1. Brief Introduction
Ceramics are widely indicated for higher temperature turbine components. Water vapor attack has become a new element of study because of volatile hydroxides that form by reaction with Al
2O
3 substrates or with SiO
2 scales that form on SiC and Si
3N
4. The severity of attack is controlled by the thermodynamics of the reaction, velocity, and water vapor pressure [
1,
2,
3,
4]. The water vapor component of combusted jet fuel is generally indicated at ~10%, with chemical activity increasing with overall system pressure. Typical volatile hydroxides of common oxides observed or projected are CrO
2(OH)
2, Si(OH)
4, TiO(OH)
2, and Al(OH)
3, in order of decreasing severity [
1,
2,
3,
4]. While Cr volatiles can occur at low temperatures, 1200 °C or more is required for the others to become a noticeable problem. Thus, widely used Al
2O
3-forming superalloys, typically limited to 1150 °C, have not previously exhibited a scale volatility problem. Also, thermal barrier coatings (TBC) allow for higher gas temperatures, but protect the metal and thermally grown oxide (TGO) from both high temperature and high velocity. Al
2O
3 and SiC ceramic matrix composites (CMC) generally need an environmental barrier coating (EBC) for continuous use above about 1200 °C [
5]. Furthermore, SiO
2 scales are known to have significantly increased growth rates in water vapor, whereas Al
2O
3 scales show more complex effects on metals than on MAX phases [
6,
7,
8,
9].
Another class of materials that presents opportunities at intermediate temperatures is that of Al
2O
3-forming MAX phases, such as Cr
2AlC, Ti
2AlC, and Ti
3AlC
2. These compounds are very oxidation resistant, some at or above 1300 °C [
10], although strength is lacking for unsupported, load-bearing use at this temperature. A broad program evolved at NASA Glenn to examine Type II low temperature hot corrosion resistance of Cr
2AlC coatings on superalloys, basic Al
2O
3 scale kinetics, and extreme durability of yttria-stabilized zirconia (YSZ) TBC on Ti
2AlC [
11]. Burner rig testing was also performed that yielded experimental results for TiO
2 and Al
2O
3 scale durability in water vapor. The purpose of this present paper is to catalogue and analyze high pressure (6 atm) and high velocity (100 m/s) burner results with regards to scale volatility issues. Part of the motivation relates to the behavior of Al
2O
3 scales in high velocity water vapor at 1300 °C, an environment not typically used for standard metal alloy tests. The materials and processes were described in detail from a number of studies comprising the source data for this compilation [
12,
13,
14,
15]. All the results subsequently presented derive from these studies. For further reference, those studies included broad literature surveys and more in-depth discussions.
3. Results
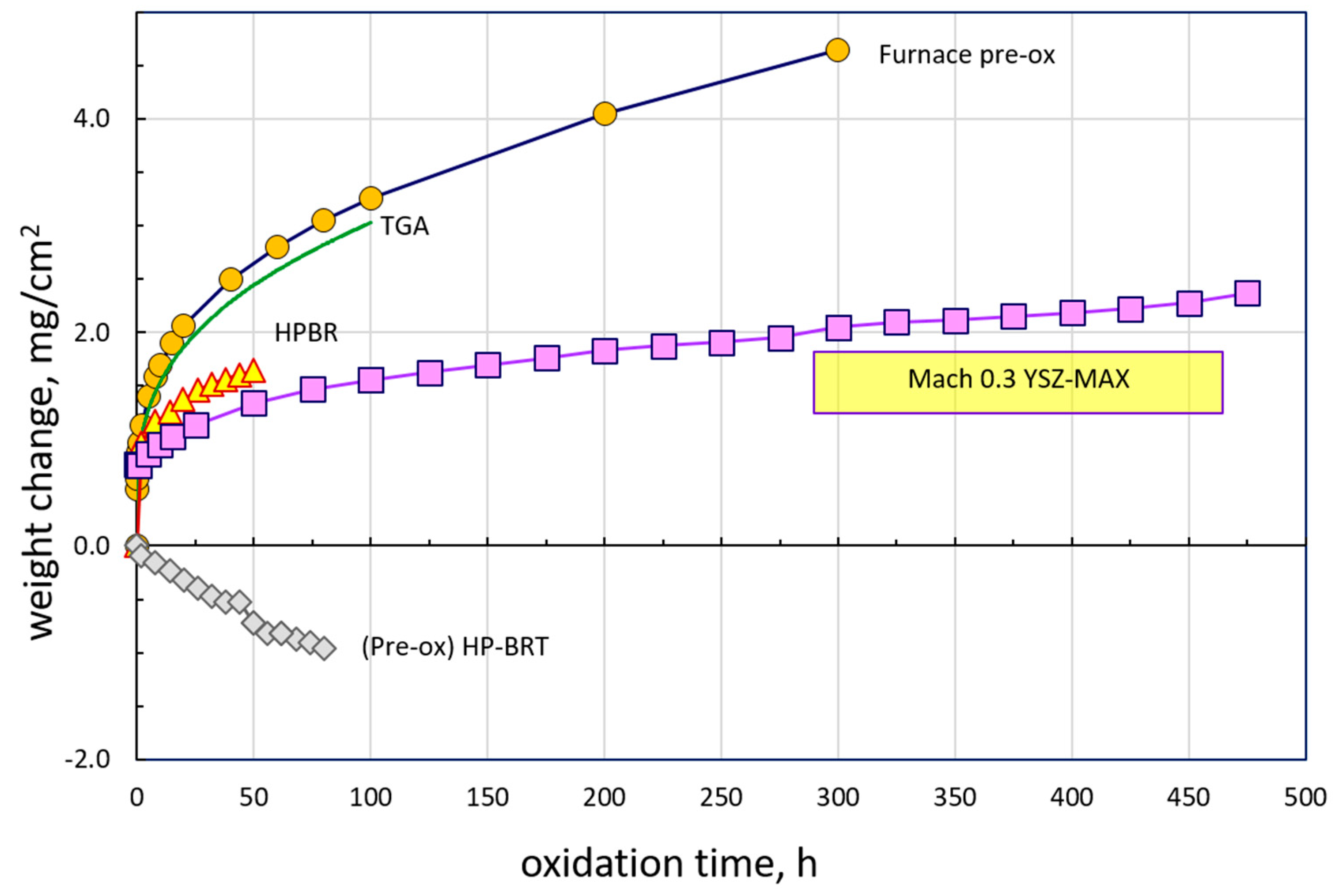
The weight change results from a suite of 1300 °C furnace and jet fuel burner tests are presented in
Figure 1. Test times were dictated by the specific study they addressed: standard 100 h TGA, extended 300 h furnace pre-oxidation to produce a very slow growing Al
2O
3 scale, short 50 h HP-BRT to demonstrate kinetics in a labor-intensive, expensive, pressurized apparatus, and 500 h to demonstrate long term TBC durability in the convenient, available, Mach 0.3 high velocity cyclic rig.
The ambient air box furnace (pre-ox) and dry air TGA show similar gains. These are compared to lower curves for the two burner tests. All results indicate a rapid initial uptake in the order of 1 mg/cm2 within the first hour of exposure. This has often been associated with a rapid transient growth of discontinuous TiO2 scales that are then undercut by a healing layer of slow-growing, steady-state Al2O3. Nearly linear weight loss was observed in the HP-BRT for a pre-oxidized sample, that would otherwise have been masked by the high initial growth rate. Long-term testing was conveniently enabled by the box furnace, used for a pre-oxidation treatment here, and by the accessibility of the open Mach 0.3 burner test. Weight gain of ~2.4 mg/cm2 was achieved in the Mach 0.3 BRT as compared to about 1.6 mg/cm2 for the much shorter HP-BRT.
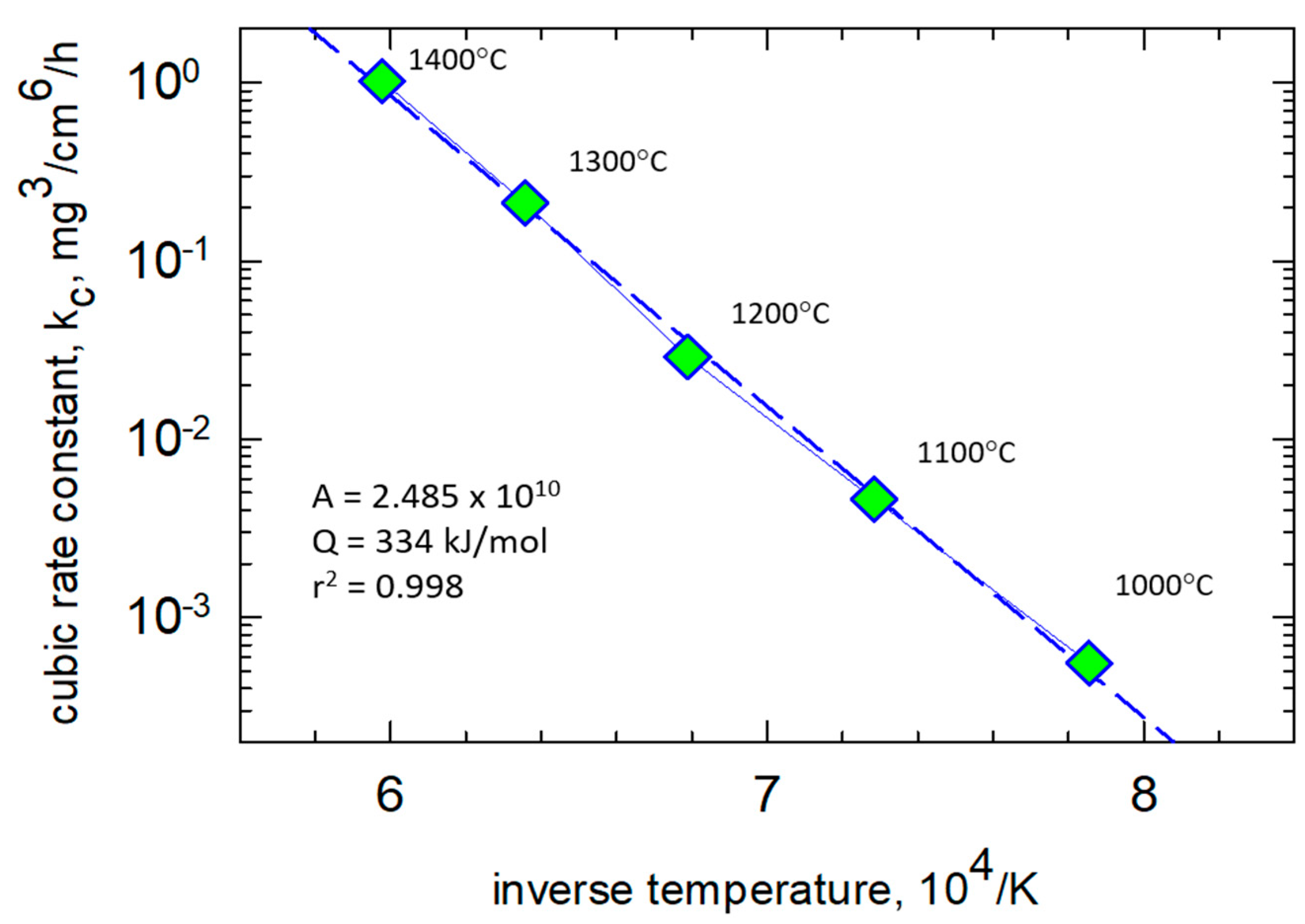
The cubic scaling kinetics were easily treated by correcting (subtracting) the amount of transient TiO
2, as graphically interpolated on log-log plots [
12,
14]. In the case of TGA tests, it was shown that most of the transient growth, w
0, took place in the first 10 min. Good linearized fits of (
w − w0) to (
t − t0)
1/3 cubic kinetics could be obtained in these well-controlled TGA furnace tests. The cubic scaling constants, extracted from the (
w − w0) offset-corrected mass gain curves show a very well-behaved, single-mechanism Arrhenius dependency,
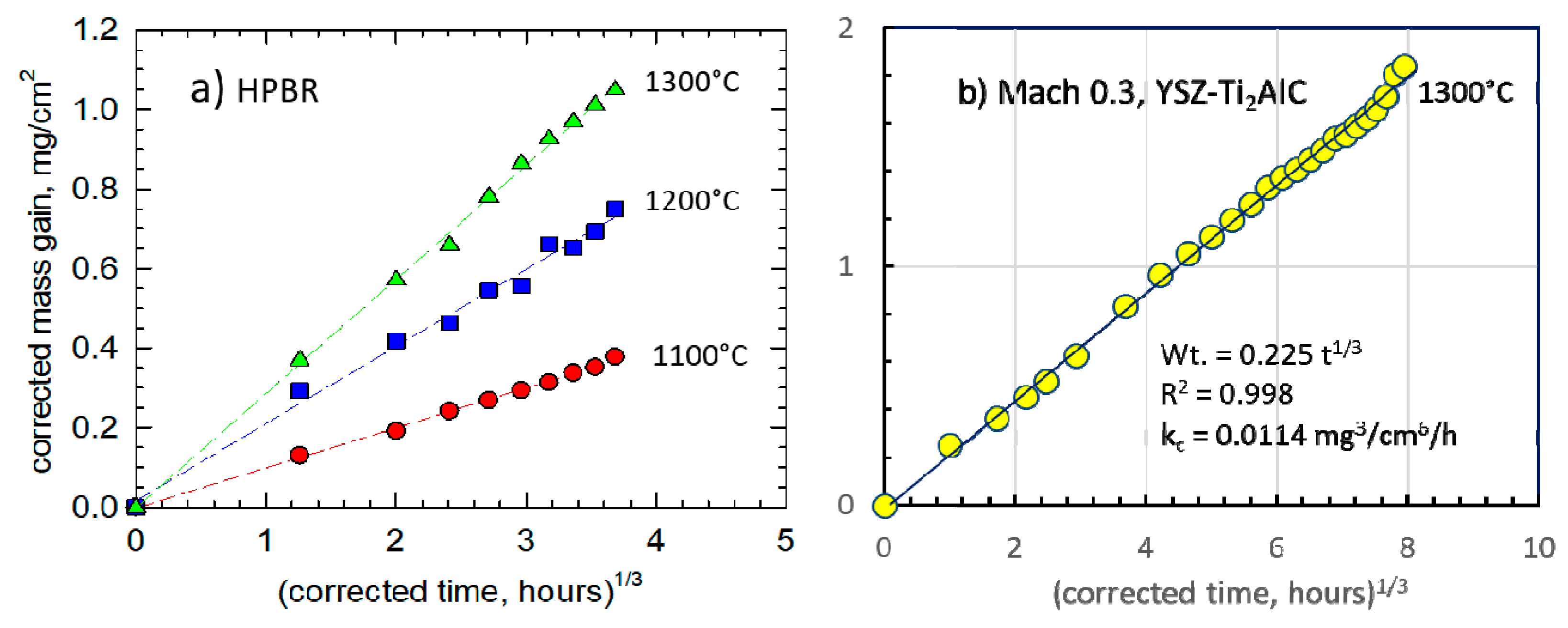
Figure 2. Over the temperature range of 1000 °C–1400 °C, an activation energy of 334 kJ/mol·K is seen to apply. Accordingly, the same graphical approach was applied to the 1300 °C test data for HP-BRT (
Figure 3a) and Mach 0.3 BRT (
Figure 3b). These yielded
kc of 0.024 and 0.011 mg
3/cm
6·h, respectively, compared to 0.212 mg
3/cm
6·h determined by TGA [
12,
13]. These BRT reductions reflect losses due to scale volatility effects. The Mach 0.3 test also incorporates protective effects of the YSZ face-coat and lower temperatures (100 °C) away from the hot zone.
Visual confirmation of TiO
2 volatility can be surmised from the low-magnification optical micrographs in
Figure 4. Here, the scattered, sometimes oriented, initial clusters of light scale phases are seen to coarsen with furnace exposure time and decrease or disappear with HP-BRT exposure time. This effect was semi-quantitatively verified by Rietveld analyses of X-ray diffractometer scans. While TiO
2 (rutile) was the primary transient identified at 1200 °C and below, the reaction phase of TiAl
2O
5 was also identified after 1300 °C exposures. Here, initial scale quantities of 20% TiO
2 and 10% TiAl
2O
5 were determined after just 0.2 h of furnace exposure. These decreased dramatically to only 0.1% and 1%, respectively, after 80-h HP-BRT exposures (300 h furnace pre-oxidation), the remainder being α-Al
2O
3 [
13].
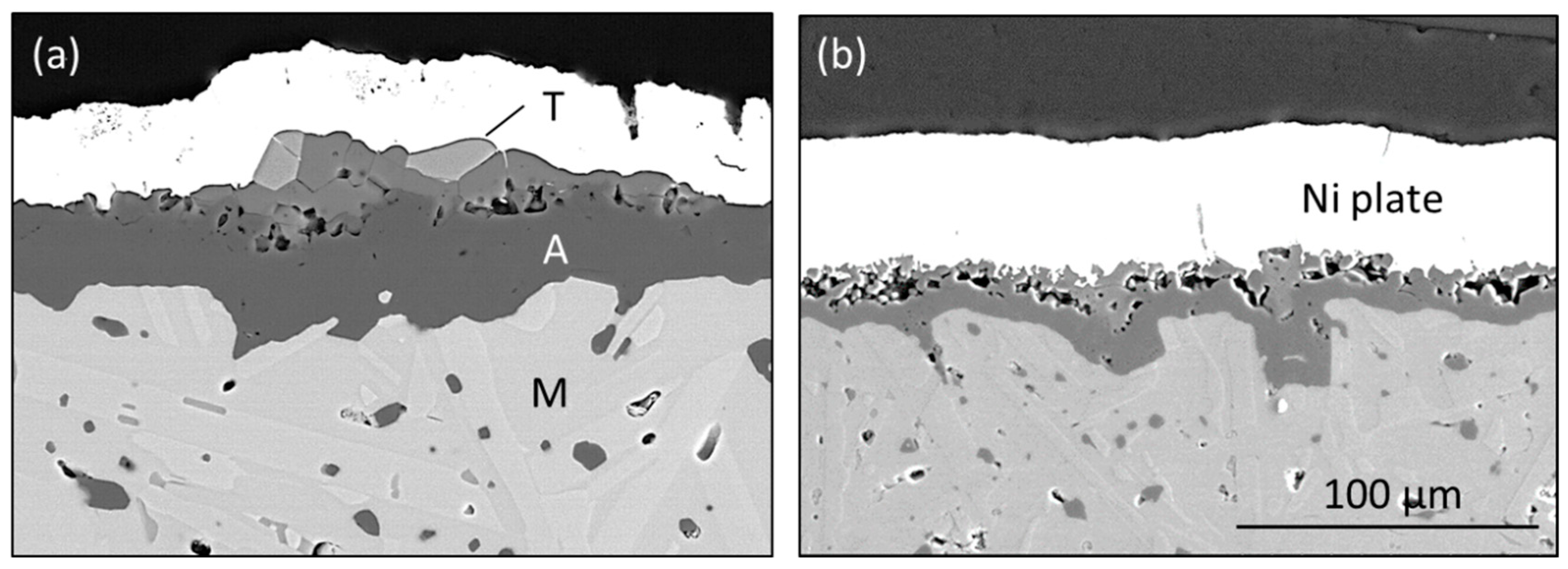
The effect of this HP-BRT exposure on the surface structure can be seen in
Figure 5. After 300 h pre-oxidation at 1300 °C, transient TiO
2 and TiAl
2O
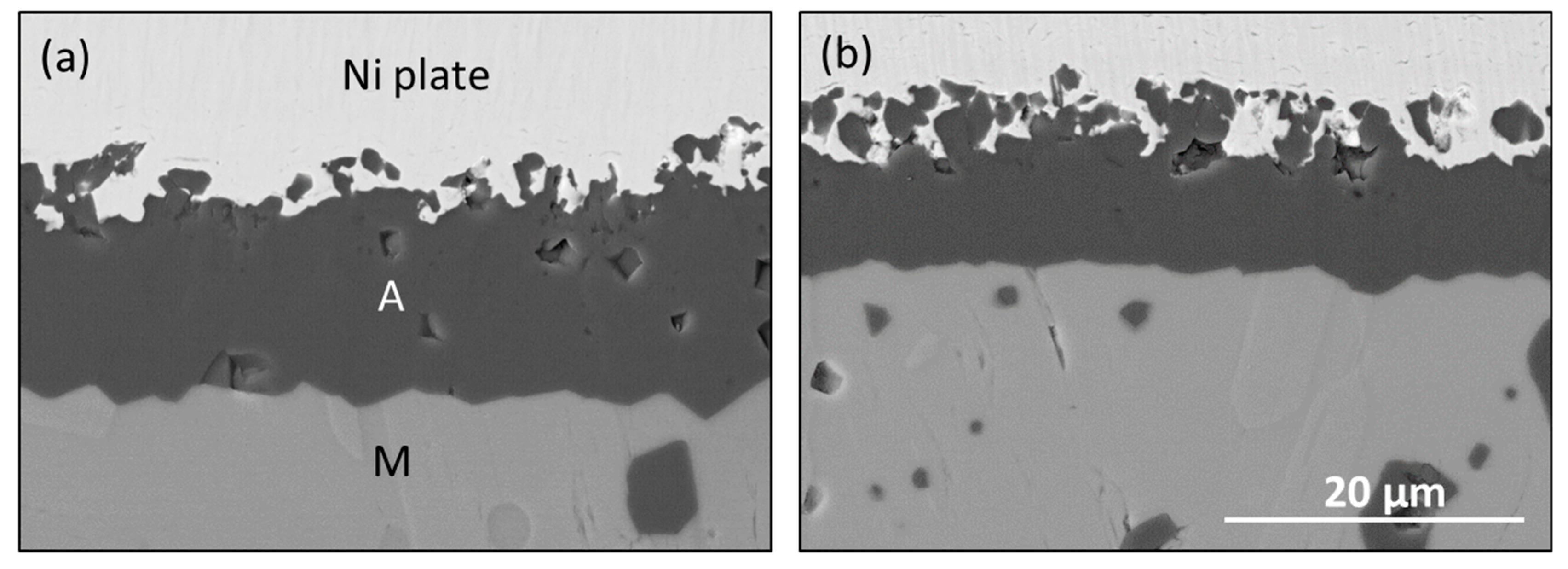
5 bright clusters (T) were retained in (a), but then largely removed by HP-BRT testing for 80 h at 1300 °C in (b). Distinct underlying grains of Al
2O
3 (A) could then be discerned with a much lower Ti EDS signal overall. A linear weight loss rate of 0.012 mg/cm
2·h was also measured, as shown by the lower curve in
Figure 1. Since this included some modest scale growth, the total removal rate was surmised to be about 0.017 mg/cm
2·h. (Pre-oxidation was required to produce a thick scale with a low instantaneous growth rate less than the volatility rate).
A direct comparison of scales formed in 1300°C TGA (100 h) and HP-BRT (50 h) in cross-section is presented in
Figure 6. The TGA structure shows the Ti-rich remnants of scattered transient scale colonies, with a dense underlayer of Al
2O
3. The HP-BRT sample exhibits a rather discontinuous surface scale with less distinct Ti-rich regions, if at all. HP-BRT scale volatility is again suggested. The inner Al
2O
3–Ti
2AlC interface is completely intact with no porosity or cracking.
Mach 0.3 BRT (1 atm., 100 m/s, 500 h) exposures produced similar effects on surface scale microstructure (uncoated sample backside) (
Figure 7). However, since the burner nozzle was about 2.5 cm in diameter centered on the 5 cm long exposed sample length, the sample temperature at the top (a) and bottom (grip end, c) was about 100°C cooler. This resulted in a less severe attack, with some remnants of bright Ti-rich particles atop Al
2O
3 grains, the latter exhibiting grain boundary porosity.
In contrast, the hot section (b) showed little vestige of Ti-rich scales, but a highly irregular, open Al
2O
3 scale. Some grains appeared to be etched crystallographically, forming lamellae, possibly along the hexagonal (0001) basal planes. The platelets retained a slight Ti level; they may have been derived from TiAl
2O
5 grains where Ti was removed by selective water vapor corrosion. Xrd analyses of the oxidized surface showed ~10% TiO
2 after the initial 20 min at 1000 °C, then removed by volatile reactions to just 0.1% after the 1300 °C exposure, the remainder of the scale being α-Al
2O
3 [
15]. No phase change in the Ti
2AlC substrate was apparent other than reduced x-ray diffraction intensity due to absorption from the thickening scale.
In cross-section,
Figure 8, little indication of Ti-rich scales remains, and the scale is thicker in the hot zone region (a). The surfaces are very irregular and open, consistent with scale removal by volatile products. Less of this structure remains in the hot zone region compared to the grip end (b). Again, the scale-substrate interface is seen to be completely intact.
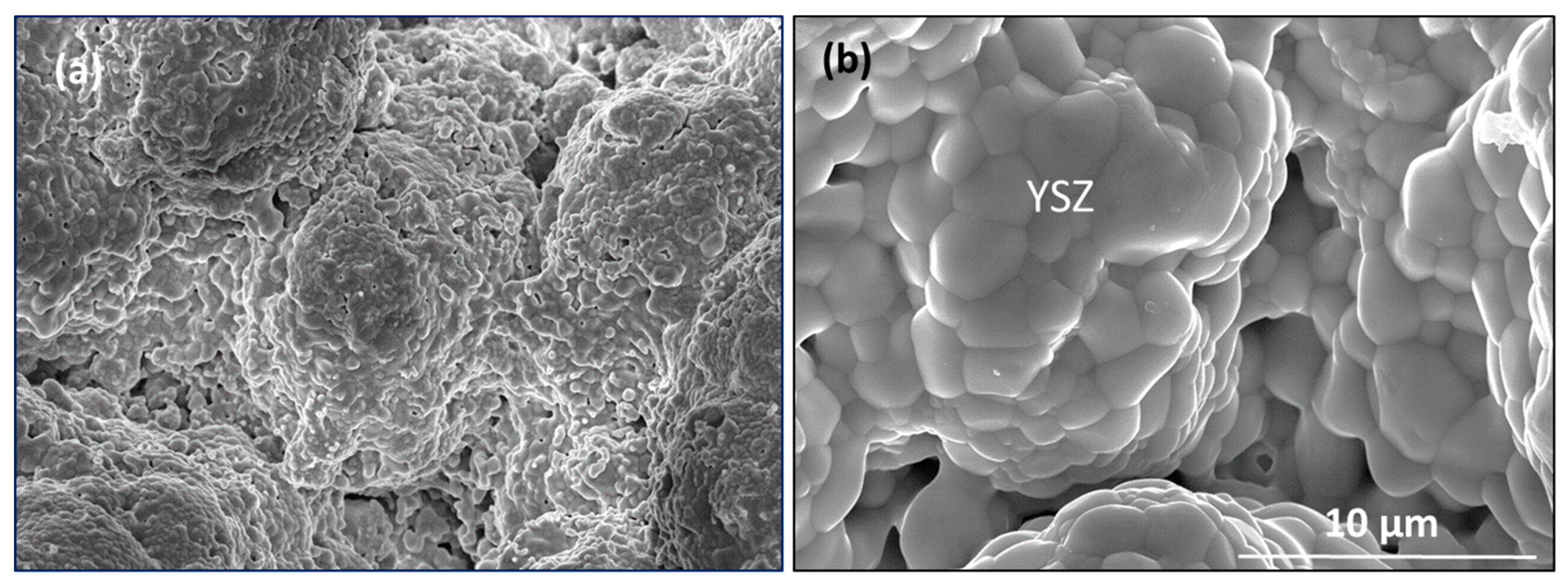
Figure 9 presents coating structures typifying the as-sprayed (a) and the hot zone region (b) for the coated face of the Mach 0.3 BRT sample. The coating exhibits deposition columns, first textured by the deposition process (a), then by grain growth and surface smoothing during thermal annealing (b). Here, the flame directly impinged on the YSZ coating face, which shows no features of oxide removal by volatility: the zirconia grains and PS-PVD coating columns are basically intact. In the cross-section (
Figure 10), the coating, TGO, and MAX phase substrate are also intact with no interfacial porosity, cracks, or delamination. Porosity and metallographic pullout, however, is observed within the scale. Volatility issues have therefore been prevented on the coated face. Coating survival after 500 h testing at 1300 °C and 100 m/s is thus indicated on all accounts. Further testing would not be “cost effective” or especially productive as there was little indication of imminent failure, i.e., the same results are expected for 1000 h testing and beyond. Furnace testing has shown similar durability for the YSZ/Ti
2AlC system, surviving 2500 h total, including 500 h at 1300 °C and scales up to 40 μm thick [
14]. While little evidence is seen for detrimental interface reactions, it can be surmised that sustained Al
2O
3 growth will be limited by the Al reservoir in the MAX phase substrate.
4. Discussion
The previous assemblage of results compared the high temperature scaling characteristics of the oxidation resistant Ti
2AlC phase under moist, high velocity burner conditions to those from static and dry atmospheres. The distinct appearance and removal of the initial TiO
2 transient scale by moisture in a high velocity gas stream was highlighted. The relative susceptibility of TiO
2 to TiO(OH)
2 formation in water vapor appears preferential compared to that of Al
2O
3 via Al(OH)
3 volatiles. Such a condition has been examined in concert with Jacobson’s thermodynamic treatment of various oxides in flowing moist gases [
13]. It was predicted that TiO(OH)
2 losses would be on the same order as Al(OH)
3 losses, but it is now acknowledged that some uncertainties still remain regarding TiO
2 volatiles [
16,
17]. While the Ti-oxides appeared to be removed preferentially, some losses of Al
2O
3 are also indicated. Critical studies have indeed demonstrated volatile losses and crystallographic etching for bulk Al
2O
3 [
18,
19,
20].
The general removal rate of various scales in various water vapor environments can be modeled according to v
1/2p
H2On/p
total1/2, using the original thermochemical-diffusional approach developed by Opila et al. for various oxides [
1]. Here,
n = 1 for TiO
2,
n = 3/2 for Al
2O
3, and
n = 2 for SiO
2, as dictated by the chemical reaction with water vapor. Accordingly, the relative severity of the Mach 0.3 test (100 m/s, 1 atm) to the HP-BRT (25 m/s, 6 atm) shown here produces relative rig factors of 0.82, 0.33, and 0.14 for the three scales, respectively [
15]. Thus, in the Mach 0.3 test, TiO
2 is expected to show similar attack severity as in the HP-BRT, while SiO
2 is expected to show less attack, with Al
2O
3 intermediate. This is consistent with efficient removal of TiO
2 observed in both tests and more severe removal of SiO
2 in the HP-BRT.
An attempt was made to extract volatility kinetics from the weight change curves using a cubic-linear fit (Chen-Tedmon) [
21]. This was partially enabled using COSP for Windows originally designed for cyclic oxidation spalling models. For the present case, cubic growth and uniform scale removal is the model selected [
13,
22]. To account for a decreasing amount of TiO
2 with time, the “spall” (removed) thickness exponent (α) is addressed as a negative number (−3). It is recognized that a constant quotient of the ”spall” fraction,
Q0, and cycle duration, τ, yield identical loss rates per hour. (The response basically converges to continuous curves for τ ≤ 1 h and reproduce their analytical expression).
Some solutions are presented in
Figure 11 for (a) the HP-BRT and (b) Mach 0.3 BRT results. Both model curves (dashed) are reasonable fits for the experimental data (symbols). The fitting parameters were
kc = 0.212 mg
3/cm
6/h,
Q0 = 0.220 for the HP-BRT [
13] and
kc = 0.050 mg
3/cm
6/h,
Q0 = 0.038 for the Mach 0.3 BRT. Both sets fixed a cubic growth exponent,
m = 3.0, and used a decreasing spall exponent,
α = −3. The HP-BRT fit initiated with the same
kc determined by TGA in dry air. The resulting
Q0 was shown to be consistent with a linear volatility weight loss of 0.01–0.02 mg/cm
2·h produced for the pre-oxidized sample [
13]. The Mach 0.3 BRT, however, maintained face-to-back and center-to-top/bottom temperature gradients. Accordingly, the net Mach 0.3 growth constant was considerably less, being only about one-quarter that of the HP-BRT. Also, the COSP fit projected a low Al volatility weight loss rate of ~0.002 mg/cm
2·h after 100 h, converging to just ~0.001 mg/cm
2·h after 500 h, or about one-tenth that of the 50 h HP-BRT rates. Remarkably, the Al
2O
3 scale thickness under the YSZ in the hot zone (at ~1244 °C) after 500 h (~20 μm) was basically the same as that produced in the HP-BRT (at ~1300 °C) after just 50 h.