Superhydrophobic Surface with Gamma Irradiation Resistance and Self-Cleaning Effect in Air and Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
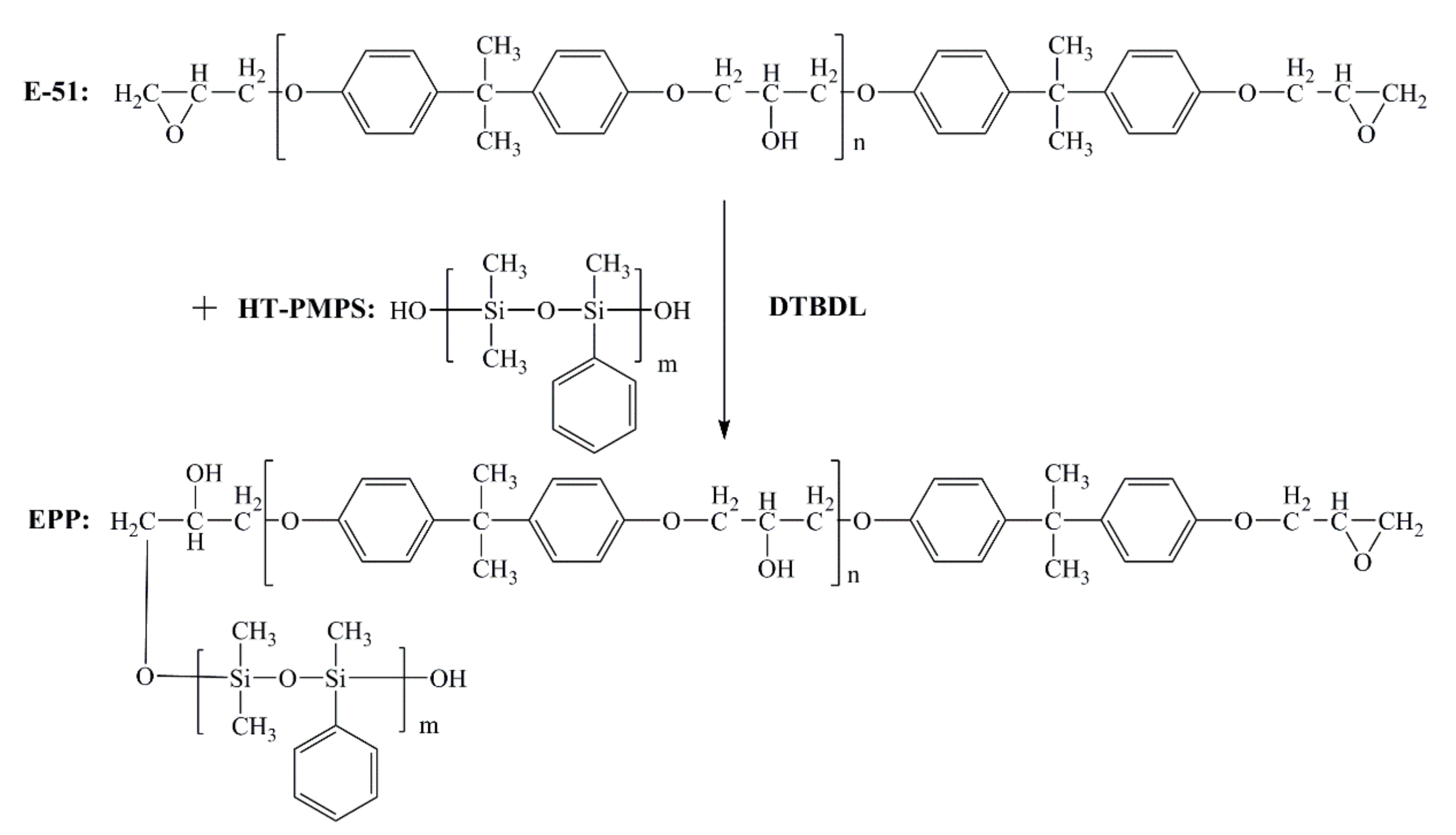
2.2. Synthesis of Polymethylphenylsiloxane-Modified Epoxy Resin
2.3. Preparation of Dual-Scale Silica Particles
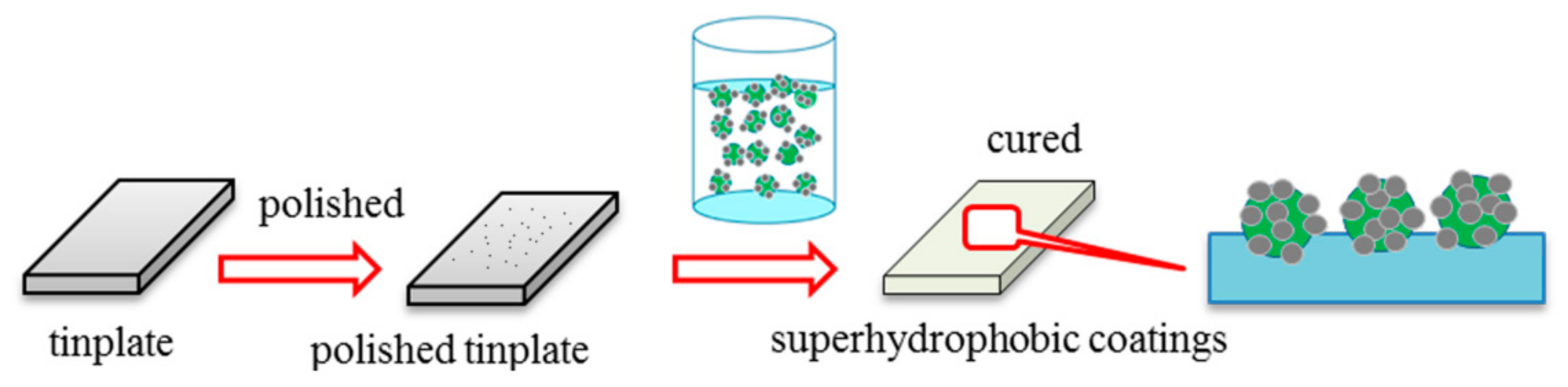
2.4. Fabrication of Superhydrophobic Surface
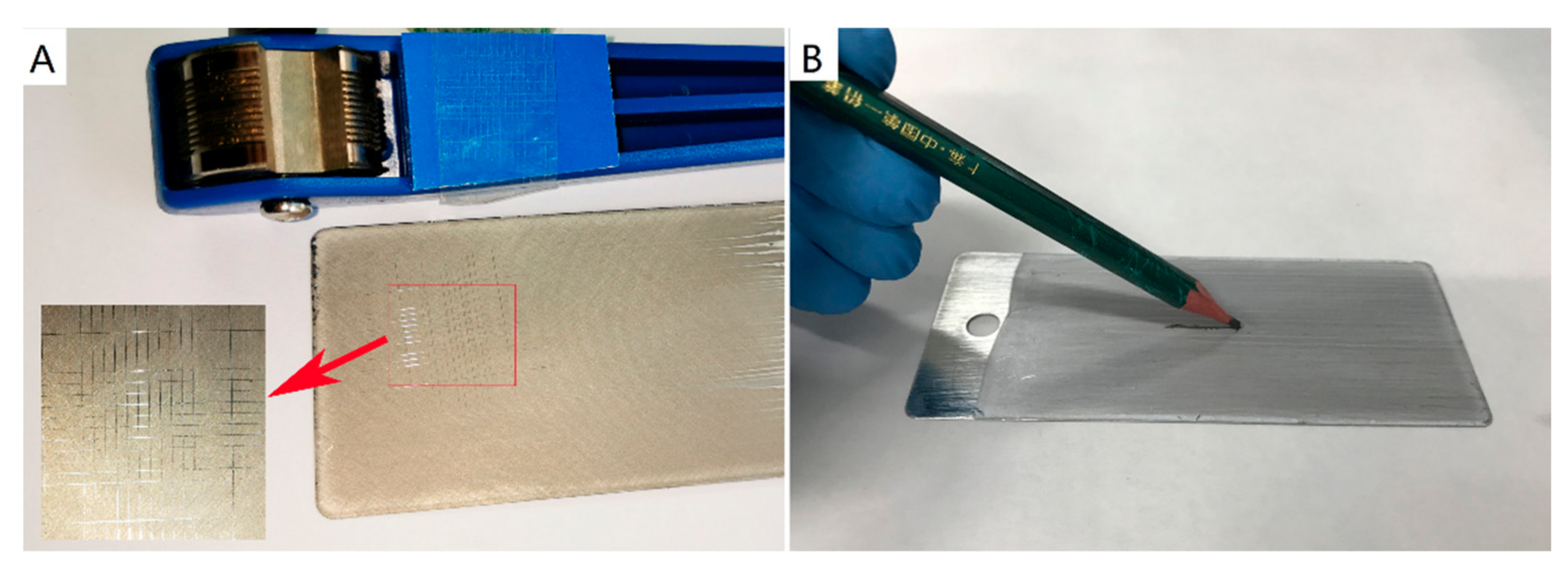
2.5. Characterization
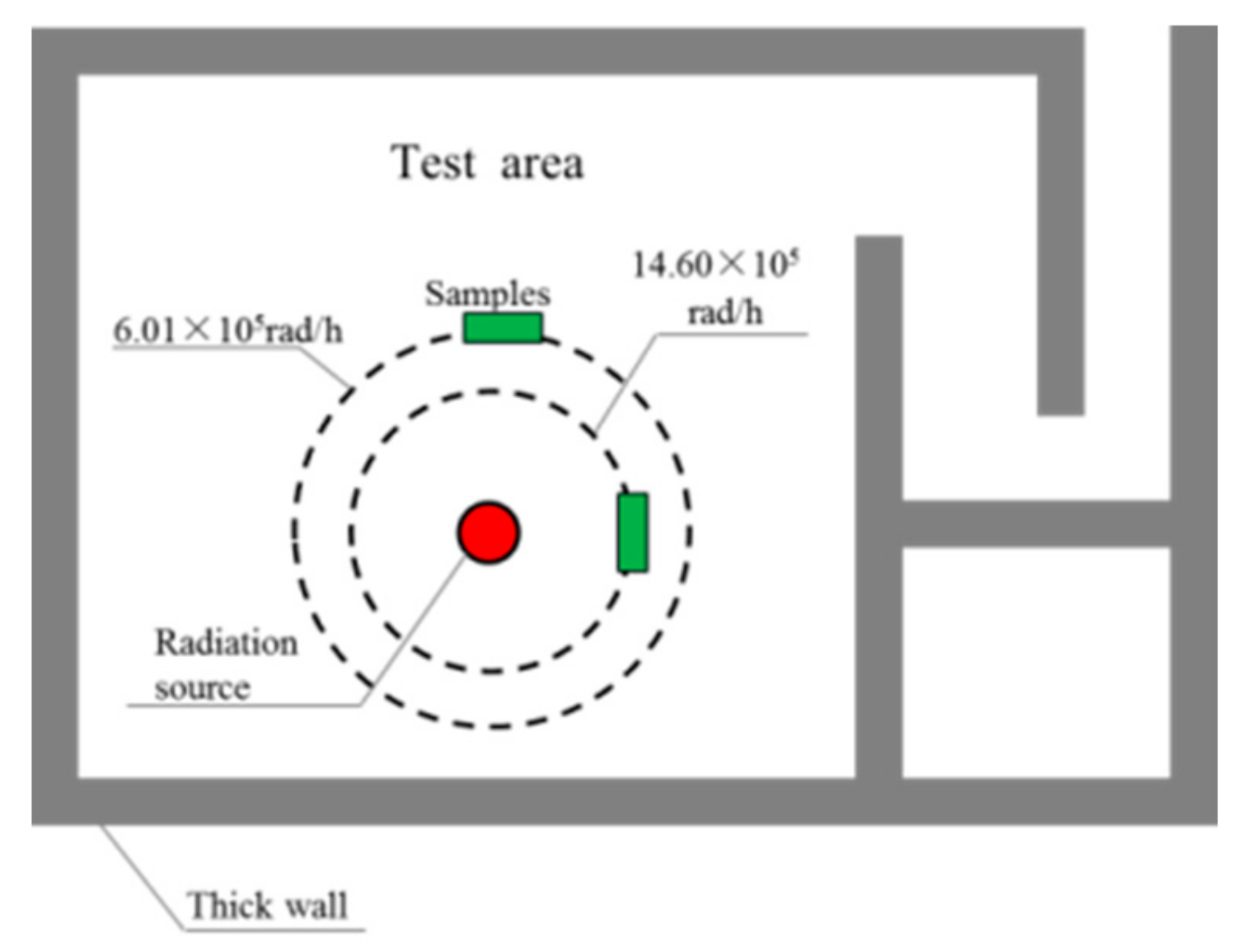
2.6. Irradiation
3. Results and Discussions
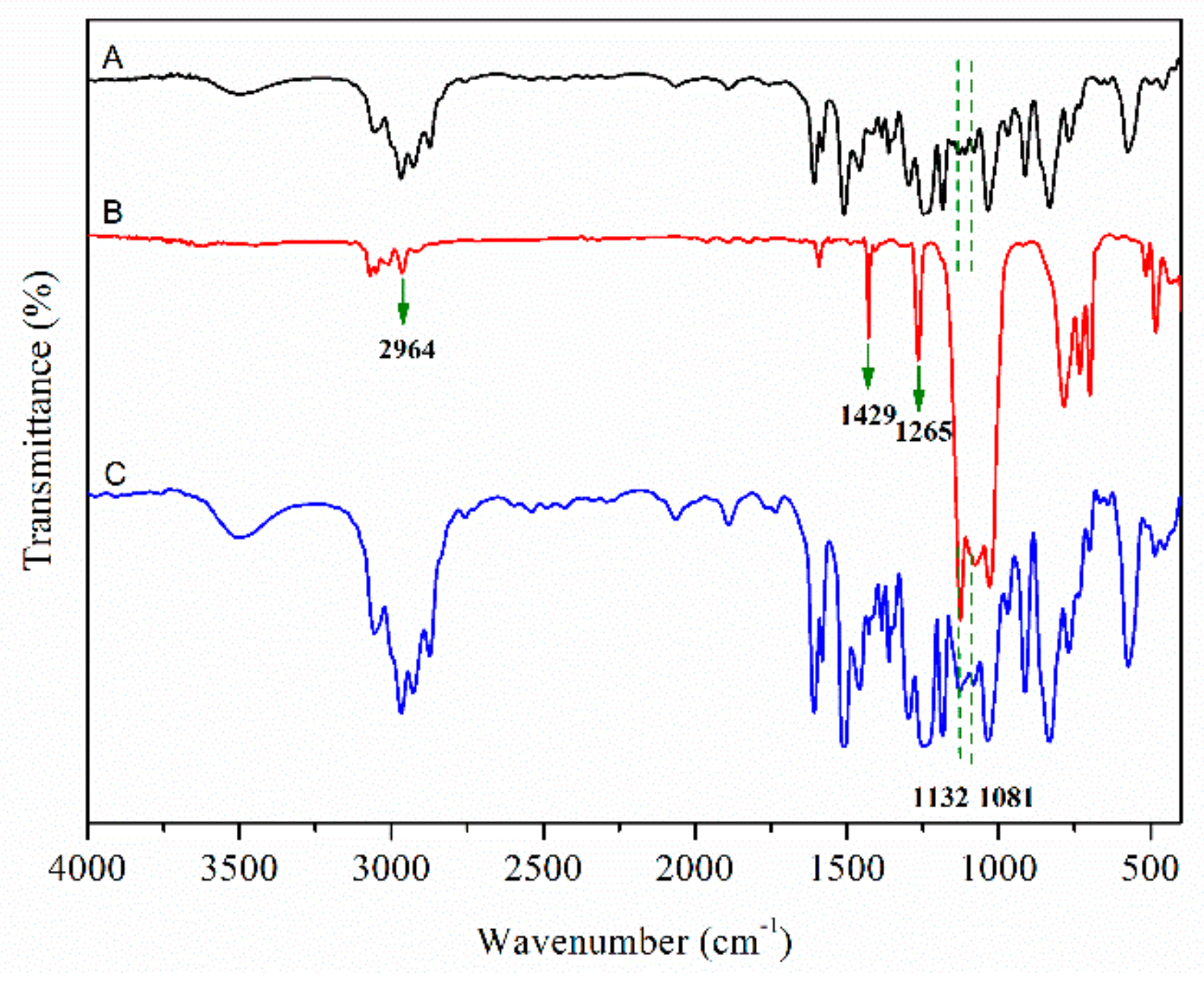
3.1. Silicone Modified Epoxy Resin
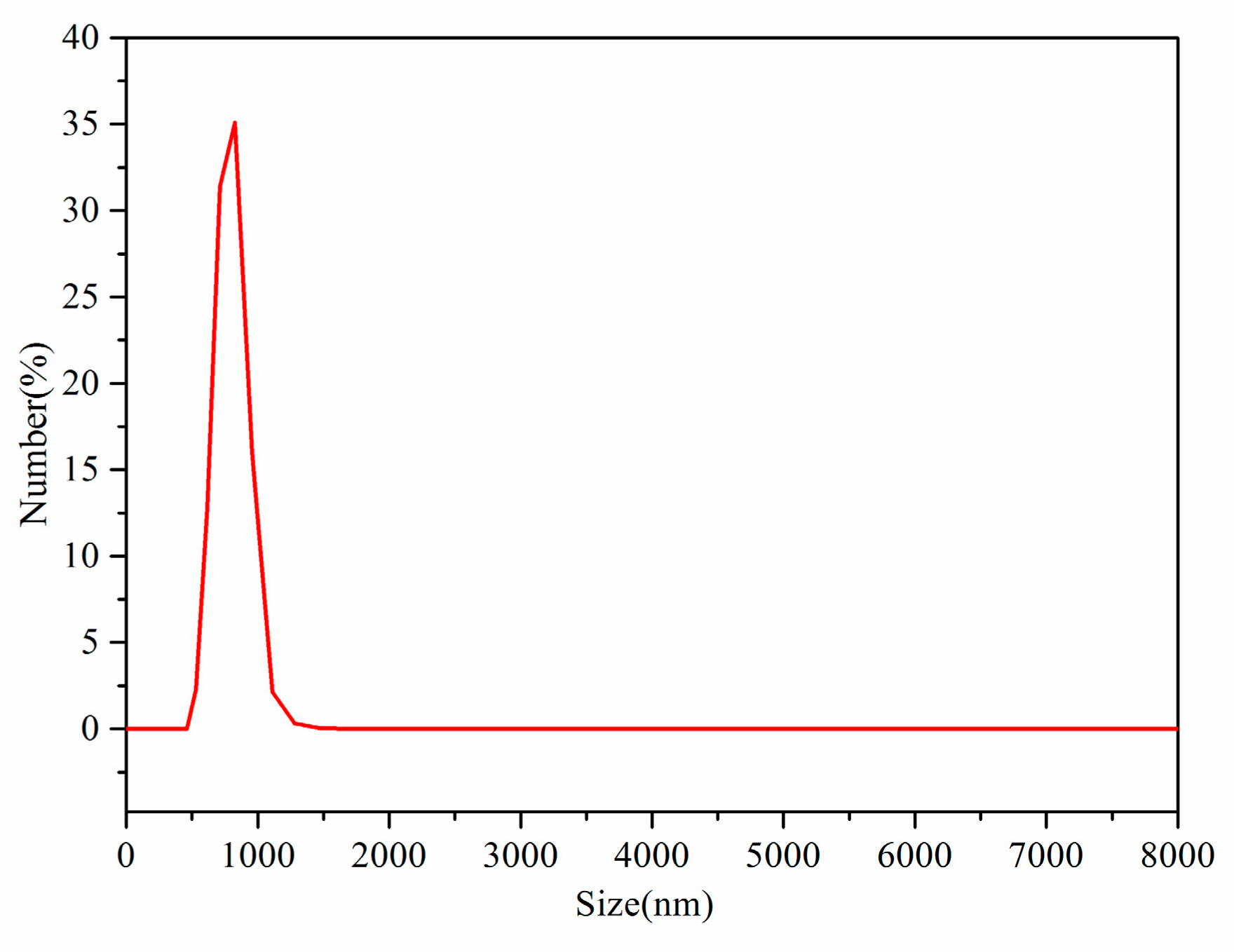
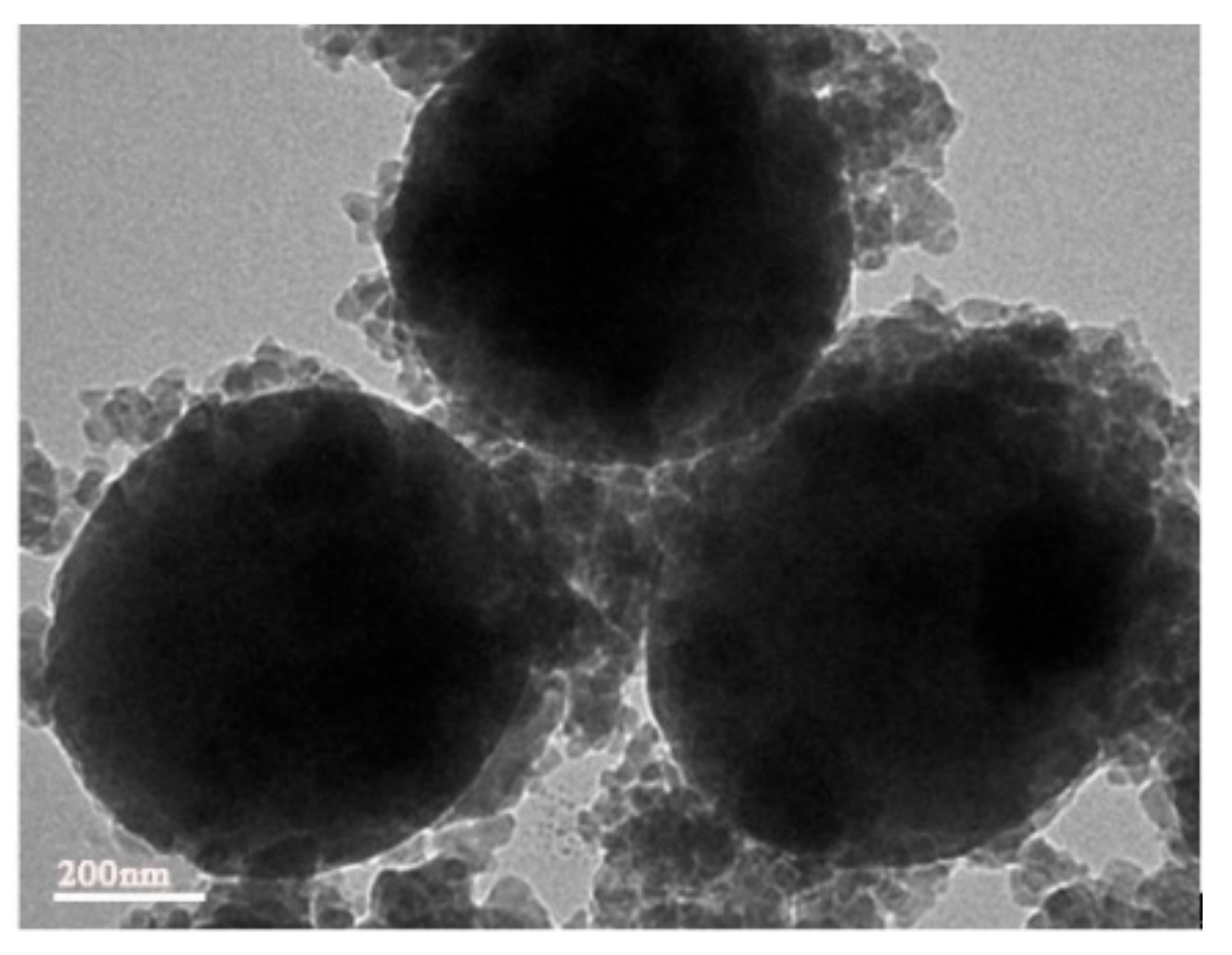
3.2. Dual-Scale Silica Particles
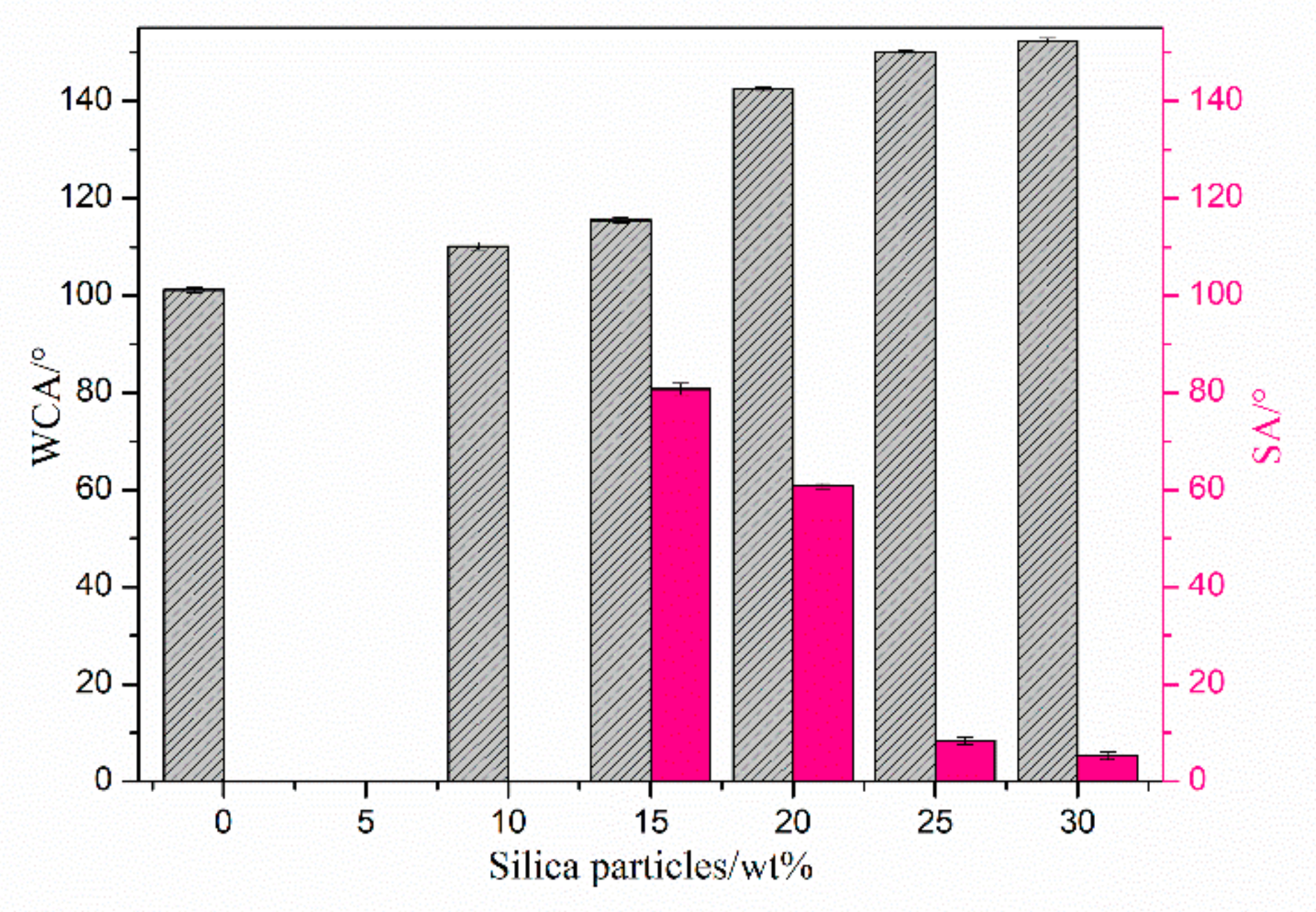
3.3. Static Water Contact Angle of the Surfaces
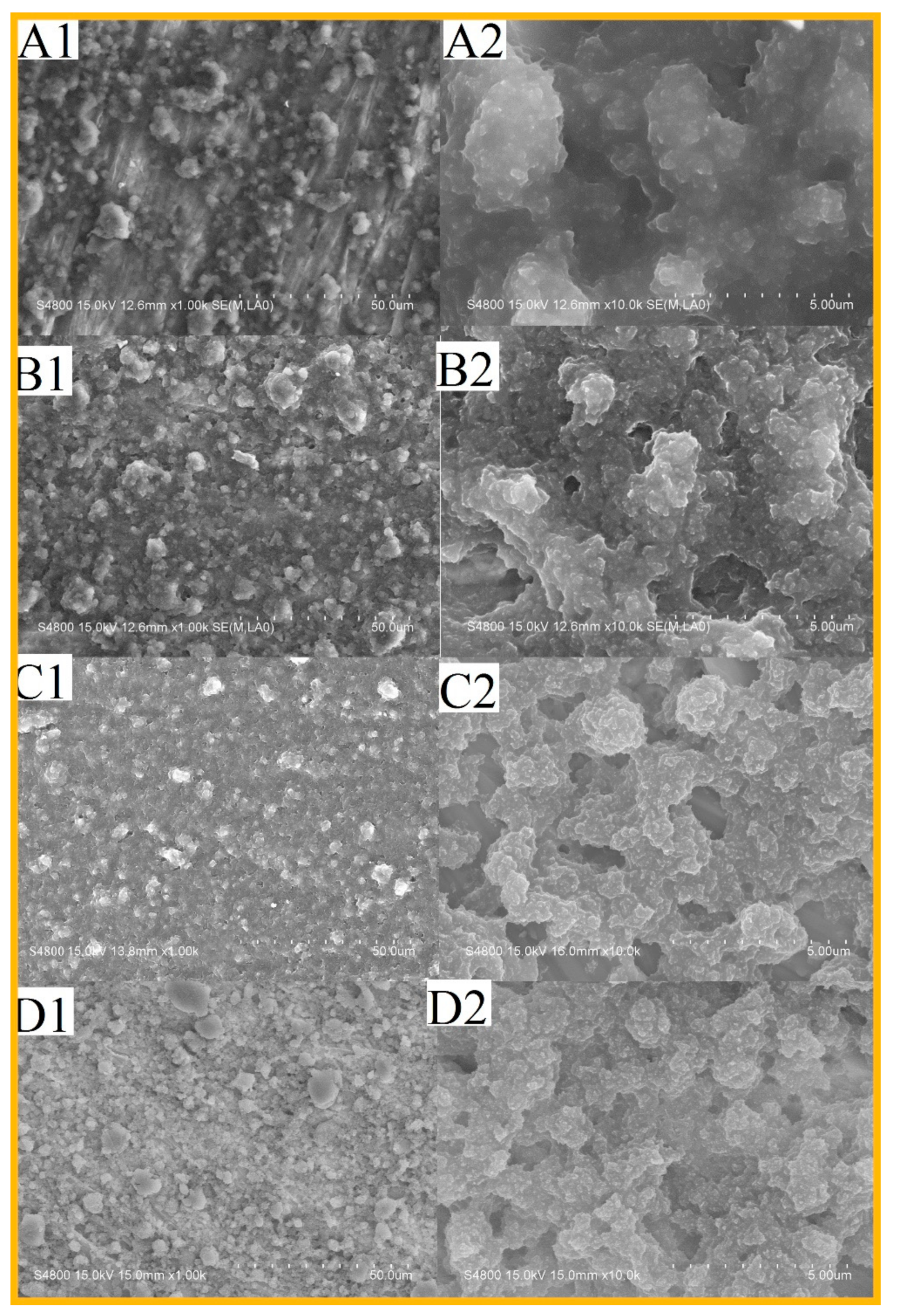
3.4. Morphology and Roughness of the Surfaces
3.5. Self-Cleaning Effect
3.6. Gamma Irradiation Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, W.K. Nuclear energy for the United States. Energy 1979, 4, 1053–1062. [Google Scholar] [CrossRef]
- Chang, Y.C.; Zhao, Y. The Fukushima Nuclear Power Station incident and marine pollution. Mar. Pollut. Bull. 2012, 64, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Comans, R.N.J.; Beresford, N.A.; Wright, S.M.; Camplin, W.C.; Howard, B.J. Pollution: Chernobyl’s legacy in food and water. Nature 2000, 405, 141. [Google Scholar] [CrossRef] [PubMed]
- Roger, J.L.; Nicole, F.D.S.; Henson, B.L. Incidence of thyroid cancer surrounding three mile island nuclear facility: The 30-year follow-up. Laryngoscope 2013, 123, 2064–2071. [Google Scholar]
- Komarneni, S.; Roy, R. Alternative radwaste solidification route for three mile island wastes. J. Am. Ceram. Soc. 1982, 65, C198. [Google Scholar] [CrossRef]
- Hlgye, Z.; Malátová, I. Estimation of intakes of 131I, 137Cs and 134Cs after the Chernobyl accident. Radiat. Prot. Dosim. 2012, 150, 504–507. [Google Scholar] [CrossRef]
- Chino, M.; Nakayama, H.; Nagai, H.; Terada, H.; Katata, G. Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Daiichi Nuclear Power Plant into the atmosphere. J. Nucl. Sci. Technol. 2011, 48, 1129–1134. [Google Scholar] [CrossRef]
- EL-Zayat, M.M.; Abdel-Hakim, A.; Mohamed, M.A. Effect of gamma radiation on the physico mechanical properties of recycled HDPE/modified sugarcane bagasse composite. J. Macromol. Sci. Part A 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Liu, G.; Liu, H.; Liu, Y.; He, S. The degradation of DGEBA/DICY under 100 keV proton irradiation. Polym. Degrad. Stab. 2011, 96, 732–738. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nishikawa, N.; Mayama, H.; Nonomura, Y.; Yokojima, S.; Nakamura, S.; Uchida, K. Theoretical explanation of the lotus effect: Superhydrophobic property changes by removal of nanostructures from the surface of a lotus leaf. Langmuir 2015, 31, 7355–7363. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, D.; Lee, J.B. Hierarchical micro/nano structures for super-hydrophobic surfaces and super-lyophobic surface against liquid metal. Micro Nano Syst. Lett. 2014, 2, 3. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Bioinspired rice leaf and butterfly wing surface structures combining shark skin and lotus effects. Soft Matter 2012, 8, 11271–11284. [Google Scholar] [CrossRef]
- Wang, F.; Li, S.; Wang, L. Fabrication of artificial super-hydrophobic lotus-leaf-like bamboo surfaces through soft lithography. Colloids Surf. A 2017, 513, 389–395. [Google Scholar] [CrossRef]
- Hardman, S.J.; Muhamad-Sarih, N.; Riggs, H.J.; Thompson, R.L.; Rigby, J.; Bergius, W.N.A.; Hutchings, L.R. Electrospinning superhydrophobic fibers using surface segregating end-functionalized polymer additives. Macromolecules 2011, 44, 6461–6470. [Google Scholar] [CrossRef]
- Zander, N.E.; Orlicki, J.A.; Karikari, A.S.; Long, T.E.; Rawlett, A.M. Super-Hydrophobic surfaces via micrometer-scale templated pillars. Chem. Mater. 2007, 19, 6145–6149. [Google Scholar] [CrossRef]
- Farzaneh, M.; Menini, R.; Jafari, R. Superhydrophobic and icephobic surfaces prepared by RF-sputtered polytetrafluoroethylene coatings. Appl. Surf. Sci. 2010, 257, 1540–1543. [Google Scholar]
- He, G.; Kaige, W. The super hydrophobicity of ZnO nanorods fabricated by electrochemical deposition method. Appl. Surf. Sci. 2011, 257, 6590–6594. [Google Scholar] [CrossRef]
- Shieh, J.; Hou, F.J.; Chen, Y.C.; Chen, H.M.; Yang, S.P.; Cheng, C.C.; Chen, H.L. Robust airlike superhydrophobic surfaces. Adv. Mater. 2010, 22, 597–601. [Google Scholar] [CrossRef]
- Ren, L.F.; Xia, F.; Shao, J.; Zhang, X.; Li, J. Experimental investigation of the effect of electrospinning parameters on properties of superhydrophobic PDMS/PMMA membrane and its application in membrane distillation. Desalination 2017, 404, 155–166. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Jiangab, T.; Guoab, Z. Robust superhydrophobic tungsten oxide coatings with photochromism and UV durability properties. Appl. Surf. Sci. 2016, 387, 412–418. [Google Scholar] [CrossRef]
- Pintoa, D.; Bernardob, L.; Amaroa, A.; Lopesa, S. Mechanical properties of epoxy nanocomposites using titanium dioxide as reinforcement—A review. Constr. Build. Mater. 2015, 95, 506–524. [Google Scholar] [CrossRef]
- Xia, W.; Xue, H.; Wang, J.; Wang, T.; Song, L.; Guo, H.; Fan, X.; Gong, H.; He, J. Functionlized graphene serving as free radical scavenger and corrosion protection in gamma-irradiated epoxy composites. Carbon 2016, 101, 315–323. [Google Scholar] [CrossRef]
- Li, R.; Gu, Y.; Wang, Y.; Yang, Z.; Li, M.; Zhang, Z. Effect of particle size on gamma radiation shielding property of gadolinium oxide dispersed epoxy resin matrix composite. Mater. Res. Express 2017, 4, 035035. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Y.; Liu, Y.; Fang, J.; Luan, W.; Yang, X.; Zhang, W. Preparation and characterization of tungsten/epoxy composites for γ-rays radiation shielding. Nucl. Instrum. Methods Phys. Res. Sect. B 2015, 356–357, 88–93. [Google Scholar] [CrossRef]
- Nagatani, K.; Kiribayashi, S.; Okada, Y.; Otake, K.; Yoshida, K.; Tadokoro, S.; Nishimura, T.; Yoshida, T.; Koyanagi, E.; Fukushima, M.; et al. Emergency response to the nuclear accident at the Fukushima Daiichi Nuclear Power Plants using mobile rescue robots. J. Field Robot. 2013, 30, 44–63. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- GB/T 1677–2008 Determinating the Epoxy Value of Plasticizers; SAC: Beijing, China, 2008.
- D3359-09 Standard Test Methods for Measuring Adhesion by Tape Test; ASTM: West Conshohocken, PA, USA, 2009.
- D3363-00 Standard Test Method for Film Hardness by Pencil Test; ASTM: West Conshohocken, PA, USA, 2000.
- Gao, Y.Y.; Zhang, Z.L.; Chen, W.X. Several dose meters for radiation processing-the first intercomparison held in Beijing. J. Rad. Res. Rad. Proc. 1988, 6, 37–41. [Google Scholar]
- Ming, W.; Wu, D.; van Benthem, R.; de With, G. Superhydrophobic films from raspberry-like particles. Nano Lett. 2005, 5, 2298–2301. [Google Scholar] [CrossRef]
- Puretskiy, N.; Ionov, L. Synthesis of robust raspberry-like particles using polymer brushes. Langmuir 2011, 27, 3006–3011. [Google Scholar] [CrossRef]
- Wang, C.F.; Hung, S.W.; Kuo, S.W.; Chang, C.J. Combining hierarchical surface roughness with fluorinated surface chemistry to preserve superhydrophobicity after organic contamination. Appl. Surf. Sci. 2014, 320, 658–663. [Google Scholar] [CrossRef]
- Li, F.; Du, M.; Zheng, Q. Dopamine/Silica nanoparticle assembled, microscale porous structure for versatile superamphiphobic coating. ACS Nano 2016, 10, 2910–2921. [Google Scholar] [CrossRef]
- Yang, J.; Pi, P.; Wen, X.; Zheng, D.; Xu, M.; Cheng, J.; Yang, Z. A novel method to fabricate superhydrophobic surfaces based on well-defined mulberry-like particles and self-assembly of polydimethylsiloxane. Appl. Surf. Sci. 2009, 255, 3507–3512. [Google Scholar] [CrossRef]
- Bobji, M.S.; Kumar, S.V.; Asthana, A.; Govardhan, R.N. Underwater sustainability of the “Cassie” state of wetting. Langmuir 2009, 25, 12120–12126. [Google Scholar] [CrossRef] [PubMed]
- Latthe, S.S.; Sutar, R.S.; Bhosale, A.K.; Nagappan, S.; Ha, C.S.; Sadasivuni, K.K.; Liu, S.; Xing, R. Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog. Org. Coat. 2019, 137, 1–17. [Google Scholar] [CrossRef]
- Lee, S.G.; Ham, D.S.; Lee, D.Y.; Bong, H.; Cho, K. Transparent superhydrophobic/translucent superamphiphobic coatings based on silica-fluoropolymer hybrid nanoparticles. Langmuir 2013, 29, 15051–15057. [Google Scholar] [CrossRef] [PubMed]
- Vignoud, L.; Sixou, B.; David, L. Influence of electron irradiation on the mobility and on the mechanical properties of DGEBA/TETA epoxy resins. Polymer 2001, 42, 4657–4665. [Google Scholar] [CrossRef]
- Diao, F.; Zhang, Y.; Liu, Y.; Fang, J.; Luan, W. γ-Ray irradiation stability and damage mechanism of glycidyl amine epoxy resin. Nucl. Instrum. Methods Phys. Res. Sect. B 2016, 383, 227–233. [Google Scholar] [CrossRef]
- Qi, Y.; Ping, C.; Gao, Y.; Ma, K.; Chun, L.; Xuhuai, X. Effects of electron irradiation in space environment on thermal and mechanical properties of carbon fiber/bismaleimide composite. Nucl. Instrum. Methods Phys. Res. Sect. B 2014, 336, 158–162. [Google Scholar]

| Sample | EPPS10 | EPPS15 | EPPS20 | EPP25 | EPP30 |
|---|---|---|---|---|---|
| Rq (nm) | 240 | 267 | 416 | 480 | 726 |
| Ra (nm) | 188 | 207 | 335 | 505 | 526 |
| Radiation Dose (Mrad) | 0.83 | 1.54 | 2.26 | 5.11 | 12.30 |
| Static contact angle (°) | 152 ± 0.3 | 152 ± 0.6 | 153 ± 0.6 | 153 ± 0.5 | 152 ± 0.3 |
| Adhesion | 5B | 5B | 5B | 5B | 5B |
| Hardness | 6H | 6H | 6H | 6H | 6H |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, J.; Liu, Y. Superhydrophobic Surface with Gamma Irradiation Resistance and Self-Cleaning Effect in Air and Oil. Coatings 2020, 10, 106. https://doi.org/10.3390/coatings10020106
Zhang Y, Zhang J, Liu Y. Superhydrophobic Surface with Gamma Irradiation Resistance and Self-Cleaning Effect in Air and Oil. Coatings. 2020; 10(2):106. https://doi.org/10.3390/coatings10020106
Chicago/Turabian StyleZhang, Yan, Jing Zhang, and Yujian Liu. 2020. "Superhydrophobic Surface with Gamma Irradiation Resistance and Self-Cleaning Effect in Air and Oil" Coatings 10, no. 2: 106. https://doi.org/10.3390/coatings10020106
APA StyleZhang, Y., Zhang, J., & Liu, Y. (2020). Superhydrophobic Surface with Gamma Irradiation Resistance and Self-Cleaning Effect in Air and Oil. Coatings, 10(2), 106. https://doi.org/10.3390/coatings10020106





