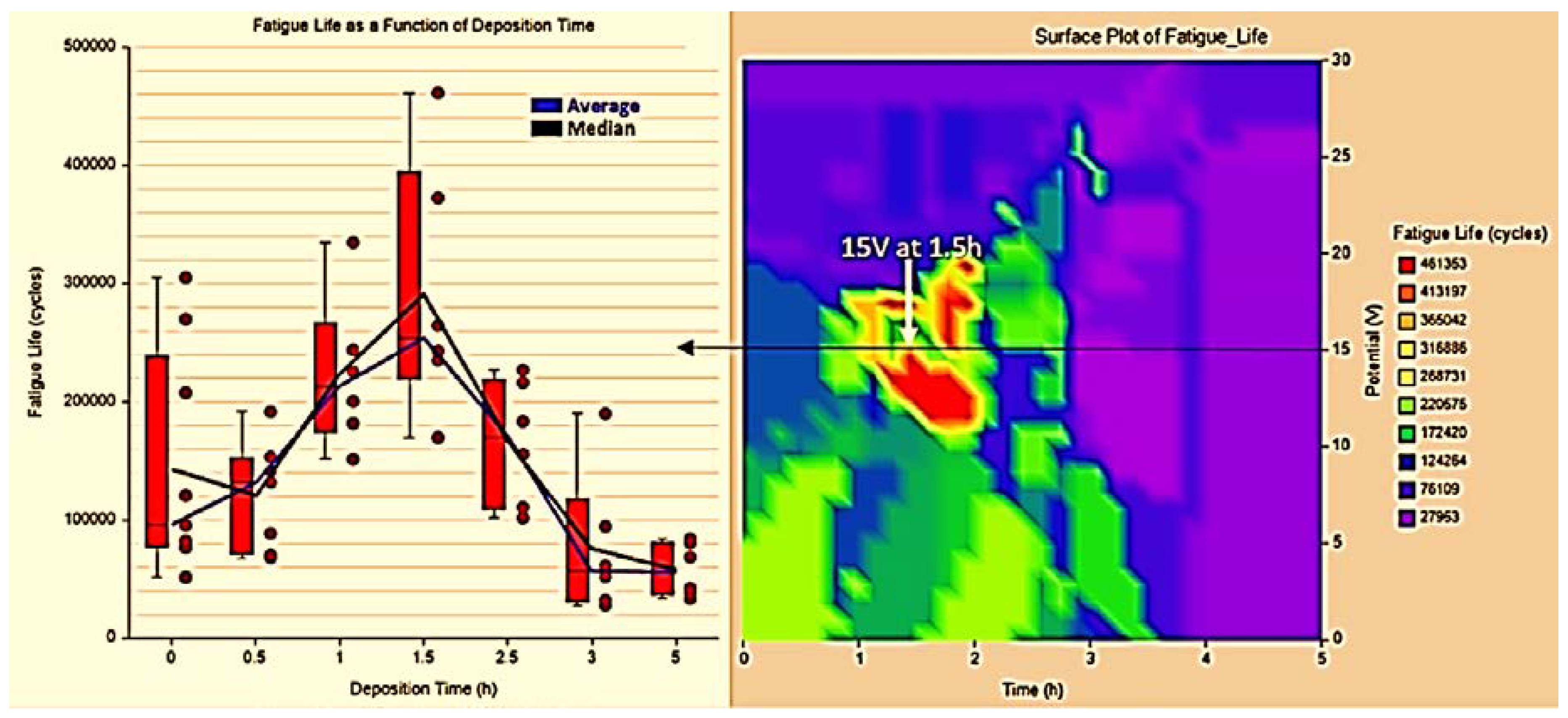
3.2. Fatigue Life Results
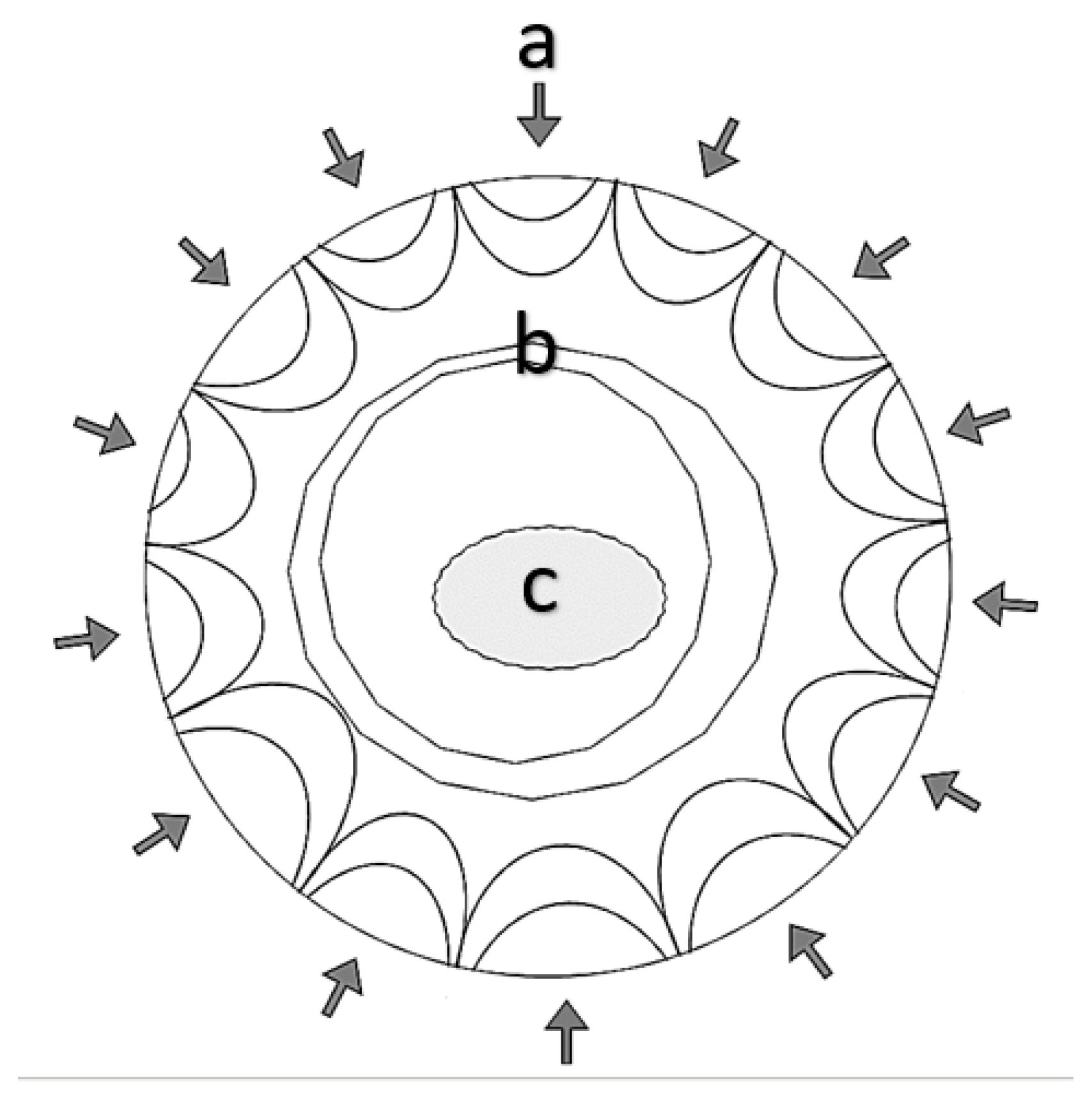
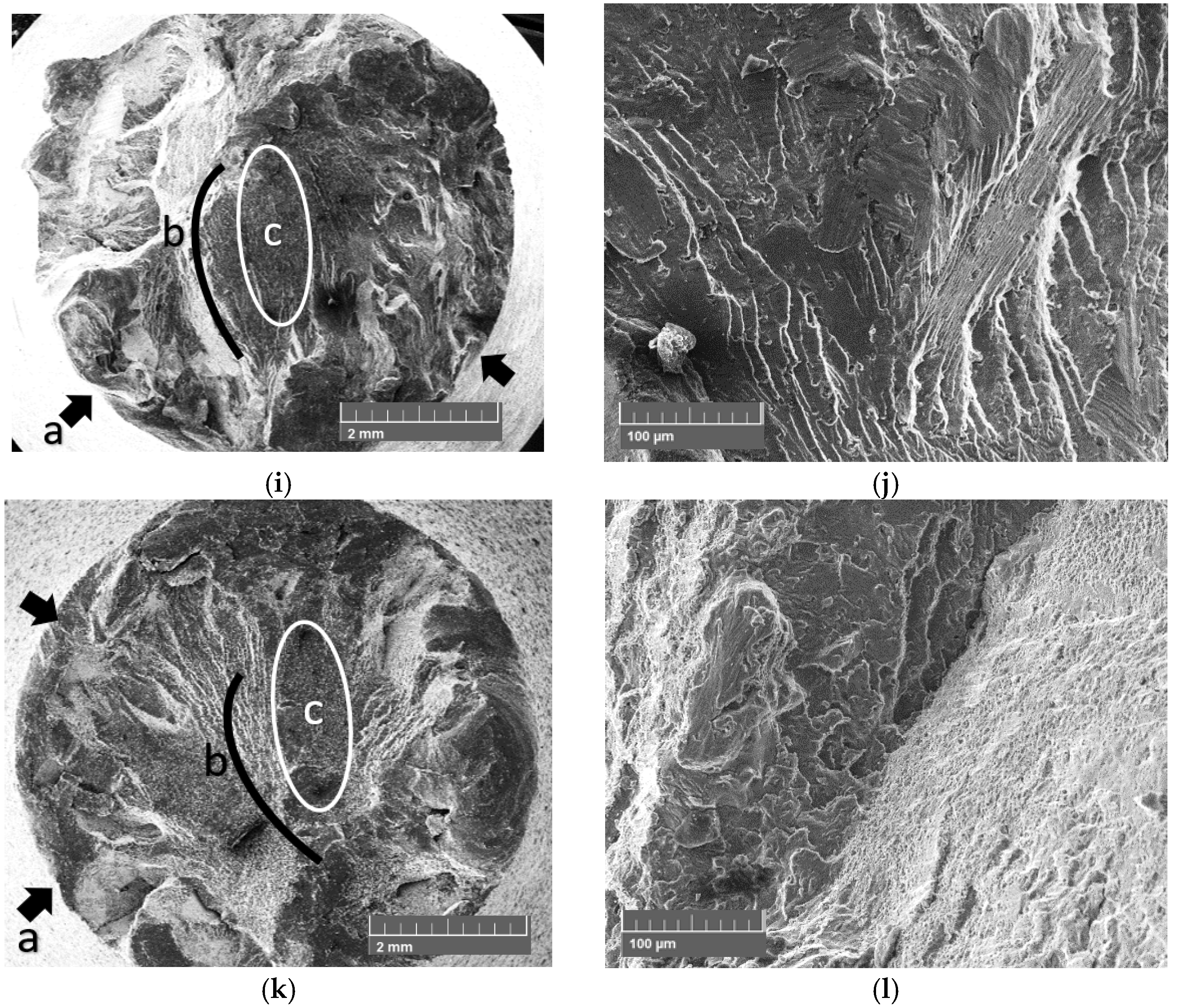
3.3. SEM Fracture Surface Characterization
3.4. Polarization Resistance Results
4. Conclusions
- The C-based coating obtained by this electrochemical route has not been previously reported in the literature;
- The obtained coating increases fatigue life by up to 100% compared with the uncoated alloy at a critical thickness of 3.9 µm and 20.5 µm for roughness;
- The fracture surface characterization reveals that the failure mechanism is the same for all samples, which indicates that the coating is a retardant of nucleation and propagation of short cracks until a critical thickness and roughness is achieved; after crossing those critical conditions, the experimental evidence suggest that the stress concentration increases due to roughness;
- Corrosion resistance is affected by the proposed C-based coating. Hence, a coating system composed of a C-based layer, primer, and topcoat must be designed and developed to overcome this drawback.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peralta, P.; Laird, C. Fatigue of Metals. In Physical Metallurgy, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 1765–1880. ISBN 9780444537713. [Google Scholar]
- Park, C.K.; Kim, D.G. Historical background. In Current and Future Management of Brain Metastasis; Springer: Dordrecht, The Netherlands, 2012; Volume 25, pp. 1–12. ISBN 9783805596183. [Google Scholar]
- Cui, W. A state-of-the-art review on fatigue life prediction methods for metal structures. J. Mar. Sci. Technol. 2002, 7, 43–56. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. Modern Electroplating, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9780470167786. [Google Scholar]
- Zhang, S.; Zhao, D. Aerospace Materials Handbook; Taylor & Francis: Boca Raton, FL, USA, 2013; ISBN 1439873305. [Google Scholar]
- Hirsch, J. Aluminium Alloys for Automotive Application. Mater. Sci. Forum 1997, 242, 33–50. [Google Scholar] [CrossRef]
- Giummarra, C.; Thomas, B.; Rioja, R. New Aluminum alloys for aerospace applications. In Proceedings of the Light Metals Technology Conference 2007, Saveur, QC, Canada, 24–26 September 2007. [Google Scholar]
- Wang, Y. A study of PVD coatings and die materials for extended die-casting die life. Surf. Coat. Technol. 1997, 94–95, 60–63. [Google Scholar] [CrossRef]
- Peter, I.; Rosso, M.; Gobber, F.S. Study of protective coatings for aluminum die casting molds. Appl. Surf. Sci. 2015, 358, 563–571. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Djokić, S. Electrodeposition and Surface Finishing; Springer: New York, NY, USA, 2014. [Google Scholar]
- Chen, K.-W.; Lin, J.-F. The study of adhesion and nanomechanical properties of DLC films deposited on tool steels. Thin Solid Films 2009, 517, 4916–4920. [Google Scholar] [CrossRef]
- Vanhulsel, A.; Velasco, F.; Jacobs, R.; Eersels, L.; Havermans, D.; Roberts, E.W.; Sherrington, I.; Anderson, M.J.; Gaillard, L. DLC solid lubricant coatings on ball bearings for space applications. Tribol. Int. 2007, 40, 1186–1194. [Google Scholar] [CrossRef]
- Lettington, A.H. Applications of diamond-like carbon thin films. Carbon N. Y. 1998, 36, 555–560. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon: State of the art. Diam. Relat. Mater. 2002, 8, 428–434. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Caschera, D.; Federici, F.; Kaciulis, S.; Pandolfi, L.; Cusmà, A.; Padeletti, G. Deposition of Ti-containing diamond-like carbon (DLC) films by PECVD technique. Mater. Sci. Eng. C 2007, 27, 1328–1330. [Google Scholar] [CrossRef]
- Li, R.S.; Zhou, M.; Pan, X.J.; Zhang, Z.X.; Lu, B.A.; Wang, T.; Xie, E.Q. Simultaneous deposition of diamondlike carbon films on both surfaces of aluminum substrate by electrochemical technique. J. Appl. Phys. 2009, 105, 1–4. [Google Scholar] [CrossRef]
- Roy, R.K.; Deb, B.; Bhattacharjee, B.; Pal, A.K. Synthesis of diamond-like carbon film by novel electrodeposition route. Thin Solid Films 2002, 422, 92–97. [Google Scholar] [CrossRef]
- Hassannejad, H.; Bogani, F.; Boniardi, M.; Casaroli, A.; Mele, C.; Bozzini, B. Electrodeposition of DLC films on carbon steel from acetic acid solutions. Trans. IMF 2014, 92, 183–188. [Google Scholar] [CrossRef]
- Manhabosco, T.M.; Muller, I.L. Electrodeposition of diamond-like carbon (DLC) films on Ti. Appl. Surf. Sci. 2009, 255, 4082–4086. [Google Scholar] [CrossRef]
- Ribeiro, H.; Cardoso, P.; Falcade, T.; Kunst, S.R. Corrosion and Wear Resistance of Carbon Films Obtained by Electrodeposition on Ferritic Stainless Steel. Mater. Res. 2015, 18, 292–297. [Google Scholar]
- Wang, H.; Shen, M.R.; Ning, Z.Y.; Ye, C.; Dang, H.Y.; Cao, C.B.; Zhu, H.S. Deposition of unhydrogenated diamond-like amorphous carbon films by electrolysis of organic solutions. Thin Solid Films 1997, 293, 87–90. [Google Scholar] [CrossRef]
- Jiu, J.T.; Li, L.P.; Cao, C.B.; Zhu, H.S. Deposition of diamond like carbon films by using liquid phase electrodeposition technique and its electron emission properties. J. Mater. Sci. 2001, 36, 5801–5804. [Google Scholar] [CrossRef]
- Sreejith, K.; Nuwad, J.; Pillai, C.G.S. Low voltage electrodeposition of diamond like carbon (DLC). Appl. Surf. Sci. 2005, 252, 296–302. [Google Scholar] [CrossRef]
- Gupta, S.; Pal Chowdhury, M.; Pal, A.K. Synthesis of DLC films by electrodeposition technique using formic acid as electrolyte. Diam. Relat. Mater. 2004, 13, 1680–1689. [Google Scholar] [CrossRef]
- Namba, Y. Attempt to grow diamond phase carbon films from an organic solution. J. Vac. Sci. Technol. A Vacuum Surf. Film. 1992, 10, 3368–3370. [Google Scholar] [CrossRef]
- Kulak, A.I.; Kokorin, A.I.; Meissner, D.; Ralchenko, V.G.; Vlasov, I.I.; Kondratyuk, A.V.; Kulak, T.I. Electrodeposition of nanostructured diamond-like films by oxidation of lithium acetylide. Electrochem. Commun. 2003, 5, 301–305. [Google Scholar] [CrossRef]
- Gupta, S.; Roy, R.K.; Deb, B.; Kundu, S.; Pal, A.K. Low voltage electrodeposition of diamond-like carbon films. Mater. Lett. 2003, 57, 3479–3485. [Google Scholar] [CrossRef]
- He, W.; Yu, R.; Wang, H.; Yan, H. Electrodeposition mechanism of hydrogen-free diamond-like carbon films from organic electrolytes. Carbon N. Y. 2005, 43, 2000–2006. [Google Scholar] [CrossRef]
- Mayen, J.; Gallegos-Melgar, A.; Abúndez, A.; Alcudia, E.; Colín, J.; Barredo, E. Yield Strength Improvement of an Al-6063 Alloy by Applying a Novel Combination of Cold Working and Heat Treatments for Die Casting of Plastic Parts. Trans. Indian Inst. Met. 2019, 72, 1211–1220. [Google Scholar] [CrossRef]
- Weibull, W. Fatigue Testing and Analysis of Results; Published for Advisory Group for Aeronautical Research and development, North Atlantic Treaty Organization; Pergamon Press: Oxford, UK, 1961; ISBN 9781483154169. [Google Scholar]
- Ferrari, A.C.; Robertson, J.; Trans, P.; Lond, R.S. Raman spectroscopy of amorphous, nanostructured, diamond—Like carbon, and nanodiamond Raman spectroscopy of amorphous. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 2, 2477–2512. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C. Determination of bonding in diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2002, 11, 1053–1061. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Characterization of graphite fiber surfaces with Raman spectroscopy. J. Compos. Mater. 1970, 4, 492–497. [Google Scholar] [CrossRef]
- Washer, G.; Blum, F. Raman spectroscopy for the nondestructive testing of carbon fiber. Adv. Mater. Sci. Eng. 2008, 2008, 2–5. [Google Scholar] [CrossRef]
- Pozegic, T.R.; Hamerton, I.; Anguita, J.V.; Tang, W.; Ballocchi, P.; Jenkins, P.; Silva, S.R.P. Low temperature growth of carbon nanotubes on carbon fibre to create a highly networked fuzzy fibre reinforced composite with superior electrical conductivity. Carbon N. Y. 2014, 74, 319–328. [Google Scholar] [CrossRef]
- Street, T. Raman spectroscopy of graphite. Philos. Trans. R. Soc. London Ser. A Math. Phys. Eng. Sci. 2004, 2271–2288. [Google Scholar]
- Okuda, H.; Young, R.J.; Wolverson, D.; Tanaka, F. Investigating nanostructures in carbon fi bres using Raman spectroscopy. Carbon N. Y. 2018, 130, 178–184. [Google Scholar] [CrossRef]
- Rahim, I.; Dresselhaus, M.S. Thermal conductivity and Raman spectra of carbon fibers. Phys. Rev. B 1985, 32, 6742–6747. [Google Scholar]
- Wang, H.; Shen, M.R.; Ning, Z.Y.; Ye, C.; Cao, C.B.; Dang, H.Y.; Zhu, H.S. Deposition of diamond-like carbon films by electrolysis of methanol solution. Appl. Phys. Lett. 1996, 69, 1074–1076. [Google Scholar] [CrossRef]
- Falcade, T.; Shmitzhaus, T.E.; Dos Reis, O.G.; Vargas, A.L.M.; Hübler, R.; Müller, I.L.; De Fraga Malfatti, C. Electrodeposition of diamond-like carbon films on titanium alloy using organic liquids: Corrosion and wear resistance. Appl. Surf. Sci. 2012, 263, 18–24. [Google Scholar] [CrossRef]
- Fatigue Fracture—An overview | ScienceDirect Topics. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014.
- Stern, M.; Geary, A.L. Electrochemical Polarization. J. Electrochem. Soc. 1957, 104, 559. [Google Scholar] [CrossRef]






| Substrate | Electrolyte (%) | Temperature | Anode | Distance | Time | Voltage/Current Density | Year |
|---|---|---|---|---|---|---|---|
| Silicon (100) | Pure methanol, ethanol, 2-propanol, acetone and tetrahydrofuran | 50 °C and 60 °C | - | 10 mm | - | 0 to 3000 V | 1997 [23] |
| Si (100) | Methanol (99.5) | Graphite | 4 mm | 1000 V–0.2 mA/mm2 | 2001 [24] | ||
| SnO2-coated glass | CH3COOH and deionized water (0.5 to 20) | 30 and 85 °C. | Graphite sheet | 7 mm | 1 h | 1000 V | 2002 [19] |
| Nickel of 99% purity and stainless steel | Oxidation of 4 M solution of lithium acetylide in dimethyl-sulfoxide | 25 °C | Current densities (0.2–2.0 mA/cm2) in the range of electrode potentials 0.3–2.5 V | 2003 [28] | |||
| SnO2-coated glass | CH3COOH and deionized water | 358 K. | Graphite sheet | 7 mm | 0.5 h | 0.8 mA/cm2 | 2003 [29] |
| SnO2-coated glass | From 0.5 to 10% v/v using formic acid and water as electrolyte | at 300 and 360 K. | Graphite | 7 mm | (0 –30 V; 1 A) f16 V/Am and 5.44 mA/m2 | 2004 [26] | |
| Si (100) | Methanol, ethanol, acetonitrile and DMF (Dimethylformamide) | 50 °C | Graphite | 10 mm | 5h | 1600 V | 2005 [30] |
| Pure titanium (Grade 1) | Acetonitrile and DMF | 25 °C | Graphite | 4 mm | 4h | 1200 V | 2009 [21] |
| A284 steel | CH3COOH (1 to 10%) | 25 °C | High-purity graphite plates | 4 mm | 1 to 5h | 8 to 20V | 2014 [20] |
| AISI 430 | DMF | 20 °C | 7 mm | 24 h | 1200 V | 2015 [22] | |
| Al6063-T6 | CH3COOH (1%) | 25 °C | Graphite | 10 mm | 0.5 h to 5 h | 2020 |
| Sample | Roughness (Profilometer) | Thickness (Profilometer) |
|---|---|---|
| 0.5 h | 1.2 µm | 4.4 µm |
| 1.5 h | 3.9 µm | 20.5 µm |
| 3 h | 4 µm | 30 µm |
| 5 h | 13.9 µm | 51 µm |
| Sample | Grain Size (µm) |
|---|---|
| 0.5 h | 76.4 |
| 1.5 h | 88.8 |
| 3 h | 91.6 |
| 5 h | 94 |
| Sample | Ecorr (V) | icorr (µA/cm2) | Rp (KΩ-cm2) | Np (%) |
|---|---|---|---|---|
| 0.5 h | −1.281 | 12.204 | 2.309 | 61 |
| 1.5 h | −1.360 | 1101.734 | 0.059 | 2 |
| 3 h | −1.224 | 89.978 | 0.449 | 12 |
| 5 h | −0.890 | 9.864 | 5.931 | 156 |
| Al6063 | −1.298 | 20.708 | 3.799 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-Melgar, A.; González-López, Y.; Abúndez, A.; Flores-Ruiz, F.J.; Díaz-Guillén, J.C.; Betancourt-Cantera, J.A.; Hernández-Hernández, M.; Trápaga-Martínez, G.; Poblano-Salas, C.A.; Acevedo-Dávila, J.L.; et al. Characterization of a C-Based Coating Applied on an AA6063 Alloy and Developed by a Novel Electrochemical Synthesis Route. Coatings 2020, 10, 145. https://doi.org/10.3390/coatings10020145
Gallegos-Melgar A, González-López Y, Abúndez A, Flores-Ruiz FJ, Díaz-Guillén JC, Betancourt-Cantera JA, Hernández-Hernández M, Trápaga-Martínez G, Poblano-Salas CA, Acevedo-Dávila JL, et al. Characterization of a C-Based Coating Applied on an AA6063 Alloy and Developed by a Novel Electrochemical Synthesis Route. Coatings. 2020; 10(2):145. https://doi.org/10.3390/coatings10020145
Chicago/Turabian StyleGallegos-Melgar, Adriana, Yael González-López, Arturo Abúndez, Francisco Javier Flores-Ruiz, Juan C. Díaz-Guillén, José. A. Betancourt-Cantera, Maricruz Hernández-Hernández, Gerardo Trápaga-Martínez, Carlos. A. Poblano-Salas, Jorge. L. Acevedo-Dávila, and et al. 2020. "Characterization of a C-Based Coating Applied on an AA6063 Alloy and Developed by a Novel Electrochemical Synthesis Route" Coatings 10, no. 2: 145. https://doi.org/10.3390/coatings10020145
APA StyleGallegos-Melgar, A., González-López, Y., Abúndez, A., Flores-Ruiz, F. J., Díaz-Guillén, J. C., Betancourt-Cantera, J. A., Hernández-Hernández, M., Trápaga-Martínez, G., Poblano-Salas, C. A., Acevedo-Dávila, J. L., & Mayen, J. (2020). Characterization of a C-Based Coating Applied on an AA6063 Alloy and Developed by a Novel Electrochemical Synthesis Route. Coatings, 10(2), 145. https://doi.org/10.3390/coatings10020145








